Background: Alterations in hERG-encoded K+ channel current can cause fatal cardiac electrical disturbances.
Results: Caveolin-3 enhances ubiquitin ligase Nedd4-2 interaction with mature hERG channels in the plasma membrane, leading to decreased channel expression.
Conclusion: Caveolin-3 regulates hERG expression and thus function via Nedd4-2.
Significance: Understanding of hERG regulation pathway is important for cardiac electrophysiology and antiarrhythmic strategies.
Keywords: Caveolin, hERG, Patch Clamp, Protein Expression, Ubiquitin Ligase
Abstract
The human ether-a-go-go-related gene (hERG) encodes the rapidly activating delayed rectifier potassium channel (IKr) which plays an important role in cardiac repolarization. A reduction or increase in hERG current can cause long or short QT syndrome, respectively, leading to fatal cardiac arrhythmias. The channel density in the plasma membrane is a key determinant of the whole cell current amplitude. To gain insight into the molecular mechanisms for the regulation of hERG density at the plasma membrane, we used whole cell voltage clamp, Western blotting, and immunocytochemical methods to investigate the effects of an integral membrane protein, caveolin-3 (Cav3) on hERG expression levels. Our data demonstrate that Cav3, hERG, and ubiquitin-ligase Nedd4-2 interact with each other and form a complex. Expression of Cav3 thus enhances the hERG-Nedd4-2 interaction, leading to an increased ubiquitination and degradation of mature, plasma-membrane localized hERG channels. Disrupting Nedd4-2 interaction with hERG by mutations eliminates the effects of Cav3 on hERG channels. Knockdown of endogenous Cav3 or Nedd4-2 in cultured neonatal rat ventricular myocytes using siRNA led to an increase in native IKr. Our data demonstrate that hERG expression in the plasma membrane is regulated by Cav3 via Nedd4-2. These findings extend our understanding of the regulation of hERG channels and cardiac electrophysiology.
Introduction
The rapidly activating delayed rectifier K+ channel (IKr),3 encoded by the human ether-a-go-go-related gene (hERG), plays an important role in cardiac repolarization (1, 2). A reduction in the hERG current (IhERG) delays cardiac repolarization and causes long QT syndrome (3). However, an increase in IhERG can cause short QT syndrome (4). Both long QT syndrome and short QT syndrome predispose individuals to high risk of lethal arrhythmias and sudden death (3, 5). Although mutation- or drug-mediated alterations in IhERG have been studied extensively, little is known about how the density of wild-type (WT) hERG channels in the plasma membrane is regulated. In particular, the mechanism for degradation of mature hERG channels in the plasma membrane is unknown.
We demonstrated previously that a reduction in extracellular K+ concentration, clinically known as hypokalemia, induces endocytic degradation of the cell surface hERG channels (6). There are many ways that endocytic cargo molecules can be internalized. Clathrin-dependent endocytosis is the best characterized route, which mediates degradation of various membrane proteins including ion channels such as cystic fibrosis transmembrane conductance regulator and connexin 43 (7, 8). Caveolin-dependent endocytosis represents another pathway for membrane protein internalization (9, 10). We have previously demonstrated that caveolin-1 (Cav1), but not clathrin, is involved in the low K+-induced endocytosis of hERG channels expressed in HEK 293 cells (11, 12). However, the degradation routes for hERG channels in normal (5 mm K+) culture conditions are unknown.
The caveolin protein family contains three distinct isoforms. Whereas Cav1 and caveolin-2 (Cav2) are expressed in nonmuscle cells such as adipocytes, endothelial and epithelial cells as well as fibroblasts, caveolin-3 (Cav3) is muscle-specific. In particular, HEK 293 cells express Cav1, and cardiomyocytes express Cav3 (13). In the present study, we investigated the effects of Cav3 on hERG expression and function. Using electrophysiological, Western blotting, and immunocytochemical analyses, our data demonstrate that overexpression of Cav3 significantly decreases hERG channel density in the plasma membrane. Mechanistically, Cav3-induced hERG reduction is accompanied by enhanced hERG ubiquitination through the ubiquitin (Ub) ligase, Nedd4-2 (neural precursor cell expressed developmentally down-regulated protein 4 subtype 2). In cultured neonatal rat ventricular myocytes, knockdown of endogenous Cav3 or Nedd4-2 leads to an increase in IKr. Our data indicate that Cav3-mediated endocytic degradation may play a role in the homeostatic regulation of IKr expression at the plasma membrane.
EXPERIMENTAL PROCEDURES
Molecular Biology
hERG cDNA was provided by Dr. Gail Robertson (University of Wisconsin-Madison). Human ether-a-go-go (EAG) cDNA was provided by Dr. Luis Pardo (Max-Planck Institute of Experimental Medicine, Göttingen, Germany). Kv1.5 cDNA (encoding the cardiac ultrarapidly activating delayed rectifier K+ channel) was provided by Dr. Michael Tamkun (Colorado State University, Fort Collins, CO). Cav3 plasmid and Myc-tagged plasmid of Nedd4-1 were obtained from Origene Technologies. The human Nedd4-2 plasmid in pBluescript II was obtained from Kazusa DNA Research Institute (Chiba, Japan). The open reading frame was amplified using PCR and cloned into HA-pcDNA3 (Invitrogen) to generate HA-tagged Nedd4-2 (Nedd4-2-HA). The hERG point mutation Y1078A and C-terminal truncation mutation Δ1073 were generated using PfuUltra Hotstart PCR Master Mix (Agilent Technologies, Santa Clara, CA) and confirmed by DNA sequencing (Eurofins MWG Operon, Huntsville, AL). A HEK 293 cell line stably expressing hERG channels (hERG-HEK cells) was obtained from Dr. Craig January (University of Wisconsin-Madison). Lipofectamine 2000 (Invitrogen) was used for transfecting various plasmids and siRNAs into HEK 293 cells. Stable cell lines were generated using G418 for selection (1 mg/ml) and maintenance (0.4 mg/ml). HEK 293 cells were cultured in minimum essential medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). For electrophysiological studies with transiently expressed channels, GFP cDNA (pIRES2-EGFP, Clontech) was co-transfected for selection of transfected cells. Neonatal rat ventricular myocytes were isolated from 1-day-old Sprague-Dawley rats of either sex by enzymatic dissociation as described previously (14). Lipofectamine RNAiMAX (Invitrogen) was used for transfecting siRNA into cultured neonatal rat ventricular myocytes.
Patch Clamp Recording Method
The whole cell patch clamp method was used to record the hERG (IhERG), EAG (IEAG), and Kv1.5 currents (IKv1.5). The pipette solution contained 135 mm KCl, 5 mm EGTA, 1 mm MgCl2, and 10 mm HEPES (pH 7.2 with KOH). The bath solution contained 135 mm NaCl, 5 mm KCl, 10 mm HEPES, 10 mm glucose, 1 mm MgCl2, and 2 mm CaCl2 (pH 7.4 with NaOH). IhERG, IEAG, or IKv1.5 was evoked by incremental depolarization to voltages between −70 and 70 mV. The tail currents were recorded upon a repolarizing step to −50 mV. The holding potential was −80 mV. For the current amplitude analyses, the peak tail current at −50 mV following the 50-mV depolarizing step for the hERG channel, and the pulse current at the end of the depolarizing step to 50 mV for the Kv1.5 and EAG channel were used. Isolated IKr in neonatal ventricular myocytes was recorded using symmetrical Cs+ solutions by depolarizing pulses to voltages between −70 and +70 mV in 10-mV increments. Tail currents upon repolarization to the holding potential of −80 mV after depolarizing pulse to +50 mV were used to measure the current amplitude (15). Patch clamp experiments were performed at room temperature (22 ± 1 °C).
Western Blot Analysis and Co-immunoprecipitation (Co-IP)
Membrane protein of ventricular tissues from adult Sprague-Dawley rats and New Zealand White rabbits as well as whole cell protein from hERG-HEK cells were used. Western blot analysis was performed using the previously described procedure (6, 11, 16). The Precision Plus Protein Dual Color Standards (161-0374; Bio-Rad) was used as protein ladder. To confirm that the 155-kDa hERG protein is localized at the plasma membrane, cell surface proteins were digested by proteinase K treatment; live cells were washed with PBS and treated with 200 μg/ml proteinase K (Sigma) in a physiological buffer, containing 10 mm HEPES, 150 mm NaCl, and 2 mm CaCl2 (pH 7.4) at 37 °C for 30 min. The reaction was terminated by adding ice-cold PBS containing 6 mm phenylmethylsulfonyl fluoride and 25 mm EDTA. The hERG expression levels of whole cell proteins extracted from control and proteinase K-treated hERG-HEK cells were analyzed using Western blotting. For co-IP analysis of Ub-hERG interactions, 50 μm ALLN was added to the culture medium to inhibit hERG degradation. For co-IP, 0.5 mg of the protein was incubated with the appropriate primary antibody overnight at 4 °C and then precipitated with protein A/G Plus-agarose beads at 4 °C for 4 h. The beads were washed three times with ice-cold radioimmuneprecipitation assay lysis buffer, resuspended in 2× Laemmli sample buffer, and boiled for 5 min. The samples were centrifuged at 20,000 × g for 5 min, and the supernatants were analyzed using Western blotting. An anti-hERG antibody (C-20, sc-15968; Santa Cruz Biotechnology) was used for co-IP analyses between ERG and Cav3 in samples from rat and rabbit ventricular tissues. This antibody binds to an epitope between residues 1129 and 1159 in humans. This region of residues has 30/31 amino acids in common with rats and is identical with rabbits.
Immunofluorescence Microscopy
hERG-HEK or HEK 293 cells were transiently transfected with Nedd4-2-HA or Cav3 plasmid, respectively. Twenty four hours after transfection, cells were fixed and permeabilized. hERG channels were stained with a rabbit anti-hERG primary and Alexa Fluor 488-conjugated donkey anti-rabbit antibodies. Nedd4-2-HA protein was detected with mouse anti-HA primary and Alexa Fluor 594-conjugated goat anti-mouse antibodies. The endogenous Nedd4-2 was stained with a rabbit anti-Nedd4-2 antibody and an Alexa Fluor 594-conjugated goat anti-rabbit antibody. Cav3 was stained with a mouse anti-Cav3 antibody and an Alexa Fluor 488-conjugated donkey anti-mouse antibody. Nuclei were stained with Hoechst 33342 (0.2 μg/ml; Sigma). Cultured neonatal rat ventricular myocytes were transfected with Cav3 siRNA, Nedd4-2 siRNA, or scrambled control siRNA and cultured for 24–48 h. Cell surface IKr protein was labeled with a rabbit anti-hERG (anti-Kv11.1, P0749; Sigma) antibody at room temperature for 20 min. This antibody corresponds to residues 430–445 of human ERG (AFLLKETEEGPPATEC), which shares 11/16 residues with rat and 15/16 residues with rabbit. After labeling, the unbound antibody was washed away, and the cells were cultured at 37 °C for 4 h, fixed, and permeabilized. Alexa Fluor 488-conjugated donkey anti-rabbit antibody was used to stain ERG. Hoechst 33342 (0.2 μg/ml) was used to stain nuclei. Images were acquired using a Leica TCS SP2 Multi Photon confocal microscope.
Reagents and Antibodies
Rabbit anti-Kv11.1 (hERG), anti-Kv10.1 (EAG-1), mouse anti-Myc, anti-HA, and anti-actin antibodies, G418, proteinase K, and ALLN were purchased from Sigma. Goat anti-hERG (C-20 and N-20), anti-actin and anti-GAPDH, rabbit anti-Kv1.5 and anti-GAPDH, mouse anti-Cav1, anti-Cav3, anti-Na-K ATPase, and anti-GAPDH; donkey anti-goat IgG, goat anti-mouse IgG and anti-rabbit IgG; siRNAs for Cav3, Nedd4-2 and scrambled control; and protein A/G Plus-agarose for immunoprecipitation assay were purchased from Santa Cruz Biotechnology. Rabbit anti-Nedd4-2 antibodies were obtained from Abcam and New England Biolabs. The biotinylation kit for surface protein isolation was purchased from Pierce.
All data are expressed as the mean ± S.E. A one-way ANOVA or two-tailed Student's t test was used to determine statistical significance between the control and test groups. A p value ≤0.05 was considered significant.
RESULTS
Cav3 Decreases hERG Expression in the Plasma Membrane
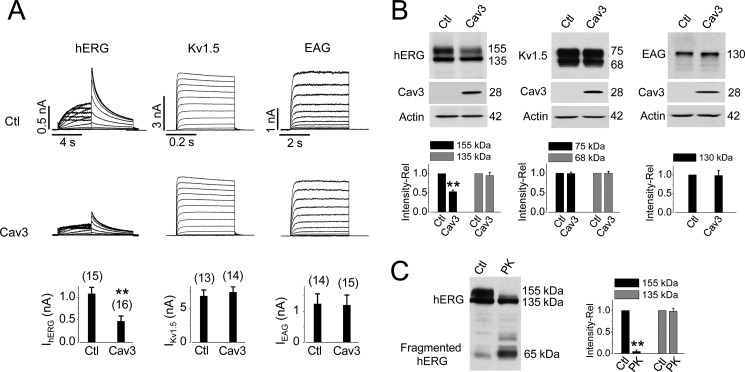
Fig. 1 illustrates the effects of Cav3 expression on the function and expression of hERG, Kv1.5, and EAG channels stably expressed in HEK 293 cells. Cav3 or empty pcDNA3 plasmid (control, Ctl) was transiently transfected into each of the stable cell lines. Transfected cells were cultured in regular minimum essential medium for 24 h and then collected for whole cell patch clamp experiments. Cav3 expression significantly decreased IhERG. However, it did not affect the Kv1.5 current (IKv1.5) or EAG current (IEAG) (Fig. 1A). Cav3 expression did not affect the biophysical properties of IhERG. The half-activation voltage and the slope factor of IhERG were −9.1 ± 0.9 mV and 7.6 ± 0.6 mV in control cells, and −11.2 ± 0.7 mV and 8.1 ± 0.7 mV in Cav3-transfected cells (p > 0.05).
FIGURE 1.
Overexpression of Cav3 reduces the hERG expression at the plasma membrane. A, effects of Cav3 on hERG, Kv1.5, and EAG currents. Representative currents in pcDNA3-transfected (control, Ctl) or Cav3-transfected cells are shown. The current amplitudes under various conditions are shown below the current traces. The numbers in parentheses above each bar indicate the number of cells tested. B, effects of Cav3 on the expression levels of hERG, Kv1.5, and EAG channel proteins. The relative band intensities of various channel proteins in the presence of Cav3 versus those in the absence of Cav3 are summarized beneath the representative Western blot images (n = 8 for hERG, n = 5 for Kv1.5, and n = 4 for EAG). C, hERG 155-kDa band localized on the plasma membrane. The relative intensities of the 155-kDa and 135-kDa band from hERG-HEK cells treated with proteinase K (PK) versus those from control cells are shown. n = 5; **, p < 0.01 versus control (Ctl).
To determine whether Cav3 decreases IhERG through reducing the hERG channel density in the plasma membrane, hERG protein expression was detected using Western blot analysis. Whole cell protein was extracted from hERG-HEK cells after transfection with either Cav3 or an empty pcDNA3 plasmid (control) for 24 h. The effects of Cav3 on the Kv1.5 and EAG expression were also investigated. The hERG protein extracted from hERG-HEK cells displayed two bands with molecular masses of 155 and 135 kDa, representing the mature, fully glycosylated form in the plasma membrane and the immature, core-glycosylated form in the endoplasmic reticulum, respectively (14, 17). To quantify the effects of Cav3 on the expression level of various channels, the intensity of the respective protein band from cells transfected with Cav3 was expressed as a value relative to that from control cells. Expression of Cav3 significantly reduced the intensity of the 155-kDa hERG band but did not affect the 135-kDa hERG band. Expression of Cav3 did not affect the expression of Kv1.5 or EAG channels stably expressed in HEK 293 cells (Fig. 1B).
We have shown previously that the 155-kDa hERG band represents the mature channels localized in the plasma membrane, and the 135-kDa band represents immature channels within the cells (14, 16). As shown in Fig. 1C, exposure of live hERG-HEK cells to a broad spectrum serine protease, proteinase K, resulted in a selective digestion of the 155-kDa band protein, confirming that the 155-kDa hERG protein is localized in the plasma membrane. Thus, expression of Cav3 selectively decreases the expression level of plasma membrane-localized mature (155-kDa) hERG channels without affecting the expression level of the 135-kDa hERG channels.
Cav3 Accelerates hERG Degradation and Enhances hERG Ubiquitination
One possible way for Cav3 to decrease expression levels of the mature hERG band is to facilitate hERG degradation. To test this, we used brefeldin A (10 μm), which inhibits transport of newly synthesized proteins from the endoplasmic reticulum to Golgi, to block the conversion of hERG from its immature 135-kDa form to its mature 155-kDa form (11, 17, 18). IhERG in brefeldin A-treated hERG-HEK cells displayed a time-dependent decline, reflecting the natural degradation of the existing hERG channels in the plasma membrane (11, 18). Expression of Cav3 significantly accelerated the rate of IhERG decay. As shown in Fig. 2A, the relative reductions in IhERG after 6 h of brefeldin A treatment were more pronounced in Cav3-expressing cells than that in the control cells.
FIGURE 2.
Cav3 accelerates hERG degradation and enhances hERG-Ub interaction. A, time-dependent decrease in IhERG in brefeldin A (BFA)-treated cells transfected with or without Cav3. hERG tail currents at each recording time point in both groups are summarized on the left, and the relative current amplitudes normalized to the initial values are summarized on the right below the traces (n = 6–9 cells at each point). B, Cav3 interacting with hERG channels. Cav3 or hERG was detected in proteins precipitated with an anti-hERG or anti-Cav3 antibody from whole cell proteins extracted from hERG-HEK cells 24 h after Cav3 transfection (n = 6 or 8, respectively). A fraction of proteins used for the pulldown assay was also immunoblotted for hERG expression in lane 1 of the right panel in B. C, Cav3-enhanced hERG-Ub interaction. hERG was detected in proteins precipitated with an anti-Ub antibody from whole cell proteins extracted from hERG-HEK cells but not from HEK cells transfected with Cav3. Compared with pcDNA3 transfection, Cav3 expression increases the hERG-Ub interaction (n = 9). D, Nedd4-2 targeting PPXY motif in the hERG channel. Alignment of the PY motif (bold) of the Nav1.5 channel, ENaC subunits, and the hERG channel (GenBank numbers in italics) is shown. Position number indicates the location of first proline (P) in the PPXY sequence.
To investigate the mechanism of Cav3-induced hERG reduction, we performed co-IP analysis to study the association between hERG channels and Cav3. Whole cell lysates were extracted from hERG-HEK cells 12 h after Cav3 transfection. An anti-hERG antibody was used to immunoprecipitate hERG and its associated proteins. The proteins were then immunoblotted to detect Cav3; a strong Cav3 band was detected in the hERG antibody-precipitated proteins (Fig. 2B, left). Inversely, when the cell lysates were immunoprecipitated with an anti-Cav3 antibody, the 155-kDa but not 135-kDa band of hERG proteins was detected in the precipitated proteins (Fig. 2B, right). Thus, Cav3 interacts with the mature hERG channels.
Covalent binding of Ub to proteins, a process known as ubiquitination, is well known for labeling proteins for degradation (19). To test whether Cav3 promotes hERG degradation through enhancing hERG ubiquitination, we performed co-IP experiments to determine the effects of Cav3 on the Ub-hERG interactions. Whole cell lysates were immunoprecipitated with an anti-Ub antibody, and hERG expression levels in the precipitates were detected. As shown in Fig. 2C, Cav3-transfected cells displayed a darker band close to 155 kDa, likely reflecting the monoubiquitinated mature hERG channels (20). Thus, Cav3 promotes hERG degradation through enhancing ubiquitination of hERG.
Ubiquitination is carried out by a series of enzymes including the E3 Ub ligases which selectively attach Ub to target proteins through recognition motifs (21). Of particular interest is the Ub ligase Nedd4-2 which regulates the epithelial Na+ channel (ENaC) (22). Nedd4-2 binds to the PPXY (PY) motif of ENaC via its WW domains (22), leading to internalization of ENaC subunits from the cell surface (23). Mutations in the PY motif of ENaC disrupt Nedd4-2 binding and result in an accumulation of ENaC at the apical membrane of kidney epithelial cells. This increases tubular Na+ reabsorption (23) and causes Liddle syndrome, an autosomal dominant disorder characterized by early, frequently severe hypertension. Nedd4-2 also binds to the PY motif of the cardiac Na+ channel, Nav1.5, enhances channel ubiquitination, leading to a reduction of heterologously expressed Nav1.5 channels (24, 25). Notably, the conserved PY motif is present in the C terminus of the hERG channel but not present in either Kv1.5 (NCBI NP_002225.2) or EAG (NP_758872.1) channel (Fig. 2D). Indeed, we have reported previously that Nedd4-2 ubiquitinates and eliminates mature hERG channels (26). This observation was subsequently confirmed in a study reported by Albesa et al. (27).
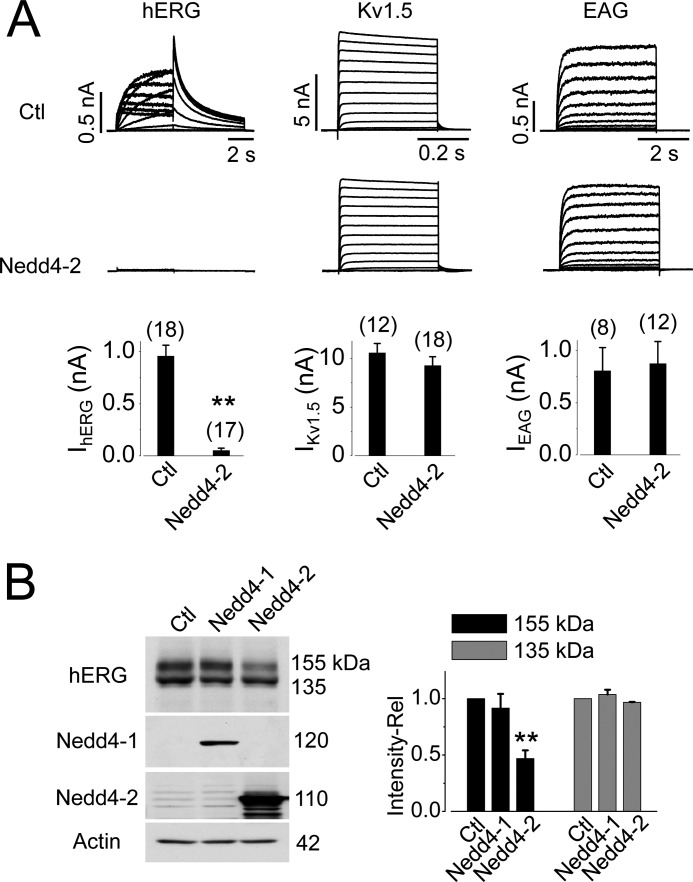
Fig. 3A shows the effects of Nedd4-2 overexpression on IhERG, IKv1.5, and IEAG. Overexpression of Nedd4-2 completely eliminated IhERG. In contrast, it had no effect on either IKv1.5 or IEAG. Thus, among three channels examined, Nedd4-2-mediated regulation is specific for the hERG channel.
FIGURE 3.
Overexpression of Nedd4-2 eliminates IhERG and decreases hERG 155-kDa expression level. A, effects of Nedd4-2 overexpression on IhERG, IKv1.5, and IEAG. Summarized current amplitudes are shown beneath the respective families of current traces. The numbers in parentheses above each bar indicate the number of cells tested. B, effects of Nedd4-1 and Nedd4-2 overexpression on hERG expression. The relative intensities of hERG bands in cells transfected with Nedd4-1 (n = 4) or Nedd4-2 (n = 7) versus those in control cells are summarized beside the representative Western blot image. **, p < 0.01 versus control (Ctl).
To determine whether the eliminated IhERG in Nedd4-2-overexpressed cells was the result of increased hERG degradation, the expression level of hERG channels was examined. Nedd4-2 overexpression significantly decreased the 155-kDa, but not the 135-kDa, band intensity of hERG channels (Fig. 3B). As well, the Nedd4 family member Nedd4-1 (28) had no effect on hERG channel expression (Fig. 3B).
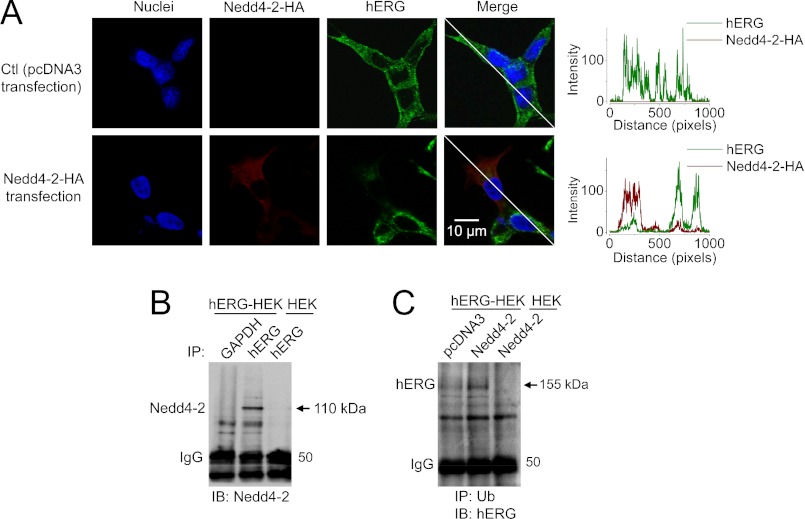
Our data show that Nedd4-2 overexpression completely eliminated IhERG (Fig. 3A) but only partially reduced the 155-kDa expression level of hERG channels (Fig. 3B). This seemingly discrepancy resulted from the fact that the transfection rate of Nedd4-2 into hERG-HEK cells is about ∼50%. Whereas single Nedd4-2-expressing (GFP-positive) cells were selected for IhERG recording, both Nedd4-2-expressing and Nedd4-2-nonexpressing cells were collected for Western blot analysis. Thus, the remaining 155-kDa protein likely reflects mature hERG channels in those cells without Nedd4-2 overexpression. This notion is directly supported by our immunocytochemical analysis shown in Fig. 4A. In the group of cells transfected with Nedd4-2-HA plasmid for 24 h, only a fraction of cells expressed Nedd4-2. Compared with Nedd4-2-HA-nonexpressing cells, Nedd4-2-HA-expressing cells displayed a diminished hERG expression (Fig. 4A).
FIGURE 4.
Interactions between hERG and Nedd4-2. A, confocal images show that Nedd4-2 overexpression reduces hERG expression. The expression of hERG and Nedd4-2-HA is demonstrated by showing their intensities in the lines across both Nedd4-2-HA-nonexpressing and Nedd4-2-HA-expressing cells. B, hERG interacts with endogenous Nedd4-2. Nedd4-2 was detected in proteins precipitated with an anti-hERG antibody from whole cell proteins extracted from hERG-HEK, but not from HEK cells (n = 5). C, Nedd4-2 enhances hERG ubiquitination. hERG was detected in proteins precipitated with an anti-Ub antibody from whole cell proteins extracted from hERG-HEK cells, but not from HEK cells. Compared with pcDNA3 transfection, Nedd4-2 overexpression increases the hERG-Ub interaction (n = 6).
Nedd4-2 is endogenously expressed in HEK 293 cells. We examined the interaction between Nedd4-2 and hERG using co-IP analysis in hERG-HEK cells. When the whole cell lysate was precipitated with anti-hERG antibody, Nedd4-2 was detected in the precipitated proteins (Fig. 4B), suggesting that hERG interacts with Nedd4-2. To further examine whether Nedd4-2 overexpression enhances hERG ubiquitination, levels of ubiquitinated hERG channels were compared between the control (pcDNA3-transfected) and Nedd4-2-transfected hERG-HEK cells. As shown in Fig. 4C, hERG was detected in Ub antibody-precipitated proteins in control cells, likely reflecting the basal level of ubiquitinated hERG channels. Nedd4-2 overexpression significantly enhanced the amount of ubiquitinated hERG channels. The intensity of the band with a molecular mass close to 155 kDa was enhanced, suggesting that Nedd4-2 promotes monoubiquitination of the mature hERG channels (20).
Cav3 Decreases hERG Channels via Nedd4-2
Among K+ channels hERG, Kv1.5, and EAG, only hERG possesses a PY motif and is sensitive to Nedd4-2 (Figs. 2D and 3). Consistent with the results reported by Albesa et al. (27), our data show that Nedd4-2 interacts with the PY motif of hERG to induce channel degradation. As shown in Fig. 5, A and B, disruption of PY motif, by point mutation Y1078A or C-terminal truncation mutation Δ1073, eliminates the effects of Nedd4-2 overexpression on the expression level and the current of hERG channels. Interestingly, these PY motif-disrupting mutations in hERG also eliminated the Cav3-induced reduction in hERG expression and IhERG (Fig. 5, C and D). These data indicate that the integrity of hERG-Nedd4-2 interaction is required for Cav3-induced reduction of hERG expression.
FIGURE 5.
Mutations disrupting Nedd4-2 targeting site in hERG eliminate both Nedd4-2 and Cav3-mediated effects on hERG channels. A and B, effects of Nedd4-2 overexpression on the protein expressions (A) and the currents (B) of WT, Y1078A, or Δ1073 hERG channels. C and D, effects of Cav3 overexpression on the protein expressions (C) and the currents (D) of WT, Y1078A, or Δ1073 hERG channels. Stable HEK cell lines expressing WT, Y1078A, or Δ1073 mutant hERG channels were transfected with pcDNA3 (Ctl), Nedd4-2, or Cav3 plasmid. Experiments were performed 24 h after transfection. For Western blot analysis, each set of experiments was repeated four to seven times, respectively. For patch clamp experiments, the number of cells examined is shown above the bars. *, p < 0.05; **, p < 0.01 versus control.
Next, we investigated how Cav3 interacts with Nedd4-2 to enhance hERG degradation. As demonstrated in Fig. 1C, the mature, 155-kDa form of hERG is localized in the plasma membrane. Interestingly, our data show that both Nedd4-2 and Cav3 overexpression selectively decrease the 155-kDa, but not the 135-kDa form of hERG (Figs. 1, 3, and 5). In other words, both Nedd4-2 and Cav3 selectively target the membrane-localized hERG channels.
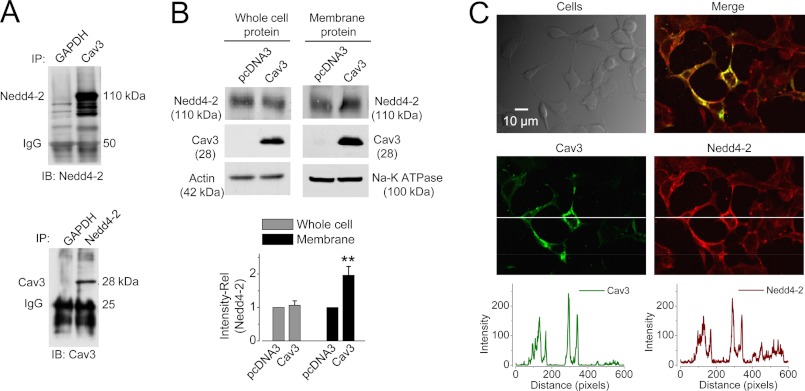
Cav3 is localized predominantly in the caveolae, small invaginations of the plasma membrane. Nedd4-2 contains a catalytic C-terminal HECT domain, a N-terminal C2 domain and several WW domains (29). Whereas WW domains are responsible for substrate recognition by binding to the PPXY motif, the C2 domain functions as a Ca2+-dependent phospholipid binding domain (29–31). It has been shown that the C1 and C2 domains target human type 6 adenylyl cyclase to lipid rafts and caveolae (32). Because caveolin is the signature component of caveolae, we reasoned that expression of Cav3 may enhance cell membrane localization of Nedd4-2 through C2 domain-mediated membrane translocation of Nedd4-2. To this end, we used co-IP analysis to investigate the interactions between Nedd4-2 and Cav3 in HEK 293 cells transfected with Cav3 plasmid for 24 h. As shown in Fig. 6A, Nedd4-2 or Cav3 was detected in Cav3- or Nedd4-2 antibody-precipitated proteins, respectively. Because our data have also shown that both Cav3 and Nedd4-2 interact with hERG (Figs. 2B and 4B), Cav3, Nedd4-2, and hERG likely form a complex in the plasma membrane.
FIGURE 6.
Interactions between Cav3 and Nedd4-2. A, Cav3 immunoprecipitates with Nedd4-2. Nedd4-2 or Cav3 was detected in proteins precipitated with an anti-Cav3 or anti-Nedd4-2 antibody, respectively, from whole cell proteins extracted from HEK 293 cells 24 h after Cav3 transfection (n = 5, for each set of experiments). B, Cav3 expression does not affect the whole cell expression level of Nedd4-2 but enhances the cell surface expression levels of Nedd4-2 (n = 4, **, p < 0.01 versus pcDNA3 (control)). C, overexpression of Cav3 enhances membrane localization of Nedd4-2 in HEK 293 cells. The expression of Cav3 and Nedd4-2 is demonstrated by showing their intensities in the line across both Cav3-nonexpressing and Cav3-expressing cells. Cav3 expression enhances the membrane expression of Nedd4-2.
To study further the interaction between Nedd4-2 and Cav3, we detected the effect of Cav3 expression on the protein expression and distribution of endogenous Nedd4-2. As shown in Fig. 6B, expression of Cav3 in HEK cells did not affect the total endogenous Nedd4-2 expression in the whole cell lysate. However, it significantly increased the Nedd4-2 expression in the cell surface proteins isolated using biotinylation method. These data suggest that Cav3 expression recruits Nedd4-2 from cytosol to the plasma membrane. This notion is supported by our immunocytochemical results shown in Fig. 6C. When HEK 293 cells were transfected with Cav3, only a fraction of cells displayed Cav3 expression. Thus, the non-Cav3-expressing cells serve as an ideal control for the Cav3-expressing cells. Compared with the non-Cav3 expressing cells, Cav3-expressing cells display enhanced Nedd4-2 expression, which is co-localized with Cav3 in the plasma membrane (Fig. 6C).
To investigate further the role of Cav3 and Nedd4-2 in the homeostasis of hERG channels, we created a HEK 293 stable cell line that concomitantly expresses both Cav3 and hERG channels. Because HEK 293 cells endogenously express Nedd4-2, this stable cell line mimics cardiac myocytes with respect to these three proteins (hERG, Cav3, and Nedd4-2). Knockdown of either Cav3 or Nedd4-2 using siRNA significantly enhanced IhERG and 155-kDa hERG expression levels (Fig. 7). We also found that co-silencing of Nedd4-2 and Cav3 resulted in increases in 155-kDa intensity and IhERG to extents similar to those of silencing of Nedd4-2 alone (data not shown), which is consistent with the notion that Cav3 decreases hERG through promoting Nedd4-2 interaction with mature hERG channels.
FIGURE 7.
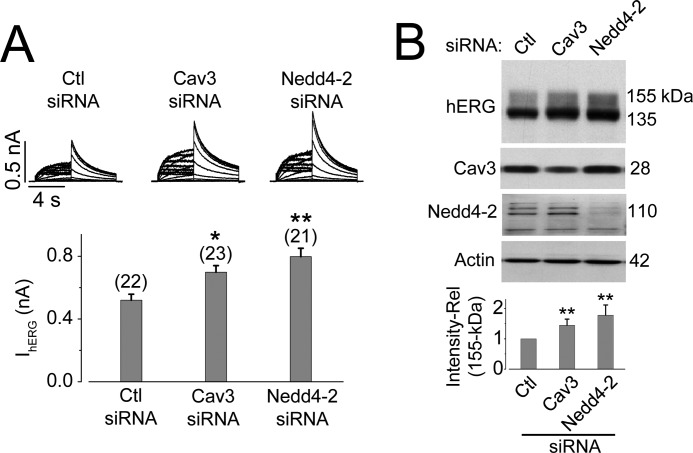
Knockdown of Cav3 or Nedd4-2 enhances IhERG (A) and the 155-kDa hERG expression (B) in a stable HEK 293 cell line expressing both hERG and Cav3. Cav3-hERG-HEK cells transfected with scrambled control siRNA, Cav3 siRNA, or Nedd4-2 siRNA for 24–48 h were collected for electrophysiology and Western blot analyses. A, effects of Cav3 siRNA or Nedd4-2 siRNA transfection on IhERG. The number above the bars in the lower panel indicates the number of cells examined. B, effects of Cav3 siRNA or Nedd4-2 siRNA transfection on the expression levels of hERG, Cav3, and Nedd4-2. The hERG 155-kDa expression levels under each condition are shown in the lower panel (n = 5). Compared with the control siRNA transfection, Cav3 siRNA transfection reduced Cav3 expression by 61% ± 13% (p < 0.01, n = 5), and Nedd4-2 siRNA transfection reduced Nedd4-2 expression by 81% ± 5% (p < 0.01, n = 5). *, p < 0.05; **, p < 0.01 versus control (Ctl) siRNA.
Knockdown of Endogenous Cav3 or Nedd4-2 Enhances IKr in Neonatal Ventricular Myocytes
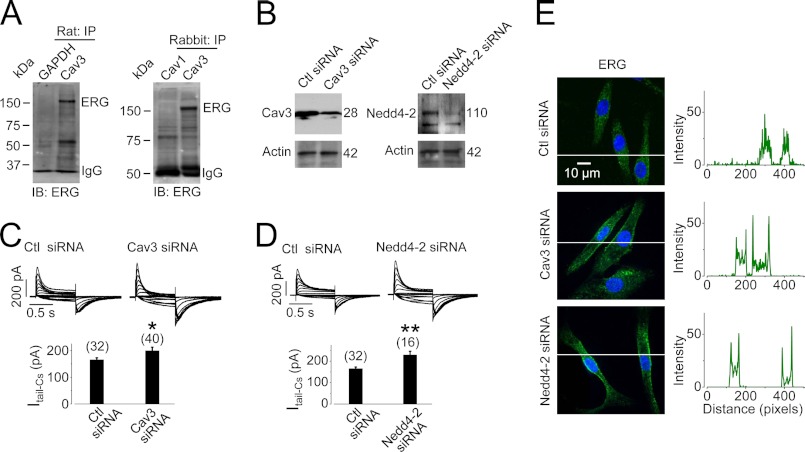
Cav3 and IKr (ERG proteins) are endogenously expressed in cardiomyocytes. We performed co-IP analysis to determine whether a native Cav3-ERG interaction exists in cardiac myocytes. Membrane proteins from rat or rabbit ventricular tissues were isolated. The proteins were precipitated with an anti-Cav3 antibody and detected for ERG expression using an anti-hERG antibody. An anti-GAPDH antibody was used as a negative control to distinguish nonspecific bindings. In addition, because Cav1 is expressed primarily in nonmuscle cells where ERG is not expressed, we also used an anti-Cav1 antibody as a negative control. As shown in Fig. 8A, a band of ERG slightly higher than 150 kDa was detected in Cav3 antibody-precipitated proteins from both rat and rabbit ventricular tissues. Thus, an interaction between native Cav3 and ERG does exist in cardiac myocytes.
FIGURE 8.
Cav3 interacts with IKr in ventricular tissues. A, Cav3 interacts with native ERG channels. ERG was detected in proteins precipitated with an anti-Cav3 antibody from membrane proteins extracted from both rat (n = 4) and rabbit ventricular tissues (n = 5). GAPDH or Cav1 was used as negative control. B, knockdown of Cav3 or Nedd4-2 expression is shown in cultured neonatal rat ventricular myocytes. C and D, knockdown of Cav3 (C) or Nedd4-2 (D) increases IKr in cultured neonatal rat ventricular myocytes. Families of Cs+-mediated IKr were recorded 24–48 h after siRNA transfection. The numbers in parentheses above the bars indicate the number of cells tested. *, p < 0.05; **, p < 0.01 versus control (Ctl) siRNA. E, knockdown of Cav3 or Nedd4-2 retains cell surface ERG proteins in cultured neonatal rat ventricular myocytes after 4-h culture. The intensities of green signals along the lines across the cells are plotted against pixel distances. Whereas the signal is evenly distributed in control siRNA-transfected cells, it peaks at the plasma membrane in Cav3- or Nedd4-2 siRNA-transfected cells.
To test whether Cav3 participates in ERG homeostatic degradation via Nedd4-2 in cardiomyocytes, we knocked down Cav3 or Nedd4-2 by transfecting the respective siRNA into cultured neonatal rat ventricular myocytes (Fig. 8B). IKr-Cs was recorded with symmetrical Cs+ solutions. Cs+ selectively permeates through IKr channels but blocks other cardiac K+ channels (15). Thus, Cs+-mediated IKr represents pure IKr isolated from other cardiac K+ currents (15). Knockdown of Cav3 or Nedd4-2 resulted in increases in IKr (Fig. 8, C and D).
To examine the role of Cav3 and Nedd4-2 in ERG homeostatic degradation, we labeled the cell surface ERG and monitored its fate in cultured neonatal rat ventricular myocytes 24–48 h after transfection with control siRNA, Cav3 siRNA, or Nedd4-2 siRNA. Live cells were treated with an anti-hERG antibody that targets an extracellularly localized motif between S1 and S2 of the channel to label cell surface ERG. After labeling, the unbound antibodies were washed away, and the cells were cultured at 37 °C for 4 h to trace the fate of labeled ERG. In control cells (transfected with control siRNA), most of the labeled ERG channels were internalized, resulting in a pattern where labeled ERG was distributed throughout the cell. In contrast, knockdown of Cav3 or Nedd4-2 by siRNA transfection caused a significant amount of labeled ERG to remain at the cell surface. These data suggest that a reduction in Cav3 or Nedd4-2 expression slows down the endocytic degradation of ERG channels (Fig. 8E).
DISCUSSION
hERG plays an important role in the repolarization of cardiac action potentials, and alterations therein can cause life-threatening cardiac arrhythmias (33). The homeostasis of hERG in the plasma membrane is determined by anterograde (targeting to plasma membrane) and retrograde trafficking (retrieval from plasma membrane). Whereas mutations and drugs disrupt forward hERG trafficking (14, 34, 35), the hERG degradation pathway remains largely unknown. In the present study, we demonstrated a novel degradation pathway of the hERG channel which involves two proteins, Cav3 and Nedd4-2.
The role of Cav3 in the homeostatic regulation of IKr has not been reported. A previous study using discontinuous sucrose density gradients has shown that IKr proteins in cardiac tissues and hERG channels expressed in HEK 293 cells are localized to cholesterol- and sphingolipid-enriched membrane fractions (36). However, a direct interaction between Cav3 and ERG protein in canine ventricular tissue was not identified (36). In the present study, our co-IP experiments revealed a Cav3-ERG interaction in rat and rabbit ventricular tissues and HEK 293 cells expressing both hERG and Cav3 (Figs. 2 and 8). Our data show that Cav3, Nedd4-2, and hERG interact with one another. Nedd4-2 targets the PPXY motif (Figs. 2 and 5) in the hERG C terminus to ubiquitinate (Fig. 4C) and therefore down-regulate hERG expression in the plasma membrane (Figs. 3 and 5). Overexpression of Cav3 similarly down-regulates mature hERG expression (Fig. 1). The following evidence suggests that Cav3 decreases hERG surface expression via Nedd4-2-mediated protein degradation. First, Cav3 enhances hERG ubiquitination (Fig. 2C). Second, disruption of Nedd4-2 binding site in hERG eliminates both Nedd4-2- and Cav3-induced hERG reduction (Fig. 5). Third, Cav3 interacts directly with Nedd4-2 (Fig. 6A). Because Cav3, an integral membrane protein, interacts with both hERG and Nedd4-2 (Figs. 2 and 6), it is likely that Cav3 recruits Nedd4-2 from the cytosol to the membrane to enhance the Nedd4-2 interaction with the membrane-localized mature hERG channels (Fig. 6, B and C).
Nedd4-2 is an E3 Ub ligase and regulates the ENaC (22), cardiac Nav1.5, and KCNQ1 channels (25, 37). The possible role of Nedd4-2 in hERG regulation is suggested from the existence of the Nedd4-2-specific binding motif (PPXY) in the C terminus of the hERG channel (Fig. 2D) and the existence of endogenous Nedd4-2 in the heart (38) (Fig. 8B). Two previous studies have reported that hERG does not interact with Nedd4-2 (39, 40). However, consistent with our previous observations (26) and the present study, a recent study also showed that Nedd4-2 overexpression down-regulates mature hERG channels expressed in HEK 293 cells (27). Thus, the hERG channel could be regulated at the cell surface via a mechanism involving Nedd4-2 alone. In addition, the present study demonstrates that Cav3 regulates hERG in HEK 293 cells and IKr in cardiac myocytes by promoting Nedd4-2-mediated ubiquitination and degradation of the target proteins.
Ub binding is known for triggering caveolin-mediated endocytosis (41). For instance, in clathrin-depleted cells, Ub can direct a caveolin-dependent endocytosis of EGFR (42, 43). Our data demonstrate that Cav3 expression results in an enhanced ubiquitination of hERG channels and decreased hERG expression. Thus, Cav3 both recruits Nedd4-2 to the plasma membrane and mediates endocytosis of hERG channels.
Caveolin is the signature protein of caveolae and plays a scaffolding role in endocytic trafficking (44). Various membrane proteins such as fibronectin (10), β1 integrins (45), transforming growth factor β (TGFβ) (46), and epidermal growth factor receptor (EGFR) (43) can be endocytosed through caveolin-dependent pathways. We recently demonstrated that, in HEK 293 cells, the endogenous form of caveolin, Cav1, is involved in the low K+-induced internalization of cell surface hERG channels (12). Because Cav3 is the isoform expressed in cardiac myocytes, we focus on the role of Cav3 in hERG regulation in the present study. In addition to demonstrating the role of Cav3 in the regulation of hERG channels heterologously expressed in HEK 293 cells, we also showed that knockdown of either Cav3 or Nedd4-2 leads to an increase in IKr in cultured neonatal rat ventricular myocytes (Fig. 8). These results indicate that Cav3- and Nedd4-2-mediated degradation plays a role in the homeostatic regulation of IKr in the plasma membrane. This information extends our understanding of cardiac electrophysiology and provides insight into new therapeutic strategies to treat cardiac electrical disorders such as long QT syndrome.
This work was supported by the Canadian Institutes of Health Research Grant MOP 72911 and the Heart and Stroke Foundation of Ontario Grant T 6612 (to S. Z.).
- IKr
- rapidly activating delayed rectifier potassium channel
- ALLN
- N-[N-(N-acetyl-l-leucyl)-l-leucyl]-l-norleucine
- Cav1
- Cav2, and Cav3, caveolin-1, -2, and -3, respectively
- co-IP
- co-immunoprecipitation
- EAG
- ether-a-go-go
- ENaC
- epithelial sodium channel
- hERG
- human ether-a-go-go-related
- Nedd4-2
- neural precursor cell expressed developmentally down-regulated protein 4 subtype 2
- Ub
- ubiquitin.
REFERENCES
- 1. Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: hERG encodes the IKr potassium channel. Cell 81, 299–307 [DOI] [PubMed] [Google Scholar]
- 2. Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995) hERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269, 92–95 [DOI] [PubMed] [Google Scholar]
- 3. Keating M. T., Sanguinetti M. C. (2001) Molecular and cellular mechanisms of cardiac arrhythmias. Cell 104, 569–580 [DOI] [PubMed] [Google Scholar]
- 4. Brugada R., Hong K., Dumaine R., Cordeiro J., Gaita F., Borggrefe M., Menendez T. M., Brugada J., Pollevick G. D., Wolpert C., Burashnikov E., Matsuo K., Wu Y. S., Guerchicoff A., Bianchi F., Giustetto C., Schimpf R., Brugada P., Antzelevitch C. (2004) Sudden death associated with short-QT syndrome linked to mutations in hERG. Circulation 109, 30–35 [DOI] [PubMed] [Google Scholar]
- 5. Patel C., Yan G. X., Antzelevitch C. (2010) Short QT syndrome: from bench to bedside. Circ. Arrhythm. Electrophysiol. 3, 401–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Massaeli H., Guo J., Xu J., Zhang S. (2010) Extracellular K+ is a prerequisite for the function and plasma membrane stability of HERG channels. Circ. Res. 106, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 7. Bradbury N. A., Cohn J. A., Venglarik C. J., Bridges R. J. (1994) Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J. Biol. Chem. 269, 8296–8302 [PubMed] [Google Scholar]
- 8. Piehl M., Lehmann C., Gumpert A., Denizot J. P., Segretain D., Falk M. M. (2007) Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol. Biol. Cell 18, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nabi I. R., Le P. U. (2003) Caveolae/raft-dependent endocytosis. J. Cell Biol. 161, 673–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sottile J., Chandler J. (2005) Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Mol. Biol. Cell 16, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo J., Massaeli H., Xu J., Jia Z., Wigle J. T., Mesaeli N., Zhang S. (2009) Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the hERG channel in human cell lines. J. Clin. Invest. 119, 2745–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Massaeli H., Sun T., Li X., Shallow H., Wu J., Xu J., Li W., Hanson C., Guo J., Zhang S. (2010) Involvement of caveolin in low K+-induced endocytic degradation of the cell surface hERG channels. J. Biol. Chem. 285, 27259–27264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams T. M., Lisanti M. P. (2004) The caveolin proteins. Genome Biol. 5, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo J., Massaeli H., Li W., Xu J., Luo T., Shaw J., Kirshenbaum L. A., Zhang S. (2007) Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J. Pharmacol. Exp. Ther. 321, 911–920 [DOI] [PubMed] [Google Scholar]
- 15. Zhang S. (2006) Isolation and characterization of IKr in cardiac myocytes by Cs+ permeation. Am. J. Physiol. Heart Circ. Physiol. 290, H1038–1049 [DOI] [PubMed] [Google Scholar]
- 16. Guo J., Wang T., Yang T., Xu J., Li W., Fridman M. D., Fisher J. T., Zhang S. (2011) Interaction between the cardiac rapidly (IKr) and slowly (IKs) activating delayed rectifier potassium channels revealed by low K+-induced hERG endocytic degradation. J. Biol. Chem. 286, 34664–34674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Z., Gong Q., Ye B., Fan Z., Makielski J. C., Robertson G. A., January C. T. (1998) Properties of hERG channels stably expressed in HEK 293 cells studied at physiological temperature. Biophys. J. 74, 230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo J., Li X., Shallow H., Xu J., Yang T., Massaeli H., Li W., Sun T., Pierce G. N., Zhang S. (2011) Involvement of caveolin in probucol-induced reduction in hERG plasma-membrane expression. Mol. Pharmacol. 79, 806–813 [DOI] [PubMed] [Google Scholar]
- 19. Piper R. C., Luzio J. P. (2007) Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr. Opin. Cell Biol. 19, 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun T., Guo J., Shallow H., Yang T., Xu J., Li W., Hanson C., Wu J. G., Li X., Massaeli H., Zhang S. (2011) The role of monoubiquitination in endocytic degradation of human ether-a-go-go-related gene (hERG) channels under low K+ conditions. J. Biol. Chem. 286, 6751–6759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rotin D., Kumar S. (2009) Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10, 398–409 [DOI] [PubMed] [Google Scholar]
- 22. Staub O., Abriel H., Plant P., Ishikawa T., Kanelis V., Saleki R., Horisberger J. D., Schild L., Rotin D. (2000) Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 57, 809–815 [DOI] [PubMed] [Google Scholar]
- 23. Abriel H., Loffing J., Rebhun J. F., Pratt J. H., Schild L., Horisberger J. D., Rotin D., Staub O. (1999) Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome. J. Clin. Invest. 103, 667–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abriel H., Kamynina E., Horisberger J. D., Staub O. (2000) Regulation of the cardiac voltage-gated Na+ channel (H1) by the ubiquitin-protein ligase Nedd4. FEBS Lett. 466, 377–380 [DOI] [PubMed] [Google Scholar]
- 25. van Bemmelen M. X., Rougier J. S., Gavillet B., Apotheloz F., Daidie D., Tateyama M., Rivolta I., Thomas M. A., Kass R. S., Staub O., Abriel H. (2004) Cardiac voltage-gated sodium channel Nav1.5 is regulated by Nedd4-2-mediated ubiquitination. Circ. Res. 95, 284–291 [DOI] [PubMed] [Google Scholar]
- 26. Shallow H., Guo J., Zhang S. (2011) Cardiac potassium channel hERG is regulated by ubiquitin ligase Nedd4L. Biophys. J. 100, 102a [Google Scholar]
- 27. Albesa M., Grilo L. S., Gavillet B., Abriel H. (2011) Nedd4-2-dependent ubiquitylation and regulation of the cardiac potassium channel hERG1. J. Mol. Cell. Cardiol. 51, 90–98 [DOI] [PubMed] [Google Scholar]
- 28. Rotin D., Staub O., Haguenauer-Tsapis R. (2000) Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176, 1–17 [DOI] [PubMed] [Google Scholar]
- 29. Ingham R. J., Gish G., Pawson T. (2004) The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23, 1972–1984 [DOI] [PubMed] [Google Scholar]
- 30. Rizo J., Südhof T. C. (1998) C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 273, 15879–15882 [DOI] [PubMed] [Google Scholar]
- 31. Wang J., Peng Q., Lin Q., Childress C., Carey D., Yang W. (2010) Calcium activates Nedd4 E3 ubiquitin ligases by releasing the C2 domain-mediated auto-inhibition. J. Biol. Chem. 285, 12279–12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thangavel M., Liu X., Sun S. Q., Kaminsky J., Ostrom R. S. (2009) The C1 and C2 domains target human type 6 adenylyl cyclase to lipid rafts and caveolae. Cell. Signal. 21, 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanguinetti M. C., Tristani-Firouzi M. (2006) hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 34. Anderson C. L., Delisle B. P., Anson B. D., Kilby J. A., Will M. L., Tester D. J., Gong Q., Zhou Z., Ackerman M. J., January C. T. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113, 365–373 [DOI] [PubMed] [Google Scholar]
- 35. Ficker E., Kuryshev Y. A., Dennis A. T., Obejero-Paz C., Wang L., Hawryluk P., Wible B. A., Brown A. M. (2004) Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol. Pharmacol. 66, 33–44 [DOI] [PubMed] [Google Scholar]
- 36. Balijepalli R. C., Delisle B. P., Balijepalli S. Y., Foell J. D., Slind J. K., Kamp T. J., January C. T. (2007) Kv11.1 (ERG1) K+ channels localize in cholesterol and sphingolipid enriched membranes and are modulated by membrane cholesterol. Channels 1, 263–272 [DOI] [PubMed] [Google Scholar]
- 37. Jespersen T., Membrez M., Nicolas C. S., Pitard B., Staub O., Olesen S. P., Baró I., Abriel H. (2007) The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc. Res. 74, 64–74 [DOI] [PubMed] [Google Scholar]
- 38. Kamynina E., Tauxe C., Staub O. (2001) Distinct characteristics of two human Nedd4 proteins with respect to epithelial Na+ channel regulation. Am. J. Physiol. Renal Physiol. 281, F469–477 [DOI] [PubMed] [Google Scholar]
- 39. Rougier J. S., van Bemmelen M. X., Bruce M. C., Jespersen T., Gavillet B., Apotheloz F., Cordonier S., Staub O., Rotin D., Abriel H. (2005) Molecular determinants of voltage-gated sodium channel regulation by the Nedd4/Nedd4-like proteins. Am. J. Physiol. Cell Physiol. 288, C692–701 [DOI] [PubMed] [Google Scholar]
- 40. Maier G., Palmada M., Rajamanickam J., Shumilina E., Böhmer C., Lang F. (2006) Up-regulation of hERG channels by the serum and glucocorticoid inducible kinase isoform SGK3. Cell Physiol. Biochem. 18, 177–186 [DOI] [PubMed] [Google Scholar]
- 41. Hurley J. H. (2008) ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 20, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen H., De Camilli P. (2005) The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc. Natl. Acad. Sci. U.S.A. 102, 2766–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Razani B., Woodman S. E., Lisanti M. P. (2002) Caveolae: from cell biology to animal physiology. Pharmacol. Rev. 54, 431–467 [DOI] [PubMed] [Google Scholar]
- 45. Bretscher M. S. (1992) Circulating integrins: α5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO J. 11, 405–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen Y. G. (2009) Endocytic regulation of TGF-β signaling. Cell Res. 19, 58–70 [DOI] [PubMed] [Google Scholar]