Background: Estrogen is involved in nociception. It is unclear whether estrogen affects spinal synaptic transmission and plasticity.
Results: Estrogen facilitates NMDA transmission and spinal LTP via membrane estrogen receptor (mER)-initiated rapid signaling.
Conclusion: Estrogen increases spinal nociceptive synaptic transmission via activation of mER and NMDA receptors.
Significance: These results reveal a spinal mechanism by which estrogen enhances pain states.
Keywords: Electrophysiology, Estrogen, Neuroendocrinology, Pain, Western Blotting, LTP, NMDA Transmission, Nociception, Spinal Cord
Abstract
Recent evidence suggests that estrogen is synthesized in the spinal dorsal horn and plays a role in nociceptive processes. However, the cellular and molecular mechanisms underlying these effects remain unclear. Using electrophysiological, biochemical, and morphological techniques, we here demonstrate that 17β-estradiol (E2), a major form of estrogen, can directly modulate spinal cord synaptic transmission by 1) enhancing NMDA receptor-mediated synaptic transmission in dorsal horn neurons, 2) increasing glutamate release from primary afferent terminals, 3) increasing dendritic spine density in cultured spinal cord dorsal horn neurons, and 4) potentiating spinal cord long term potentiation (LTP) evoked by high frequency stimulation (HFS) of Lissauer's tract. Notably, E2-BSA, a ligand that acts only on membrane estrogen receptors, can mimic E2-induced facilitation of HFS-LTP, suggesting a nongenomic action of this neurosteroid. Consistently, cell surface biotinylation demonstrated that three types of ERs (ERα, ERβ, and GPER1) are localized on the plasma membrane of dorsal horn neurons. Furthermore, the ERα and ERβ antagonist ICI 182,780 completely abrogates the E2-induced facilitation of LTP. ERβ (but not ERα) activation can recapitulate E2-induced persistent increases in synaptic transmission (NMDA-dependent) and dendritic spine density, indicating a critical role of ERβ in spinal synaptic plasticity. E2 also increases the phosphorylation of ERK, PKA, and NR2B, and spinal HFS-LTP is prevented by blockade of PKA, ERK, or NR2B activation. Finally, HFS increases E2 release in spinal cord slices, which can be prevented by aromatase inhibitor androstatrienedione, suggesting activity-dependent local synthesis and release of endogenous E2.
Introduction
Mounting evidence indicates that estrogen plays an important role in an extensive spectrum of neural functions, such as nociception (1–3). The mechanisms by which estrogen modulates pain appear to be highly complex and remain to be elucidated. In the past several decades, studies regarding estrogen's regulation of pain have largely focused on the slow genomic actions of the steroid. Recent studies have demonstrated that estrogen can act nongenomically on nociception within seconds to minutes (4–7).
Estrogen has been reported to rapidly enhance excitatory synaptic transmission, especially via NMDA receptor (NMDAR)2-mediated synaptic activity and LTP (8–13). Estrogen also promotes the formation of new dendritic spines and excitatory synapses in the hippocampus and cortex (13–16). Estrogen has also been shown to rapidly phosphorylate extracellular signal-regulated kinase (ERK) (17–19), a member of the family of mitogen-activated protein kinases (MAPKs) that is strongly implicated in NMDA-dependent synaptic plasticity and persistent pain hypersensitivity (20–22) via the activation of membrane estrogen receptors (mERs) (23), which subsequently enhance NMDAR transmission and LTP (13, 24).
LTP is a long lasting form of synaptic plasticity, and synaptic plasticity is fundamental to many neuronal functions, including learning, memory, and pain (25). In the pain pathway, activation of peripheral nociceptors results in neuronal plasticity in the CNS, which modifies the performance of the nociceptive pathway by enhancing and prolonging responses to subsequent peripheral stimuli. These changes in the spinal cord are known as spinal central sensitization (26). High frequency stimulation (HFS) of peripheral nerves produces LTP of C-fiber-evoked responses in the spinal dorsal horn, which are thought to be a substrate for spinal central sensitization (27). Considering that there are many similarities between mechanisms of synaptic plasticity in different regions of the CNS, such as the spinal dorsal horn and hippocampus (25), we propose that spinal central sensitization and hippocampal LTP share similar mechanisms. In this study, we investigated whether estrogen could acutely modulate spinal synaptic transmission and plasticity and further explored the molecular/cellular events and signaling pathways that promote synaptic plasticity. We demonstrated that estrogen might rapidly modulate spinal excitatory synaptic transmission and facilitate LTP by increasing NMDA transmission, presynaptic glutamate release, and dendritic branching/spine density. The activation of mERs, PKA/ERK, and NR2B is possibly involved in the process.
EXPERIMENTAL PROCEDURES
Animals and Reagents
Experiments were performed on young (2–3-week-old) Sprague-Dawley rats of both sexes. Rats were obtained from the Experimental Animal Center of the Chinese Academy of Science. All experimental procedures were approved by the Shanghai Animal Care and Use Committee, and all efforts were made to minimize animals' suffering and the number of animals used.
All reagents were purchased from Sigma unless otherwise noted. 17β-estradiol (E2) and ICI 182,870 were dissolved in sesame oil or ethanol. E2-BSA was dissolved in phosphate-buffered saline (PBS). Androstatrienedione (Steraloids) and PD98059 were prepared in DMSO. Stock solutions of the following drugs were prepared in double-distilled H2O: bicuculline methiodide (BMI), strychnine, APV, CNQX, NMDA, AMPA, Ro 25-6981, and H89. QX-314 and GDPβS was dissolved in an intracellular solution.
Spinal Cord Slice Preparation
Rat spinal cord slices were prepared as described previously (28). Briefly, laminectomy was performed under diethyl ether deep anesthesia, and the lumbosacral segment of the spinal cord was rapidly removed and placed in ice-cold sucrose artificial cerebrospinal fluid (ACSF) containing 80 mm NaCl, 2.5 mm KCl, 1.25 mm NaH2PO4, 0.5 mm CaCl2, 3.5 mm MgCl2, 25 mm NaHCO3, 75 mm sucrose, 1.3 mm ascorbate, 3.0 mm sodium pyruvate, oxygenated with 95% O2 and 5% CO2, pH 7.4. Transverse slices (500 μm) with attached dorsal roots (8–12 mm) were prepared and then incubated in preoxygenated recording ACSF with the following composition: 125 mm NaCl, 2.5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 1.25 mm NaH2PO4, 26 mm NaHCO3, 25 mm d-glucose, 1.3 mm ascorbate, 3.0 mm sodium pyruvate. The slices then recovered at 32 ± 1 °C for 40 min and then at room temperature for an additional 1 h before experimental recordings. Slices were then transferred into a recording chamber and continuously perfused with recording solution at a rate of 5 ml/min prior to electrophysiological recordings at room temperature.
Whole-cell Patch Clamp Recordings from Spinal Cord Slices
tk;1Whole-cell recordings were obtained from neurons of the superficial dorsal horn of the spinal cord. Neurons were identified by infrared differential interference contrast video microscopy with an upright microscope (Leica DMLFSA) equipped with a ×40, 0.8 numerical aperture water immersion objective and a CCD camera (IR-1000E). Patch pipettes (5–10 megaohms) were made from borosilicate glass on a horizontal micropipette puller (P-97, Sutter Instruments, Novato, CA) and were filled with a solution of the following composition: 120 mm potassium gluconate, 20 mm KCl, 2 mm MgCl2, 2 mm Na2ATP, 0.5 mm NaGTP, 20 mm HEPES, 0.5 mm EGTA, pH 7.28, with KOH. QX-314 (5 mm) and GDPβS (1 mm) were added when necessary. To measure EPSCs from neurons in lamina I and the outer part of lamina II, a constant current pulse (0.3–0.5 mA) at 0.05 Hz was delivered to the dorsal root by a suction electrode. EPSCs were recorded with normal ACSF (2 mm Ca2+, 1 mm Mg2+) containing BMI (10 μm) and strychnine (1 μm) to block GABAA- and glycine receptor-mediated inhibitory synaptic currents, whereas low Mg2+, high Ca2+ (2.5 mm Ca2+, 0.25 mm Mg2+) ACSF was used in the NMDAR testing experiments. The membrane potential was held at −70 mV. When recording the NMDA receptor-mediated component of EPSCs (NMDA-EPSCs), a holding potential of +40 mV was used as indicated. Only 1 cell was recorded per slice to obviate contamination due to previous treatment. Data were acquired using an Axopatch 200B amplifier and were low pass-filtered at 2 kHz and digitized at 5 kHz. The series resistance (Rs) was monitored during recording. Cells in which Rs deviated >20% or cells with Rs > 60 megaohms were excluded from analysis.
Field Potential Recordings from Spinal Cord Slices
Field potential recordings from the superficial spinal dorsal horn were obtained with glass microelectrodes (impedance 2–5 megaohms) filled with 135 mm NaCl, 5.4 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm HEPES (pH adjusted to 7.2 with NaOH). A bipolar tungsten electrode was used to stimulate Lissauer's tract (LT). The low pass filter was set to 1 kHz, and the amplification was set to ×500 (Axopatch 200B amplifier, Axon Instruments). To minimize current spread to the dorsal roots and the recording site, the electrode was placed at the ventrolateral border of LT (29). For LTP-associated experiments, recordings of field excitatory postsynaptic potentials (fEPSPs) were made in the presence of BMI (10 μm) and strychnine (1 μm) to block the tonic inhibitory actions of GABAA and glycine, respectively. The current intensity of the test stimuli was set at a current strength sufficient to excite the C-fibers (0.1 ms, 0.5–0.7 mA). LTP was induced at base-line stimulus intensity using tetanic HFS. HFS consisted of three trains of 100 pulses at 100 Hz with 10-s intertrain intervals. Before HFS, test pulses of 0.1 ms were given at 120- or 300-s intervals and recorded for at least 10 min to ensure the stability of the response. Data were collected with pClamp version 10.1 software and analyzed using Clampfit version 10.1. The magnitude of LTP was estimated by comparing averaged responses at 30 or 60 min after induction with averaged base-line responses before induction.
Immunocytochemistry/Immunohistochemistry
Rats were sacrificed with an overdose of chloral hydrate (80 mg/kg) and perfused transcardially with normal saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Spinal cords were then removed, postfixed in the same fixative for 6 h at 4 °C, and immersed in a 10–30% gradient of sucrose in phosphate buffer for 24–48 h at 4 °C for cryoprotection. Transverse sections (16 μm) were cut in a cryostat (Leica 1900) and processed for immunofluorescence. Cultured spinal dorsal horn neurons (14 days in vitro) grown on glass coverslips were fixed in 4% paraformaldehyde in 0.1 m PBS for 15 min at room temperature. The sections or cells were blocked with 10% donkey serum in 0.01 m PBS (pH 7.4) with 0.3% Triton X-100 for 1 h at room temperature. For ERα/NeuN/MAP-2, ERβ/NeuN/MAP-2, or GPER1/NeuN/MAP-2 double immunofluorescence, the sections or cells were incubated with a mixture of rabbit anti-ERα (1:50; Santa Cruz Biotechnology, Inc., or Upstate), goat anti-ERβ (1:50; Santa Cruz Biotechnology, Inc.), rabbit anti-GPER1 (1:50; Abcam), and mouse anti-NeuN (neuronal marker, 1:1000; Millipore) or with mouse anti-MAP-2 (1:1000; Millipore) overnight at 4 °C, followed by a mixture of Rhodamine Red-X- and FITC-conjugated secondary antibodies for 2 h at 4 °C. For immunohistochemical experiments on spinal cord slices, five adjacent slices (350 μm) from the same spinal segment were prepared as in the electrophysiological experiment described above. At least two slices were used for controls (non-treated and ethanol vehicle-treated) and three for E2 treatment (10 μm for 10, 30, or 60 min), and the remaining slices were used for NMDA (100 μm, 10 min). This arrangement allowed negative (untreated or ethanol-treated slice) and positive controls (NMDA-treated slice) for each animal. Subsequently, the slices were rapidly fixed in cold 4% paraformaldehyde for 60 min and then processed for immunostaining. The sections were blocked with 10% donkey serum in 0.01 m PBS with 0.3% Triton X-100 for 4 h at room temperature. For pERK or pERK/pPKA double immunofluorescence, the sections were incubated with mouse anti-pERK (1:1000; Sigma) or a mixture of mouse anti-pERK and rabbit anti-pPKA RII (Ser-96) (1:1000; Upstate), for 48 h at 4 °C, followed by FITC-conjugated or a mixture of Rhodamine Red-X- and FITC-conjugated secondary antibodies for 4 h at 4 °C. All sections were coverslipped with a mixture of 50% glycerin in PBS and then observed with a Leica SP2 confocal laser-scanning microscope. The specificity of immunostaining was verified by omitting the primary antibodies, and immunostaining signals disappeared after the omission of primary antibodies. The specificity of primary antibodies was verified by the preabsorption experiment. Sections were first incubated with a mixture of ERα, ERβ, and GPER1 primary antibody and the corresponding blocking peptide (Santa Cruz Biotechnology, Inc.; blocking peptide/primary antibody = 3:1) overnight, followed by secondary antibody incubation. ER immunostaining signals were abolished after absorption.
E2-BSA-FITC binding experiments were performed on cultured spinal dorsal horn neurons grown on glass coverslips. E2-BSA covalently coupled to FITC (E2-BSA-FITC) was resuspended at 1 mg/ml in PBS. The unfixed live cells were washed one time with ACSF and incubated with E2-BSA-FITC (10 μg/ml) in PBS with 5% BSA for 30 min in the dark at 37 °C. For E2-BSA-FITC/GluN1 or E2-BSA-FITC/GluA1 double staining, stained E2-BSA-FITC neurons were incubated with rabbit anti-GluN1 (1:500; Sigma) or rabbit anti-GluA1 (1:200; Millipore), respectively.
Western Blot
Under urethane (1.5 g/kg, intraperitoneally) anesthesia at 10 min and 30 min after intrathecal injection of E2 or E2-BSA, the animals were rapidly sacrificed by decapitation (n = 3 or 4/group). The L4–L6 lumbar spinal cord was rapidly removed, immediately frozen in liquid nitrogen, and stored at −70 °C until use. Frozen samples were homogenized in a lysis buffer (12.5 μl/mg of tissue) containing a mixture of protease inhibitors (Roche Applied Science) and PMSF (Sigma). Cultured spinal dorsal horn neurons (14 days in vitro) were lysed with radioimmune precipitation assay buffer. After incubating in ice for 30 min, samples were centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were used for Western blotting. Equal amounts of protein (∼20 μg) were loaded and separated in a 10% Tris-Tricine SDS-polyacrylamide gel. The resolved proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences). The membranes were blocked with 5% nonfat milk in Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 for 2 h at room temperature and incubated overnight at 4 °C with mouse anti-pERK (1:2000; Sigma), rabbit anti-total ERK (1:100,000; Sigma), rabbit anti-ERα (1:50 (Upstate) or 1:100 (Santa Cruz Biotechnology, Inc.)), rabbit anti-ERβ (1:200; Santa Cruz Biotechnology, Inc.), rabbit anti-GPER1 (1:200; Abcam), rabbit anti-PKA (1:500; Upstate), rabbit anti-phospho-NR2B (1:1000; Millipore), mouse anti-NR2B (1:2000; Neuromab), rabbit anti-GluN1 (1:2000; Sigma) and rabbit anti-GluA1 (1:500; Millipore), mouse anti-transferrin receptor (1:1000; Invitrogen), and rabbit anti-CREB (1:1000; Sigma). The blots were then incubated with the secondary antibody, goat anti-mouse or goat anti-rabbit IgG conjugated with horseradish peroxidase (HRP) (1:1000; Pierce), for 2 h at 4 °C. Signals were finally visualized using enhanced chemiluminescence (ECL; Pierce), and the bands were visualized with the ChemiDox XRS system (Bio-Rad). All Western blot analyses were performed at least three times, and consistent results were obtained. A Bio-Rad image analysis system was then used to measure the integrated optic density of the bands.
Cell Surface Biotinylation
Surface biotinylation experiments were performed in live transverse spinal dorsal horn slices and cultured dorsal horn neurons (14 days in vitro). After treatment, the slices or cultured neurons were incubated with PBS containing 0.5–1.0 mg/ml Sulfosuccinimidel-6-[biotin-amido] hexanoate (Pierce) for 45 min on ice and rinsed in ice-cold PBS containing 1 m glycine to quench the biotin reaction. The cultured neurons were lysed, and the slices were homogenized in modified radioimmune precipitation assay buffer (50 mm Tris (pH 7.5), 150 mm NaCl, 1% Triton X-100, 10% glycerol, 0.5 mg/ml BSA, 1 mm PMSF, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 μg/ml pepstain). The homogenates were centrifuged at 10,000 × g for 15 min at 4 °C, and the supernatant was collected. The supernatant was incubated with 50 μl of NeutrAvidin-agarose (Pierce) for 4 h at 4 °C and washed three times with radioimmune precipitation assay buffer. The total and biotinylated surface proteins were detected using quantitative Western blots as described above.
Estradiol Assay
Samples from the slice perfusate were collected. To compare E2 concentrations in the perfusate from spinal slices of different sexes, slices were incubated in the same volume of ACSF. For LT stimulation experiments, ACSF was added to the initial volume after each collection of perfusate to exclude the influence of volume on the measurement of E2 concentration. The E2 concentration was determined with double antibody radioimmunoassay kits according to protocols provided by the manufacturer (National Atomic Energy Research Institute, Beijing, China). The sensitivity of the kit was 1.4 pg/ml.
Primary Spinal Dorsal Horn Neuron Culture and Transfection
Spinal cord neurons were prepared from rat embryos at embryonic day 15. Briefly, spinal cords were removed from each embryo, and the dura was stripped. Dorsal horn tissue was isolated according to the open book technique (30). Subsequently, the spinal dorsal horn was dissociated with trypsin, and cells were plated (1 × 105 to 1 × 106 cells/ml) in Neurobasal medium (Invitrogen) supplemented with B27 (Invitrogen), l-glutamine (Invitrogen), and 5% fetal bovine serum (FBS) (Sigma) on 60-mm dishes that were precoated with poly-d-lysine (0.1 or 1 mg/ml; Sigma). After 4 h, the media (5% FBS B27 and glutamine) were changed to feeding media without 5% FBS. Cells were fed twice weekly thereafter with Neurobasal medium prepared as above but without the 5% FBS. Spinal dorsal horn neurons were grown at 37 °C in 5% CO2 on coverslips. Cultured neurons received transfection of GFP-lentivirus (Genechem, Shanghai) 3 days before imaging at 14 days in vitro.
Time Lapse Imaging
Three days after transfection, living transfected neurons on coverslips were transferred to a stage chamber filled with Neurobasal medium and imaged with a Nikon (Tokyo, Japan) TE300 inverted microscope. Images were obtained using a Yokogawa spinning disk (Solamere Technology Group). Healthy neurons expressing GFP were identified and imaged every 5–8 min for 30 min before and 30–60 min after E2 treatment at 37 °C in 5% CO2. At each time point, Z-stacks of images were collected and analyzed using Origin (Microcal Software).
Statistical Analysis
Data are presented as the mean ± S.E. Statistical comparisons were performed using Student's t test, paired t test, and one- or two-way ANOVA followed by post hoc Student-Newman-Keuls test. In all cases, p < 0.05 was considered statistically significant.
RESULTS
E2 Rapidly Enhances NMDA Transmission via G Protein-coupled Receptors in Superficial Dorsal Horn Neurons
Previous studies have shown that NMDAR and AMPAR function can be rapidly regulated by estrogen in hippocampal neurons (8, 11). To examine the effects of estrogen on NMDA- and AMPA-evoked currents in the spinal cord, whole-cell patch clamp recordings were performed on neurons from lamina I and II of the spinal cord slices. In all of the experiments, male and female rats at 2–3 weeks of age were randomly used because there were no detectable sex differences in E2 concentrations in the perfusates of the spinal slices (male 189.65 ± 25.27 pmol/liter versus female 185.93 ± 28.94 pmol/liter; t test, p = 0.93, n = 4), and no estrous cycles were observed in the infant female rats, thus excluding the fluctuations in ovarian steroid hormones.
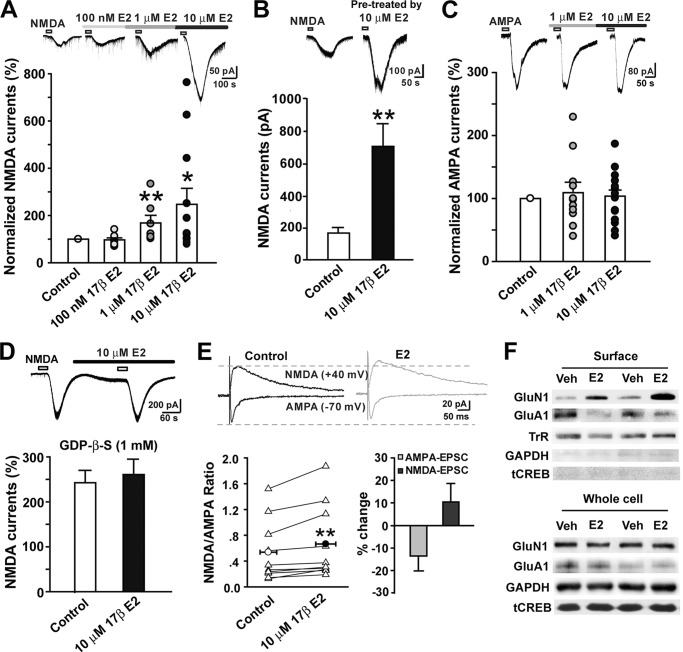
Application of NMDA (50 μm) for 30 s through adding the compound to a low Mg2+ (0.25 mm) and high Ca2+ (2.5 mm) perfusion solution in the presence of tetrodotoxin (0.5 μm), BMI (10 μm), strychnine (1 μm), and CNQX (20 μm) at −40 mV evoked inward currents. Using the >20% potentiation criterion, we found that E2 (100 nm to 10 μm) dose-dependently potentiated NMDA-currents (one-way ANOVA, F(3, 36) = 3.12, p = 0.038, n = 7–12). E2 potentiated NMDA currents in 1 of 8 (13%), 4 of 7 (57%), and 7 of 12 (58%) cells at doses of 100 nm, 1 μm, and 10 μm, respectively. The potentiation effect began within 3–5 min after bath application of E2 (Fig. 1A). Interestingly, when the slices were pretreated with 10 μm E2 for 30 min, the NMDA currents were greatly enhanced in all 11 cells recorded (Fig. 1B). In separate slices, we recorded an inward current after 30 s of AMPA (10 μm) bath application in normal ACSF with tetrodotoxin, BMI, strychnine, and APV (50 μm) at −70 mV. The AMPAR currents were diversely affected by E2 in different neurons and in general exhibited no effect (one-way ANOVA, F(2, 42) = 0.21, p = 0.811, n = 11–17) (Fig. 1C).
FIGURE 1.
Estrogen rapidly potentiates NMDA currents via G protein-coupled receptors in superficial dorsal horn neurons. NMDA and AMPA currents were recorded in lamina I and II neurons of spinal cord slices. E2 (100 nm to 10 μm) was bath-applied for 3–5 min. A, infusion of E2 at concentrations of 100 nm (n = 8), 1 μm (n = 7), and 10 μm (n = 12) caused a rapid, dose-dependent increase in NMDA current amplitude (infusion period indicated by bar). Top, NMDA current traces collected as controls and during E2 infusion. *, p < 0.05; **, p < 0.01 versus control. B, NMDA currents in neurons of control and E2 pretreatment slices. NMDA currents obtained from slices pretreated for 30 min with E2 (n = 11) were significantly higher than those from naive slices (n = 16). Top, NMDA current traces collected as control and pretreatment by E2. **, p < 0.01 versus naive control. C, E2 at concentrations of 1 μm (n = 11) and 10 μm (n = 17) had no effects on AMPA currents. Top, AMPA current traces collected as controls and during E2 infusion. D, loading neurons with 1 mm GDPβS abolished the acute action of E2 on NMDA currents (n = 9). E, E2 increases the NMDA/AMPA ratio. Left, the effects of E2 on the NMDA/AMPA ratio in individual cells (n = 11). Measurements were counted at the peak point of the traces at −70 mV for AMPA-EPSCs and at 40 ms after the stimulation artifacts for the traces at +40 mV for NMDA-EPSCs. Right, the effects of E2 on NMDA-EPSCs and AMPA-EPSCs. Top, typical NMDA-EPSCs recorded at +40 mV and AMPA-EPSCs at −70 mV before and after E2 application. **, p < 0.01 versus control. F, two examples showing the effects of E2 on expression of GluN1 and GluA1 in surface biotinylation-isolated membrane protein and whole cell protein extracts from cultured dorsal horn neurons and spinal slices. Neurons or slices were treated with vehicle or E2 (10 μm) for 10 min. Transferrin receptor (TrR), a marker for membrane proteins, served as a loading control. Error bars, S.E.
The potentiation of NMDA currents by E2 in dorsal horn neurons within 3–5 min suggests a nongenomic, rapid mechanism. Several studies have found that many rapid estrogen effects are sensitive to G protein manipulation (31, 32). We therefore tested whether the rapid enhancement of NMDA currents in dorsal horn neurons by E2 is mediated by G proteins. GDPβS (1 mm), a G protein inhibitor, was included in the pipette internal solution. As shown in Fig. 1D, the potentiation of NMDA currents by E2 was completely abolished (Student's t test, p = 0.64, n = 9).
The rapid regulation of NMDAR and AMPAR function by E2 was further confirmed by measuring the ratio of NMDAR- to AMPAR-mediated components of the dorsal root stimulation-evoked EPSCs in the spinal dorsal horn. We isolated NMDA-EPSCs and AMPA-EPSCs at +40 and −70 mV in the presence of BMI and strychnine and CNQX or APV, respectively. The NMDAR/AMPAR ratio was then calculated. As shown in Fig. 1E, a short application of E2 (10 μm, 3–5 min) significantly increased the NMDAR/AMPAR ratio in individual experiments (control 0.54 ± 0.14 versus E2 0.67 ± 0.17; paired t test, p < 0.01); this was the result of a selective increase in NMDA-EPSCs and a decrease in AMPA-EPSCs (NMDA-EPSCs, 10.35 ± 8.20% of base-line change; AMPA-EPSCs, −13.57 ± 6.63% of base-line change).
Changes in the protein levels of the NMDAR subunit GluN1 and the AMPAR subunit GluA1 in the plasma membrane due to E2 were also observed using the cell surface biotinylation technique. To ensure that only cell surface proteins were labeled with the membrane-impermeable biotin and were not contaminated by cytoplasmic proteins, Western blots of labeled protein fractions were tested. The immunoband for GAPDH was nearly negative, which verified the purity of the biotinylated sample. Transferrin receptor was used as a marker for membrane proteins and served as loading control. Expectedly, membrane GluN1 levels were increased after 10 min of E2 (10 μm) treatment compared with vehicle treatment. In contrast, the AMPAR subunit GluA1 was reduced by E2 treatment. Neither the GluN1 nor GluA1 levels in the whole-cell protein extracts were affected by E2 treatment (Fig. 1F). This result implies a possible insertion of GluN1 into the membrane and internalization of GluA1 from the membrane. This bidirectional glutamate receptor trafficking suggests the formation of silent synapses (33, 34), but this possibility remains to be investigated further.
Distribution of Estrogen Receptors in Dorsal Horn Neurons
The membrane estrogen-binding site is thought to be the molecular and cellular substructure underlying rapid estrogen action (35). The distributions of E2-BSA binding sites on the plasma membrane of cultured spinal dorsal horn neurons were detected by E2-BSA-FITC (E2-BSA conjugated to FITC). Exposure of intact cells to E2-BSA-FITC resulted in a punctate staining pattern of the plasma membrane (Fig. 2A). This punctate membrane staining pattern was remarkably similar to that previously described in other cells (36). Double staining showed that the punctuate staining was colocalized with GluN1 and GluA1 (Fig. 2A), providing a cytological basis for rapid modulation of spinal glutamate synapses by E2. Immunocytochemistry showed that all three types of ERs (ERα, ERβ, and GPER1) expressed in primary cultured rat spinal dorsal horn neurons (14 days) co-localized with the neuronal marker NeuN and the cytoskeletal marker microtubule-associated protein 2 (MAP2) (Fig. 2B). The distribution of the three ERs was also confirmed in fixed spinal sections in situ (Fig. 2C), consistent with previous reports (6, 37). Furthermore, the surface proteins of dorsal horn neurons were biotinylated, and Western blots from biotinylated proteins were examined. As expected, ERα-, ERβ-, and GPER1-immunoreactive bands were all identified, thus validating the presence of mERs (Fig. 2D). Consistent with a recent study on hypothalamic neurons, a full-length 66 kDa ERα-immunoreactive band and a 52 kDa ERα-immunoreactive band were both detected in membrane proteins (38). These data provide cytological evidence for E2-mediated, membrane-initiated, rapid actions.
FIGURE 2.
Distribution of estrogen receptors in spinal dorsal horn neurons. A, immunocytochemistry for E2-BSA-FITC and double immunofluorescence for E2-BSA-FITC and GluN1 or GluA1 in cultured spinal dorsal horn neurons. B, double immunofluorescence showed colocalization of ERα, ERβ, and GPER1 with the neuronal marker NeuN and MAP-2 in cultured dorsal horn neurons. C, double immunofluorescence showed colocalization of ERα, ERβ, and GPER1 with the neuronal marker NeuN in the spinal dorsal horn in situ. D, evidence for mERs in cultured dorsal horn neurons (a) and spinal slices (b). Surface biotinylation showed that ERα, ERβ, and GPER1 were present in the membrane protein extract. Transferrin receptor (TrR), a marker of membrane proteins, served as a loading control.
E2 Facilitates Spinal LTP of fEPSPs by mERs
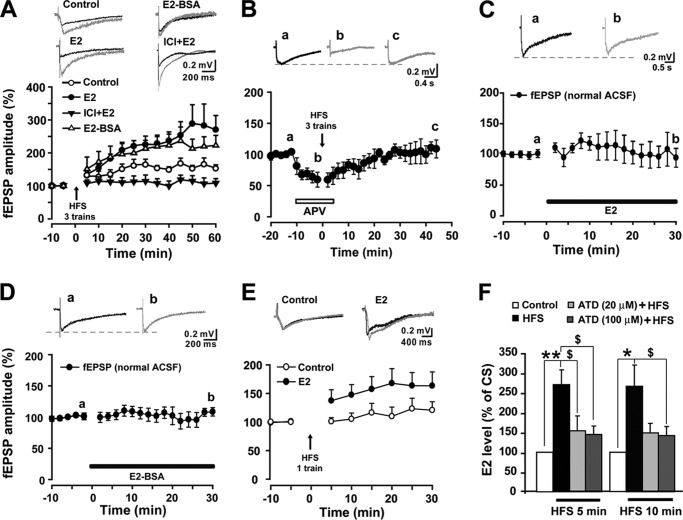
Previous studies have shown that E2 treatment significantly increased hippocampal LTP magnitude when NMDA transmission was increased relative to AMPA transmission (11). Our present finding supports these previous studies by demonstrating that E2 enhances the NMDA/AMPA ratio in spinal dorsal horn neurons and suggests that estrogens also regulate spinal LTP. fEPSPs in the superficial dorsal horn were evoked by stimulation of LT (0.1 ms, 0.5–0.7 mA, 5-min interval) via a bipolar tungsten electrode. The fEPSPs in the spinal dorsal horn were recorded in the presence of BMI (10 μm) and strychnine (1 μm) to block the tonic inhibitory action of GABAA and glycine. Three trains of HFS (100 Hz for 1 s, 10-s train intervals) were introduced to induce LTP of fEPSPs. As shown in Fig. 3A, three-train HFS reliably induced LTP of fEPSPs that could be completely impaired by the NMDA receptor antagonist APV (50 μm) (40 min point after HFS, 0 ± 14%) (Fig. 3, A and B), indicating that spinal LTP in our study is NMDAR-dependent. Preperfusion of E2 (10 μm) 10 min before the HFS robustly increased the magnitude of LTP of fEPSPs (60 min point, after HFS, +170 ± 43% versus control LTP, +54 ± 10%) (two-way ANOVA, F(1, 132) = 4.828, p = 0.048, n = 5–7) (Fig. 3A). Base-line fEPSPs were not changed by E2 (10 μm), even at 30 min after E2 application (Fig. 3C). A membrane-impermeable estrogen conjugate, E2-BSA (10 μm), which has been observed to act only on membrane ERs (39), mimicked the E2-induced enhancement of LTP magnitude without altering base-line fEPSPs (Fig. 3, A and D), strongly implicating mERs in the E2-induced facilitation of fEPSP LTP in the superficial dorsal horn of the spinal cord. The effect of E2 on the spinal LTP threshold was also tested. As shown in Fig. 3E, a single train of HFS did not produce LTP of fEPSPs in vehicle-treated slices but resulted in a significant potentiation in slices preinfused with E2 for 10 min, suggesting a reduction in the threshold of LTP induction by exogenous E2.
FIGURE 3.
The role of E2 in spinal LTP of fEPSPs. A, three trains of HFS reliably induced LTP of fEPSPs in the spinal dorsal horn (n = 7). E2 (10 μm) raised the amplitude of LTP (n = 5). E2-BSA (10 μm) induced an analogous enhancement of LTP magnitude (n = 5). E2-induced enhancement of LTP was blocked by 1 μm ICI 182,780 (n = 7). Top, traces collected during base-line recording (black line) and 60 min after three trains of HFS (gray lines) in different groups. B, pretreatment with APV (50 μm) completely impaired three-train HFS-induced LTP of fEPSPs (n = 5). Top, representative fEPSPs recorded at the times indicated by the letters. C and D, neither E2 (10 μm) nor E2-BSA (10 μm) affected basal fEPSPs in normal ACSF (n = 4–6). Top panels, representative fEPSPs recorded at the times indicated by the letters. E, one-train HFS (arrows) produced more potentiation following preinfusion with E2 (n = 12) compared with control slices (n = 13). Top, traces collected from slices during base-line recording (black line) and 30 min after delivery of one train of HFS (gray lines). F, E2 levels in the perfusate of spinal slices before and after HFS. E2 levels were significantly elevated at 5 and 10 min after HFS compared with conditioning stimulation. Preincubation of slices in ACSF containing androstatrienedione (ATD 20 and 100 μm) for 30 min significantly attenuated the HFS-induced increase in E2 concentrations. *, p < 0.05; **, p < 0.01 versus controls; $, p < 0.05 versus HFS. Error bars, S.E.
Next, we tested the effects of blocking ERs on E2-induced facilitation of LTP with the ER antagonist ICI 182,780. Infusions of ICI 182,780 (1 μm) did not affect base-line synaptic transmission (30 min after application, +8 ± 13%), but they completely abrogated the effect of E2 on spinal LTP (Fig. 3A). Interestingly, in addition to eliminating the enhancement of LTP by E2, ICI 182,870 severely impaired LTP per se (two-way ANOVA, F(1, 154) = 9.254, p = 0.009, n = 7). Three trains of HFS did not produce spinal LTP of fEPSPs following co-perfusion of ICI 182,870 and E2 for 10 min, suggesting that the existence of endogenous local estrogen may be essential for the spinal LTP induction.
To address whether the three trains of HFS that reliably induce spinal LTP of fEPSPs can elicit endogenous estrogen synthesis and release, we collected the perfusate of spinal slices within 5 and 10 min before HFS and 5 and 10 min after HFS with or without pretreatment with the aromatase inhibitor androstatrienedione to analyze levels of local spinal synthesis and release of E2. Following the HFS, E2 concentrations in the perfusate were significantly increased at 5 and 10 min compared with the 5 min (paired t test, p < 0.01, n = 7) and 10 min (paired t test, p < 0.05, n = 7) pre-HFS controls. Preincubation of slices in ACSF containing androstatrienedione (20 and 100 μm) for 30 min significantly attenuated the HFS-induced increase in E2 concentrations at 5 min (one-way ANOVA, F(3, 18) = 7.845, p = 0.001, n = 4–7) and 10 min post-HFS (one-way ANOVA, F(3, 18) = 4.864, p = 0.012, n = 4–7) (Fig. 3F), indicating that HFS induced rapid synthesis and release of spinal cord-derived estrogen.
E2-induced Facilitation of Spinal LTP Is Prevented by Blocking NR2B, PKA, and ERK Activation
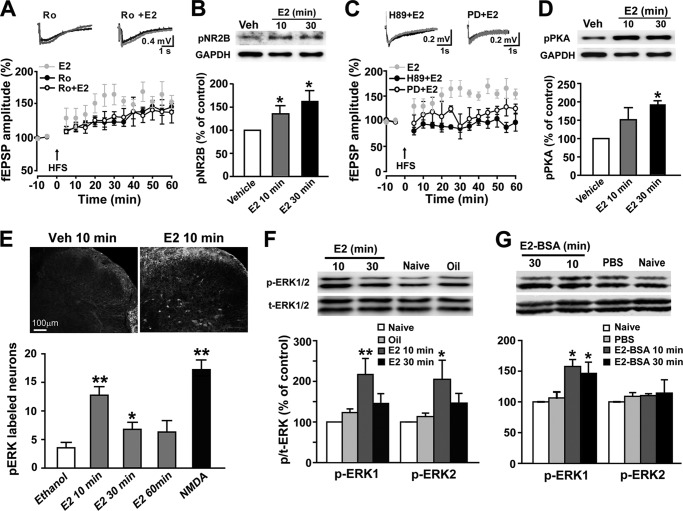
Previous studies showed that NR2B containing NMDA receptors are required for spinal LTP induction (40, 41). Here, we further demonstrated that NR2B-containing receptors also participate in E2-induced heightened LTP in the spinal dorsal horn. As shown in Fig. 4A, at a lower concentration (0.3 μm), the NR2B antagonist Ro 25-6981 did not prevent HFS-LTP induction (60 min point after HFS, +54 ± 10% for control versus +46 ± 5% for Ro 25-6981) but completely blocked E2-induced potentiation of HFS-LTP (60 min point after HFS, +170 ± 43% for E2 versus +38 ± 16% for Ro 25-6981 + E2) (two-way ANOVA, F(1, 121) = 11.559, p = 0.006, n = 5–8) (Fig. 4A). Additionally, the rapid up-regulation of pNR2B in the dorsal horn neurons with E2 exposure supports the important role of NR2B in the heightening of spinal LTP by E2 (Fig. 4B).
FIGURE 4.
Involvement of NR2B, PKA, and ERK activation in E2-induced facilitation of spinal LTP. A, at a dose of 0.3 μm, Ro 25-6981 (Ro) eliminated E2-induced potentiation of HFS-LTP (n = 8) but did not block HFS-LTP of fEPSPs in the spinal dorsal horn (n = 7). Top, representative fEPSPs recorded before HFS (black lines) and 60 min after HFS (gray lines). B, Western blot analysis revealed rapid phosphorylation of NR2B by E2 exposure in cultured spinal dorsal horn neurons (n = 4). Top, representative Western blot for pNR2B protein from vehicle- and E2-treated neurons. C, pretreatment with either H89 (1 μm) (n = 8) or PD 98059 (PD; 50 μm) (n = 4) eliminated E2-induced LTP enhancement. D, Western blot analysis showed rapid phosphorylation of PKA by E2 exposure in cultured spinal dorsal horn neurons (n = 5). E, immunohistochemistry for pERK from spinal cord slices. Following E2 or NMDA treatment, the numbers of pERK-positive cells in the superficial layers were higher than those of vehicle controls. *, p < 0.05; **, p < 0.01 versus vehicle (0.1% ethanol) controls. F and G, intrathecal injection of E2 (75 nmol) or E2-BSA (75 nmol) significantly increased expression levels of pERK in the spinal cord dorsal horn (n = 4). Top, representative Western blot for pERK expression in naive and vehicle- and E2- (or E2-BSA)-treated rats. *, p < 0.05 versus vehicle (0.1% ethanol or 0.01 m PBS). Error bars, S.E.
It has been reported that E2 exposure can activate many intracellular signaling proteins, including PKA and ERK (3, 16–18, 42). PKA and ERK activation are both involved in the induction and maintenance of LTP (43–45). We observed in the present study that both the PKA inhibitor H89 (1 μm; 60 min point after HFS, +170 ± 43% for E2 versus 92 ± 17% for H89 + E2; two-way ANOVA, F(1, 121) = 38.380, p < 0.001, n = 5–8) and the MEK inhibitor PD98059 (50 μm; 60 min point after HFS, +170 ± 43% for E2 versus +25 ± 9% for PD + E2; two-way ANOVA, F(1, 77) = 14.262, p = 0.007, n = 4–5) blocked the action of E2 on spinal LTP, indicating a potential role of PKA and ERK in the facilitation (Fig. 4C) of LTP. Furthermore, E2 exposure acutely up-regulated pPKA levels in dorsal horn neurons (Fig. 4D). Analogously, ERK activation by E2 was also observed in the spinal dorsal horn. Bath application of E2 (10 μm) for 10 min produced a robust increase in pERK in the presence of 1 μm tetrodotoxin to block action potentials (Fig. 4E). Finally, spinal application of E2 or E2-BSA up-regulated pERK levels in the spinal dorsal horn (Fig. 4, F and G).
Presynaptic Glutamate Release Contributes to E2-induced Facilitation of Spinal LTP
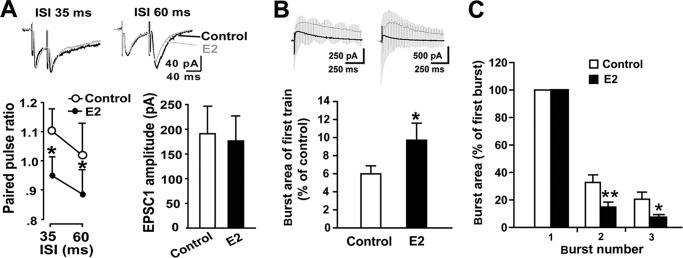
To determine the presynaptic effects of E2, we recorded EPSCs evoked by paired pulse stimulation of the dorsal root and examined the effects of E2 on the paired pulse ratio (PPR), a measure related to neurotransmitter release probability that is commonly used to assess changes in presynaptic function (46, 47). At both 35- and 60-ms intervals, PPRs were reduced by 5-min E2-treatment (paired t test, p < 0.05, n = 11) (Fig. 5A). Because the PPR is inversely related to release probability (46), our results indicate that E2 can acutely, at least in part, increase presynaptic glutamate release.
FIGURE 5.
Effects of E2 on presynaptic release probability in dorsal horn neurons. A, effects of E2 (10 μm) on the PPR. The PPR was measurably reduced at 35- and 60-ms intervals by E2 (n = 11). Top, representative traces collected as controls (black line) and after E2 application (gray line) at 30-ms (left) and 60-ms intervals (right). B, comparison of burst area during the first train of HFS relative to the area of base-line NMDA-EPSCs averaged across control slices (n = 15) and E2 pretreatment slices (n = 12). C, comparison of the extent to which the burst responses were facilitated within three trains of HFS between the two groups. The areas of burst responses 2 and 3 were estimated by expressing them as fractions of the size of the first burst in the train. The percentage increases in burst response areas across the second and third bursts in control slices were both larger than those of E2-treated slices. *, p < 0.05; **, p < 0.01 versus controls. Error bars, S.E.
Given that the burst response magnitude elicited by trains of stimulation reflects the degree of calcium influx during tetanization, the level of NMDA receptor activity, and the subsequent facilitation of LTP induction (48), we attempted to identify whether excitatory neurotransmitter release during HFS contributed to the heightened LTP. The burst area under the first train of HFS relative to the averaged area of base-line NMDA-EPSCs was calculated. E2 pretreatment produced significant enlargement of this area, which indicates the involvement of transmitter release (control 5.99 ± 0.88 versus estrogen 9.71 ± 1.88; t test, p < 0.05) (Fig. 5B). Moreover, the percentages of increase in burst response area across the second and third trains in E2-treated slices were smaller than those of control slices, indicating greater tetanus-induced depletion of neurotransmitter in E2-treated slices (Fig. 5C). Thus, excitatory neurotransmitter release during HFS possibly contributes to E2-induced increases in LTP magnitude.
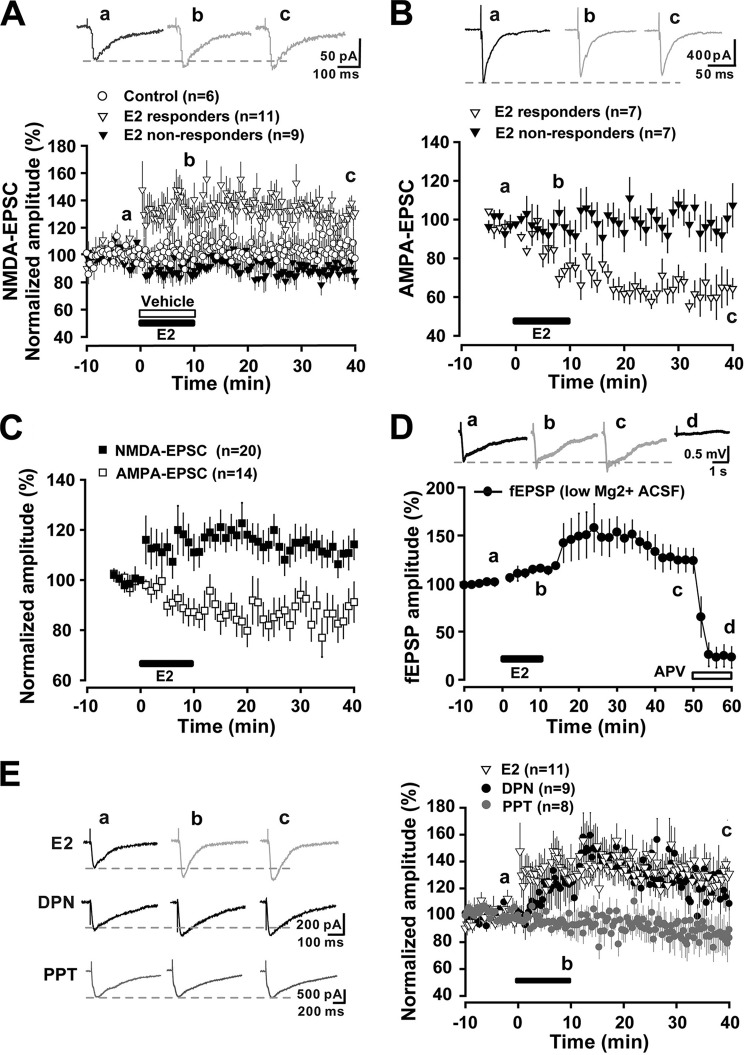
E2 Induces Chemical LTP of NMDA-EPSCs and LTD of AMPA-EPSCs
Using the >20% potentiation criterion, in 11 of 20 neurons examined, the bath application of E2 (10 μm) for 10 min rapidly and persistently enhanced the amplitude of monosynaptic NMDA-EPSCs evoked from the dorsal root for more than 40 min (responders), which was defined as the chemical LTP of NMDA-EPSCs. In the other nine neurons, the evoked monosynaptic NMDA-EPSCs were unaffected by E2 (non-responders; Fig. 6A). In contrast, in 7 of 14 neurons examined, monosynaptic AMPA-EPSCs were reduced within 10 min after E2, and this depression persisted more than 40 min, which was defined as chemical LTD of AMPA-EPSCs (Fig. 6B). This result indicated that E2 long lastingly modulated NMDA and AMPA transmission in opposite directions. We pooled all of the E2 responders and non-responders together and still found that the average amplitudes of the NMDA-EPSCs were persistently potentiated, and the AMPA-EPSCs were persistently depressed by E2 (Fig. 6C). To confirm the dominance of the potentiation of NMDA transmission by E2, fEPSPs in the superficial dorsal horn evoked by LT stimulation (0.1 ms, 0.5–0.7 mA, 2-min interval) were recorded in low Mg2+ high Ca2+ ACSF to facilitate the opening of NMDARs. Consistently, LT fEPSPs were enlarged by a brief application of E2, and this effect could be eliminated by APV (50 μm) (Fig. 6D).
FIGURE 6.
Estrogen induces chemical LTP of NMDA-EPSCs and LTD of AMPA-EPSCs. A, E2 (10 μm) induced a chemical LTP of NMDA-EPSC in 11 of 20 neurons (responders), whereas NMDA-EPSCs remained unaltered in the remaining neurons (non-responders) as compared with vehicle-treated (ethanol) neurons (n = 6). Each point represents an averaged trace of three sweeps. Top, traces collected as controls, 5 min after E2 infusion, and after 30 min of washout. B, E2 induced a chemical LTD of AMPA-EPSC in 7 of 14 neurons (responders), whereas AMPA-EPSCs were unchanged in the remaining neurons (non-responders). C, when all E2 responders and non-responders were pooled together, a persistent potentiation of NMDA-EPSCs and a long lasting depression of AMPA-EPSCs due to E2 were revealed. D, E2 rapidly increased LT stimulation-induced (0.1 ms, 0.5–0.7 mA, 2-min interval) fEPSPs (LT-fEPSPs) in the superficial dorsal horn, and this effect was eliminated by APV (50 μm). Top, traces collected as controls, 10 min after E2 infusion, 30 min after E2 washout, and 10 min after APV (50 mm) infusion. LT-fEPSPs were recorded in low Mg2+ high Ca2+ ACSF. E, comparison of the effects of E2 (n = 11), DPN (5 μm) (n = 9), and PPT (2 μm) (n = 8) on NMDA-EPSCs. Left, traces collected as controls, 10 min after E2, DPN, or PPT infusion and after 30-min washout. Error bars, S.E.
To further investigate the relative contribution of classic ERs to the acute chemical LTP of NMDA-EPSCs, the ERα- and ERβ-selective agonists PPT and DPN were used. The E2-induced potentiation of NMDA-EPSCs was mimicked by DPN (5 μm) (Fig. 6E). In contrast, the ERα-selective agonist PPT (2 μm) failed to potentiate NMDA-EPSCs in any of the neurons we recorded; PPT even resulted in a slight reduction of NMDA-EPSCs (30 min point after E2 washout, 89.13 ± 14.3% of base line) (Fig. 6E). Somewhat consistently, a recent study showed that blockade of ERα with MPP potentiates C-fiber stimulation-induced EPSCs in spinal dorsal horn neurons (49). Taken together, our present results raise the possibility that E2-induced LTP of NMDA-EPSCs in the spinal cord may be mediated by ERβ, similar to what has been observed in the hippocampus (10).
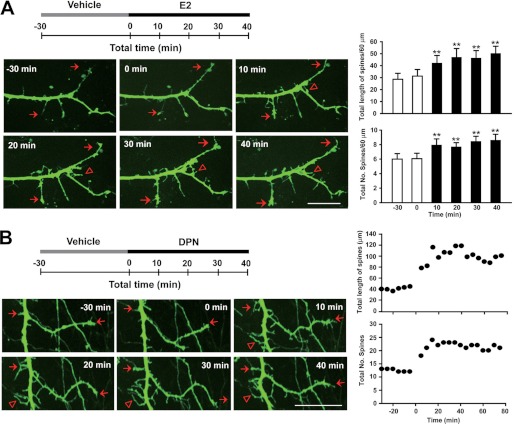
E2 Rapidly Modulates Dendritic Spine Morphogenesis
Dendritic spines are the anatomical loci of excitatory synapses on neurons, and spine formation and maintenance are thought to contribute to modified synaptic plasticity (15, 50). To investigate whether E2 can rapidly change the dendritic spine morphology of spinal dorsal horn neurons, which may contribute to enhanced NMDA transmission and plasticity as discussed above, we assessed the dendritic spine morphology of spinal dorsal horn neurons following treatment with 10 μm E2. GFP-lentivirus was transfected into cultured spinal dorsal horn neurons (14 days in vitro). Three days after transfection, the living cells emit steady green fluorescence upon illumination by light of suitable wavelengths. Neurons were pretreated for 30 min with vehicle (ethanol) as a control and with 10 μm E2 for a maximum of 40 min. After E2 treatment, the lengths (one-way ANOVA, F(5, 89) = 13.736, p = 0.017, n = 16) and densities of dendritic spines and dendritic filopodia were both increased at all time points observed (one-way ANOVA, F(5, 89) = 2.330, p = 0.049, n = 16) (Fig. 7A) (supplemental Movie S1). Given that the activation of ERβ can mimic the E2-induced LTP of NMDA-EPSCs, we also examined whether ERβ activation is capable of inducing spine morphogenesis. Cultured spinal dorsal horn neurons were exposed to the ERβ agonist DPN at 5 μm. Fig. 7B shows an example of DPN exposure increasing the total length and number of dendritic spines along the dendrites (also see supplemental Movie S2). Thus, ERβ may be involved in the E2-induced promotion of synaptic plasticity.
FIGURE 7.
Estrogen rapidly modulates dendritic spine morphogenesis. A, E2 enhances dendritic spine density and length in GFP-expressing spinal dorsal horn neurons. Top, schematic of the experiment, which involved 40-min exposure to E2 before 30-min vehicle treatment. Left, time lapse imaging of a typical neuron expressing GFP. The neuron was imaged for the 30-min vehicle treatment and then at 0, 10, 20, 30, and 40 min after treatment with E2. Triangles indicate novel spines; arrows represent persistent spines. Right, the total length and number of spines were rapidly increased by E2 at all time points observed. **, p < 0.01 versus before E2. B, an example showing the time course of DPN-induced increases in spine length and spine number. Scale bars, 20 μm. Error bars, S.E.
DISCUSSION
This study provides the first evidence that E2 in the spinal cord acutely modulates excitatory synaptic transmission and facilitates spinal LTP by increasing presynaptic glutamate release, persistently potentiating NMDAR transmission, and promoting dendritic spine formation and prolongation. This effect was mediated by membrane-initiated, G protein-dependent rapid signaling pathways. Furthermore, HFS, which reliably induced spinal LTP, rapidly increased perfusate E2 concentrations in the spinal cord slices, suggesting that spine-derived E2 may function as a neuromodulator of neuronal synaptic transmission and LTP, which are relevant to nociception.
Estrogen as a Rapid Regulator of Neuronal Synaptic Transmission and Plasticity
Accumulating evidence indicates that E2 rapidly regulates excitatory synaptic transmission and LTP in the hippocampus, hypothalamus, and medial vestibular nucleus (8, 9, 16, 18). To date, it has not been revealed whether or how estrogen in the spinal cord regulates synaptic plasticity. In the present study, estrogen robustly enhanced NMDA-evoked currents within 5 min in spinal dorsal horn neurons, suggesting that postsynaptic and/or extrasynaptic NMDAR function can be rapidly potentiated. This finding is consistent with a previous report that E2 exerts its acute effects via a postsynaptic mechanism (51). Interestingly, when the slices were pretreated by E2 for 30 min, NMDA currents exhibited larger enhancements. One possible explanation is that 30-min E2 treatment may increase externalization of ERs, leading to an exaggerated effect. In support of this idea, a recent study of hypothalamic neurons demonstrated that E2 stimulation rapidly increases the insertion of ERα proteins into the plasma membrane, and this effect peaks at 30 min (38).
We also investigated the effects of exogenous E2 on HFS-induced LTP of fEPSPs in the spinal dorsal horn. Because more than 50% of LT fibers are C-primary afferent fibers, HFS of LT-evoked LTP in vitro is thought to be linked to the central sensitization of pain (28, 52, 53). We found that E2 lowered the threshold for LTP induction and enhanced LTP magnitude, and these effects could be mimicked by E2-BSA, a mERs agonist, indicating that the heightening of HFS-LTP by E2 is mediated, at least in part, by mERs. Furthermore, the colocalization of E2-BSA-FITC with GluN1 and GluA1 provided an anatomical groundwork by which estrogen could modulate NMDAR/AMPAR transmission and synaptic plasticity of spinal dorsal horn neurons via mERs. Immunocytochemistry/immunohistochemistry and Western blot analyses of surface biotinylated proteins revealed that GPER1, ERα, and ERβ were distributed in the surface of the dorsal horn neurons. Preperfusion of ICI 182,780 (a classical ER antagonist) completely blocked the E2-induced potentiation of LTP. The effects of ICI 182,870 are not likely to be mediated via antagonism of GPER1 because ICI 182,780 is thought to be an agonist at this receptor (54). In hippocampal slices, ERβ activation significantly enhances θ burst stimulation-induced LTP in wild-type mice but not in Esr2−/− mice (15). Consistently, our current study further demonstrated that the ERβ-selective agonist DPN, but not the ERα-selective agonist PPT, could mimic the sustained effects of E2 on NMDA-EPSCs in dorsal horn neurons. Although this evidence does not rule out other candidates, such as GRER1, for mediating the rapid actions of mERs, it does suggest a critical role of ERβ in mediating the effects of estrogen on synaptic plasticity and spinally based nociceptive processing.
Potential Synaptic Underpinnings of the E2-induced Increase in Spinal LTP Magnitude
Several potential mechanisms could contribute to the enhancement of LTP magnitude by E2. In the hippocampus, E2-induced increases in NMDA transmission can directly increase LTP magnitude (11, 12). Overexpression of NR2B-containing NMDAR enhances LTP, whereas blockade of NR2B prevents the estrogen-induced increase in LTP magnitude (12, 55). In the spinal cord, this mechanism is also possible. HFS-LTP required NMDARs and NR2B activation. E2 quickly phosphorylated NR2B, persistently enhanced NMDA transmission, and depressed AMPA transmission, indicating a critical role of NMDA transmission in E2-induced increases in spinal LTP. Although E2 has been reported to inhibit GABA and glycine receptors, and this inhibition has been reported to contribute to E2-induced LTP enhancement (31, 56), it is unlikely that E2 facilitates HFS-LTP in the spinal dorsal horn by a disinhibition mechanism because, in our experiment, LTP recordings were performed in the presence of GABAA and GlyR antagonists.
E2 rapidly increases presynaptic glutamate release in the hippocampus, which may contribute to persistent potentiation of EPSC amplitude (10). In our study, E2-induced increases in glutamate release probability may also be involved in the E2-induced facilitation of HFS-LTP in the spinal cord. Moreover, an increase in steady-state depolarization during HFS occurred in spinal dorsal horn neurons following E2 treatment. The mechanism responsible for this increase in steady-state depolarization is thought to be due to mass transmitter release and activation of extrasynaptic NMDAR (57).
Synaptic structural plasticity is always in line with functional plasticity. Recent studies have shown that estrogen can rapidly influence dendritic spine structure, and the changes in connectivity contribute to modified synaptic plasticity in multiple brain regions, including the hippocampus and cortex (13, 15, 50). It has also been reported that an E2-induced increase in hippocampal LTP magnitude only occurs when spine density is increased simultaneously with an escalation in NMDA transmission relative to AMPA receptor transmission (11). Our data also revealed an acute and sustained increase in spine density and length in spinal dorsal horn neurons following treatment with E2 or the ERβ agonist DPN. Similarly, ERβ activation increases dendritic branching and the density of mushroom-type spines in hippocampal neurons and also enhances LTP magnitude (15). Thus, E2-induced dendritic spine morphogenesis may also contribute to enhancement of spinal LTP.
GPER1 belongs to the G protein-coupled, seven-membrane-spanning receptor family and has been reported to mediate the rapid effects of estrogen via generation of Gαs/Gβγ (54) and other well known membrane signaling cascades (1, 58). In addition to their nuclear localization, classical ERs (ERα and ERβ) can also be trafficked to the plasma membrane, where they may directly bind to G proteins or link to other G protein-coupled receptors (e.g. mGluRs), which then induce signaling pathways (4, 59, 60, 61). Through these signaling pathways, estrogen can not only rapidly modulate the synaptic transmission of dorsal horn neurons but also can activate transcription factors, such as cAMP-response element binding protein, to affect gene expression, leading to long term effects, including LTP. The spinal PKA-MAPK pathway is a key signaling pathway in the spinal nociceptive process (26, 62). Cross-talk between ERs, PKA, ERK, and NMDARs has been reported (3, 63, 64). In this study, E2 rapidly increased pPKA, pERK, and pNR2B levels, and blockade of PKA, ERK, or NR2B activation completely prevented spinal HFS-LTP in E2-treated slices, providing further evidence that the PKA-ERK signaling pathway is involved in estrogen-induced facilitation of spinal LTP and nociceptive behavioral responses. It is plausible that E2 activates PKA and ERK by G protein signaling and then phosphorylates NMDARs. Inhibition of spinal PKA or ERK activation has been demonstrated to attenuate NDMAR subunit phosphorylation (3, 65). Additionally, E2 increases presynaptic glutamate release, which induces the NMDAR activation-Ca2+ influx-PKA-ERK pathway. Indeed, the NMDAR-PKA-ERK pathway has also been shown to contribute to central sensitization and affective pain (66). Together, the neurosteroid E2 acts as a neuromodulator that activates a distinct suite of cellular/molecular events that increases glutamate release, facilitates NMDAR transmission, and modulates dendritic spine morphogenesis, resulting in the enhancement of spinal synaptic transmission and LTP. Thus, E2 is involved in spinal nociceptive processing.
Supplementary Material
Acknowledgments
We thank Prof. Ru-Rong Ji for critical reading of the manuscript and Prof. Wei Lu for helpful comments.
This work was supported by National Natural Science Foundation of China Grants 31271183, 31121061, and 31070973.

This article contains supplemental Movies S1 and S2.
- NMDAR
- NMDA receptor
- GDPβS
- guanosine-5′-O-(2-thiodiphosphate)
- HFS
- high frequency stimulation
- LTP
- long-term potentiation
- LTD
- long-term depression
- ER
- estrogen receptor
- mER
- membrane estrogen receptor
- E2
- 17β-estradiol
- BMI
- bicuculline methiodide
- ACSF
- artificial cerebrospinal fluid
- EPSC
- excitatory postsynaptic current
- fEPSP
- field excitatory postsynaptic potential
- LT
- Lissauer's tract
- pERK
- pPKA, and pNR2B, phosphorylated ERK, PKA, and NR2B, respectively
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine
- ANOVA
- analysis of variance
- PPR
- paired pulse ratio
- APV
- DL-2-amino-5-phosphonovaleric acid
- CNQX
- 6-Cyano-7-nitroquinoxaline-2,3-dione
- PPT
- propyl pyrazole triol
- DPN
- diarylpropionitrie.
REFERENCES
- 1. Maggiolini M., Picard D. (2010) The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 204, 105–114 [DOI] [PubMed] [Google Scholar]
- 2. Peng H. Y., Chen G. D., Tung K. C., Chien Y. W., Lai C. Y., Hsieh M. C., Chiu C. H., Lai C. H., Lee S. D., Lin T. B. (2009) Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. Am. J. Physiol. Endocrinol. Metab. 297, E416–E426 [DOI] [PubMed] [Google Scholar]
- 3. Tang B., Ji Y., Traub R. J. (2008) Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain 137, 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu N. J., Chakrabarti S., Schnell S., Wessendorf M., Gintzler A. R. (2011) Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal κ- and μ-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J. Neurosci. 31, 11836–11845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Evrard H. C., Balthazart J. (2004) Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J. Neurosci. 24, 7225–7229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evrard H. C. (2006) Estrogen synthesis in the spinal dorsal horn. A new central mechanism for the hormonal regulation of pain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R291–R299 [DOI] [PubMed] [Google Scholar]
- 7. Evrard H., Baillien M., Foidart A., Absil P., Harada N., Balthazart J. (2000) Localization and controls of aromatase in the quail spinal cord. J. Comp. Neurol. 423, 552–564 [DOI] [PubMed] [Google Scholar]
- 8. Foy M. R., Xu J., Xie X., Brinton R. D., Thompson R. F., Berger T. W. (1999) 17β-Estradiol enhances NMDA receptor-mediated EPSPs and long term potentiation. J. Neurophysiol. 81, 925–929 [DOI] [PubMed] [Google Scholar]
- 9. Kramár E. A., Chen L. Y., Brandon N. J., Rex C. S., Liu F., Gall C. M., Lynch G. (2009) Cytoskeletal changes underlie estrogen's acute effects on synaptic transmission and plasticity. J. Neurosci. 29, 12982–12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smejkalova T., Woolley C. S. (2010) Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J. Neurosci. 30, 16137–16148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith C. C., McMahon L. L. (2005) Estrogen-induced increase in the magnitude of long term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J. Neurosci. 25, 7780–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith C. C., McMahon L. L. (2006) Estradiol-induced increase in the magnitude of long term potentiation is prevented by blocking NR2B-containing receptors. J. Neurosci. 26, 8517–8522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srivastava D. P., Woolfrey K., Jones K. A., Shum C. Y., Lash L. L., Swanson G. T., Penzes P. (2008) Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc. Natl. Acad. Sci. U.S.A. 105, 14650–14655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li C., Brake W. G., Romeo R. D., Dunlop J. C., Gordon M., Buzescu R., Magarinos A. M., Allen P. B., Greengard P., Luine V., McEwen B. S. (2004) Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U.S.A. 101, 2185–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu F., Day M., Muñiz L. C., Bitran D., Arias R., Revilla-Sanchez R., Grauer S., Zhang G., Kelley C., Pulito V., Sung A., Mervis R. F., Navarra R., Hirst W. D., Reinhart P. H., Marquis K. L., Moss S. J., Pangalos M. N., Brandon N. J. (2008) Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 11, 334–343 [DOI] [PubMed] [Google Scholar]
- 16. Schwarz J. M., Liang S. L., Thompson S. M., McCarthy M. M. (2008) Estradiol induces hypothalamic dendritic spines by enhancing glutamate release. A mechanism for organizational sex differences. Neuron 58, 584–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Srivastava D. P., Woolfrey K. M., Liu F., Brandon N. J., Penzes P. (2010) Estrogen receptor β activity modulates synaptic signaling and structure. J. Neurosci. 30, 13454–13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zadran S., Qin Q., Bi X., Zadran H., Kim Y., Foy M. R., Thompson R., Baudry M. (2009) 17β-Estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc. Natl. Acad. Sci. U.S.A. 106, 21936–21941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klinge C. M., Blankenship K. A., Risinger K. E., Bhatnagar S., Noisin E. L., Sumanasekera W. K., Zhao L., Brey D. M., Keynton R. S. (2005) Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors α and β in endothelial cells. J. Biol. Chem. 280, 7460–7468 [DOI] [PubMed] [Google Scholar]
- 20. Cao H., Cui Y. H., Zhao Z. Q., Cao X. H., Zhang Y. Q. (2009) Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction of long-term potentiation in rats. Neurosci. Bull. 25, 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krapivinsky G., Krapivinsky L., Manasian Y., Ivanov A., Tyzio R., Pellegrino C., Ben-Ari Y., Clapham D. E., Medina I. (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40, 775–784 [DOI] [PubMed] [Google Scholar]
- 22. Peng G., Han M., Du Y., Lin A., Yu L., Zhang Y., Jing N. (2009) SIP30 is regulated by ERK in peripheral nerve injury-induced neuropathic pain. J. Biol. Chem. 284, 30138–30147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh M., Sétáló G., Jr., Guan X., Warren M., Toran-Allerand C. D. (1999) Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants. Convergence of estrogen and neurotrophin signaling pathways. J. Neurosci. 19, 1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bi R., Broutman G., Foy M. R., Thompson R. F., Baudry M. (2000) The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 97, 3602–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji R. R., Kohno T., Moore K. A., Woolf C. J. (2003) Central sensitization and LTP. Do pain and memory share similar mechanisms? Trends Neurosci. 26, 696–705 [DOI] [PubMed] [Google Scholar]
- 26. Ji R. R., Zhang Y. Q. (2005) The role of MAP kinase cascades in cell signaling, neural plasticity and pain facilitation. Neurosci. Bull. 21, 3–9 [Google Scholar]
- 27. Sandkühler J. (2007) Understanding LTP in pain pathways. Mol. Pain 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng L. Z., Lü N., Zhang Y. Q., Zhao Z. Q. (2010) Ryanodine receptors contribute to the induction of nociceptive input-evoked long-term potentiation in the rat spinal cord slice. Mol. Pain 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Terman G. W., Eastman C. L., Chavkin C. (2001) μ opiates inhibit long term potentiation induction in the spinal cord slice. J. Neurophysiol. 85, 485–494 [DOI] [PubMed] [Google Scholar]
- 30. Peterson E. R., Crain S. M. (1982) Nerve growth factor attenuates neurotoxic effects of taxol on spinal cord-ganglion explants from fetal mice. Science 217, 377–379 [DOI] [PubMed] [Google Scholar]
- 31. Jiang P., Kong Y., Zhang X. B., Wang W., Liu C. F., Xu T. L. (2009) Glycine receptor in rat hippocampal and spinal cord neurons as a molecular target for rapid actions of 17-β-estradiol. Mol. Pain 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiu J., Bosch M. A., Tobias S. C., Grandy D. K., Scanlan T. S., Ronnekleiv O. K., Kelly M. J. (2003) Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 23, 9529–9540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liao D., Scannevin R. H., Huganir R. (2001) Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J. Neurosci. 21, 6008–6017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu W., Man H., Ju W., Trimble W. S., MacDonald J. F., Wang Y. T. (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29, 243–254 [DOI] [PubMed] [Google Scholar]
- 35. Collins P., Webb C. (1999) Estrogen hits the surface. Nat. Med. 5, 1130–1131 [DOI] [PubMed] [Google Scholar]
- 36. Russell K. S., Haynes M. P., Sinha D., Clerisme E., Bender J. R. (2000) Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc. Natl. Acad. Sci. U.S.A. 97, 5930–5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shughrue P. J., Lane M. V., Merchenthaler I. (1997) Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J. Comp. Neurol. 388, 507–525 [DOI] [PubMed] [Google Scholar]
- 38. Dominguez R., Micevych P. (2010) Estradiol rapidly regulates membrane estrogen receptor α levels in hypothalamic neurons. J. Neurosci. 30, 12589–12596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kow L. M., Pfaff D. W. (2004) The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc. Natl. Acad. Sci. U.S.A. 101, 12354–12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pedersen L. M., Gjerstad J. (2008) Acta Physiol. (Oxf.) Spinal cord long term potentiation is attenuated by the NMDA-2B receptor antagonist Ro 25–6981. 192, 421–427 [DOI] [PubMed] [Google Scholar]
- 41. Qu X. X., Cai J., Li M. J., Chi Y. N., Liao F. F., Liu F. Y., Wan Y., Han J. S., Xing G. G. (2009) Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp. Neurol. 215, 298–307 [DOI] [PubMed] [Google Scholar]
- 42. Lagrange A. H., Ronnekleiv O. K., Kelly M. J. (1997) Modulation of G protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol. Pharmacol. 51, 605–612 [DOI] [PubMed] [Google Scholar]
- 43. Xin W. J., Gong Q. J., Xu J. T., Yang H. W., Zang Y., Zhang T., Li Y. Y., Liu X. G. (2006) Role of phosphorylation of ERK in induction and maintenance of LTP of the C-fiber-evoked field potentials in spinal dorsal horn. J. Neurosci. Res. 84, 934–943 [DOI] [PubMed] [Google Scholar]
- 44. Yang H. W., Hu X. D., Zhang H. M., Xin W. J., Li M. T., Zhang T., Zhou L. J., Liu X. G. (2004) Roles of CaMKII, PKA, and PKC in the induction and maintenance of LTP of C-fiber-evoked field potentials in rat spinal dorsal horn. J. Neurophysiol. 91, 1122–1133 [DOI] [PubMed] [Google Scholar]
- 45. Tallent M. K., Varghis N., Skorobogatko Y., Hernandez-Cuebas L., Whelan K., Vocadlo D. J., Vosseller K. (2009) In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J. Biol. Chem. 284, 174–181 [DOI] [PubMed] [Google Scholar]
- 46. Zucker R. S., Regehr W. G. (2002) Short-term synaptic plasticity. Annu. Rev. Physiol. 64, 355–405 [DOI] [PubMed] [Google Scholar]
- 47. Dobrunz L. E., Stevens C. F. (1997) Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron 18, 995–1008 [DOI] [PubMed] [Google Scholar]
- 48. Arai A., Lynch G. (1992) Factors regulating the magnitude of long-term potentiation induced by θ pattern stimulation. Brain Res. 598, 173–184 [DOI] [PubMed] [Google Scholar]
- 49. Zhong Y. Q., Li K. C., Zhang X. (2010) Potentiation of excitatory transmission in substantia gelatinosa neurons of rat spinal cord by inhibition of estrogen receptor α. Mol. Pain 6, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chklovskii D. B., Mel B. W., Svoboda K. (2004) Cortical rewiring and information storage. Nature 431, 782–788 [DOI] [PubMed] [Google Scholar]
- 51. Gu Q., Moss R. L. (1996) 17β-estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 16, 3620–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chu Y. X., Zhang Y., Zhang Y. Q., Zhao Z. Q. (2010) Involvement of microglial P2X7 receptors and downstream signaling pathways in long term potentiation of spinal nociceptive responses. Brain Behav. Immun. 24, 1176–1189 [DOI] [PubMed] [Google Scholar]
- 53. Ying B., Lü N., Zhang Y. Q., Zhao Z. Q. (2006) Involvement of spinal glia in tetanically sciatic stimulation-induced bilateral mechanical allodynia in rats. Biochem. Biophys. Res. Commun. 340, 1264–1272 [DOI] [PubMed] [Google Scholar]
- 54. Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 55. Barria A., Malinow R. (2005) NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48, 289–301 [DOI] [PubMed] [Google Scholar]
- 56. Murphy D. D., Cole N. B., Greenberger V., Segal M. (1998) Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J. Neurosci. 18, 2550–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zamani M. R., Levy W. B., Desmond N. L. (2004) Estradiol increases delayed, N-methyl-d-aspartate receptor-mediated excitation in the hippocampal CA1 region. Neuroscience 129, 243–254 [DOI] [PubMed] [Google Scholar]
- 58. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 59. Mermelstein P. G. (2009) Membrane-localized oestrogen receptor α and β influence neuronal activity through activation of metabotropic glutamate receptors. J. Neuroendocrinol. 21, 257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levin E. R. (2009) G protein-coupled receptor 30. Estrogen receptor or collaborator? Endocrinology 150, 1563–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Micevych P., Dominguez R. (2009) Membrane estradiol signaling in the brain. Front. Neuroendocrinol. 30, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ji R. R., Kawasaski Y., Zhuang Z. Y., Wen Y. R., Zhang Y. Q. (2007) Protein kinases as potential targets for the treatment of pathological pain. Handb. Exp. Pharmacol. 177, 359–389 [DOI] [PubMed] [Google Scholar]
- 63. Ji Y., Tang B., Traub R. J. (2011) Spinal estrogen receptor α mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain 152, 1182–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Adams M. M., Fink S. E., Janssen W. G., Shah R. A., Morrison J. H. (2004) Estrogen modulates synaptic N-methyl-d-aspartate receptor subunit distribution in the aged hippocampus. J. Comp. Neurol. 474, 419–426 [DOI] [PubMed] [Google Scholar]
- 65. Fu Y., Han J., Ishola T., Scerbo M., Adwanikar H., Ramsey C., Neugebauer V. (2008) PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol. Pain 4, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao H., Gao Y. J., Ren W. H., Li T. T., Duan K. Z., Cui Y. H., Cao X. H., Zhao Z. Q., Ji R. R., Zhang Y. Q. (2009) Activation of extracellular signal-regulated kinase in the anterior cingulate cortex contributes to the induction and expression of affective pain. J. Neurosci. 29, 3307–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.