Background: Oxa1 mediates the insertion of mitochondrion-encoded precursors into the inner mitochondrial membrane.
Results: Oxa1 forms a voltage- and substrate-dependent membrane pore.
Conclusion: The channel properties of the Oxa1 pore are compatible with the membrane-potential regulated protein insertase.
Significance: This is the first report on the pore-forming capacity of Oxa1, providing mechanistic insight into the insertase mechanism of Oxa1.
Keywords: Electrophysiology, Membrane Bilayer, Membrane Biophysics, Mitochondria, Protein Export, Mitochondrion-encoded Precursors, Planar Bilayer
Abstract
The inner membrane of mitochondria is especially protein-rich. To direct proteins into the inner membrane, translocases mediate transport and membrane insertion of precursor proteins. Although the majority of mitochondrial proteins are imported from the cytoplasm, core subunits of respiratory chain complexes are inserted into the inner membrane from the matrix. Oxa1, a conserved membrane protein, mediates the insertion of mitochondrion-encoded precursors into the inner mitochondrial membrane. The molecular mechanism by which Oxa1 mediates insertion of membrane spans, entailing the translocation of hydrophilic domains across the inner membrane, is still unknown. We investigated if Oxa1 could act as a protein-conducting channel for precursor transport. Using a biophysical approach, we show that Oxa1 can form a pore capable of accommodating a translocating protein segment. After purification and reconstitution, Oxa1 acts as a cation-selective channel that specifically responds to mitochondrial export signals. The aqueous pore formed by Oxa1 displays highly dynamic characteristics with a restriction zone diameter between 0.6 and 2 nm, which would suffice for polypeptide translocation across the membrane. Single channel analyses revealed four discrete channels per active unit, suggesting that the Oxa1 complex forms several cooperative hydrophilic pores in the inner membrane. Hence, Oxa1 behaves as a pore-forming translocase that is regulated in a membrane potential and substrate-dependent manner.
Introduction
Membrane protein biogenesis demands the translation on ribosomes, insertion into a target membrane in the topologically correct orientation, and proper three-dimensional folding into a functional state. In particular, the latter two processes, membrane insertion and folding of membrane proteins, are only poorly understood. The bacterial inner membrane and the endoplasmic reticulum of eukaryotic cells contain structurally related translocation machineries referred to as SecY or Sec61 complexes, respectively (1, 2). These complexes represent pore-forming structures that not only allow translocation of substrate proteins across the lipid bilayer but also their lateral integration into the membrane. In bacteria, membrane protein insertion is assisted by the conserved protein YidC (3, 4). YidC is present in the inner membrane at much higher concentrations than SecY (5). YidC facilitates SecY-independent membrane insertion of membrane proteins that do not expose large hydrophilic domains into the periplasmic space (6, 7). Moreover, YidC increases the folding rate of membrane proteins, suggesting that it enables membrane proteins to fold into their native structure (8, 9).
The inner membrane of mitochondria lacks a SecY-like translocation complex (10). Instead, the membrane protein Oxa1, which is closely related to YidC, facilitates protein insertion in mitochondria (11, 12). Oxa1 and YidC share a conserved domain consisting of five transmembrane spans, which catalyze protein insertion reactions. In contrast to YidC, Oxa1 contains a positively charged C-terminal domain of helical structure (13, 14). This matrix-exposed region binds mitochondrial ribosomes to facilitate co-translational protein insertion (13–15). Subunit 2 of cytochrome c oxidase (Cox2) of yeast is the best studied substrate of Oxa1. This mitochondrion-encoded protein directly interacts with Oxa1 early during its biosynthesis and is inserted in a strictly Oxa1-dependent manner. Cox2 contains an N-terminal leader peptide that is removed after translocation by the intermembrane space peptidase Imp1 (16, 17).
Although the relevance of Oxa1 in regard to the biogenesis of membrane proteins is well documented by many studies, the molecular function of Oxa1 in the process of substrate integration into the lipid bilayer is not understood. It was speculated that Oxa1 might form a pore to allow for the translocation of hydrophilic domains of its substrates (14). To test directly the pore-forming ability of Oxa1, we purified Oxa1 and reconstituted it into planar lipid bilayers for electrophysiological analyses. Our experiments show that Oxa1 forms a membrane channel in bilayers resembling the lipid composition of the inner mitochondrial membrane. As expected of an inner membrane channel, it was shown to maintain an electrochemical gradient, as seen in the inner membrane of mitochondria. The Oxa1 channel closes at the physiological membrane potential; however, Oxa1 responds to a substrate-peptide indicating its substrate-induced activation. Electrophysiological single channel analyses show that the active unit of the native Oxa1 complex contains four distinct pores, two of which display cooperativity in their gating behavior. Each pore is large enough to accommodate polypeptides in an unfolded or secondary structured form. Thus, our analyses provide the first experimental evidence that the Oxa1 insertase can act as a signal-regulated protein-conducting channel for the export of mitochondrion-encoded precursor proteins.
EXPERIMENTAL PROCEDURES
Purification of Oxa1 from Isolated Yeast Mitochondria
Oxa1 was purified from yeast mitochondria via a three-step isolation by combined Ni-NTA4/IgG chromatography and TEV cleavage elution. Mitochondria were resuspended in SDS-buffer (50 mm Na2HPO4, 100 mm NaCl, 10% glycerol, 10 mm imidazole, 1 mm PMSF, 1% SDS, pH 8.0) at a concentration of 1 mg of protein/100 μl at RT for 15 min. Samples were solubilized by adding 0.9 ml of TX-buffer/1 mg of protein (50 mm Na2HPO4, 100 mm NaCl, 10% glycerol, 10 mm imidazole, 1 mm PMSF, 0.2% Triton X-100). Soluble fractions were incubated with Ni-NTA resin. Columns were washed with TX-buffer containing 0.1% Triton X-100. Bound proteins were eluted in Imidazole buffer (50 mm Na2HPO4, 100 mm NaCl, 10% glycerol, 300 mm Imidazol, 1 mm PMSF, 0.1% Triton-X-100, pH 8.0), diluted with IP-buffer (50 mm Na2HPO4, 100 mm NaCl, 10% glycerol, 1 mm PMSF, 0.1% Triton X-100, pH 8.0), and loaded onto IgG-Sepharose. After washing, bound material was eluted with TEV protease in IP-buffer.
For complex isolation of Oxa1, mitochondria were solubilized in buffer (20 mm Tris, 50 mm NaCl, 10% glycerol, 0.5 mm EDTA, 1 mm PMSF, 1% digitonin, pH 7.4) at a concentration of 1 mg of protein/ml. After a clarifying spin, samples were incubated with IgG-Sepharose. Upon extensive washing with solubilization buffer containing 0.3% digitonin, bound proteins were eluted by TEV-protease cleavage.
Purification of Recombinant Oxa1
His10Oxa1 was expressed from the plasmid pEH1 in BL21(DE3) Escherichia coli cells. Cells were grown overnight at 37 °C in Terrific Broth medium supplemented with kanamycin. Cultures were diluted to an A600 of 0.4 and grown to an A600 of 0.8 at 30 °C. His10Oxa1 expression was induced by the addition of 0.5 mm isopropyl 1-thio-β-d-galactopyranoside and allowed to proceed for 4 h at 30 °C. Cells were harvested and incubated in extraction buffer (10 mm Tris, pH 7.0, 1 mm EDTA, 15% glycerol, 1 mm PMSF, 1 mg/ml lysozyme, 5 g/ml DNase I, 1× Complete Protease Inhibitor (Roche Applied Science)) for 30 min at 4 °C. Cells were broken by sonication, and cell debris was removed by centrifugation (4000 × g, 30 min, 4 °C). Subsequently, membranes were extracted by ultracentrifugation (100,000 × g, 30 min, 4 °C). Membranes were solubilized for 30 min on ice in lysis buffer (50 mm sodium phosphate buffer, pH 7.9, 500 mm NaCl, 5 mm imidazole, pH 7.9, 1% DDM, 1 mm PMSF, 1 Complete Protease Inhibitor). The lysate was cleared by ultracentrifugation (100,000 × g, 30 min, 4 °C) and His10Oxa1 was bound to a Ni-NTA column (Qiagen). The column was washed with 30 ml of washing buffer (50 mm sodium phosphate buffer, pH 7.9, 500 mm NaCl, 20 mm imidazole, pH 7.9, 0.1% DDM). Bound His10Oxa1 was recovered from the Ni-NTA resin by adding elution buffer (50 mm sodium phosphate buffer, pH 7.9, 500 mm NaCl, 500 mm imidazole, pH 7.9, 0,1% DDM). Fractions were collected and analyzed by SDS-PAGE.
Reconstitution of Channels into Liposomes
Small unilamellar liposomes were produced from synthetic lipids (Avanti Polar Lipids). A lipid composition resembling the mitochondrial inner membrane was utilized (45% phosphatidylcholine, 25% 1,2-dipalmitoleoyl-sn-glycero-3-phosphoethanolamine, 10% 1,2-dipalmitoyl-sn-glycero-3-phospho-(1′-myo-inositol), 5% 1,2-dimyristoyl-sn-glycero-3-phospho-l-serine, 15% cardiolipin). The lipid mixture at a concentration of 20 mg/ml was dissolved in 100 mm KCl, 10 mm Mops/Tris, pH 7.0, and small unilamellar liposomes were formed by subsequent freeze-thaw cycles. Recombinant channels were reconstituted using the detergent nonanoyl-N-methylglucamide (Mega-9). Liposomes and purified protein were resuspended in 80 mm Mega-9 and mixed at a protein to lipid ratio of 1:25. Proteoliposomes were formed by dialyzing the mixture against 100 mm KCl, 10 mm Mops/Tris, pH 7.0, for 1 h at room temperature and overnight at 4 °C.
For proteins purified from Saccharomyces cerevisiae, liposomes were resuspended in DDM buffer (100 mm KCl 0.4% DDM, 20 mm Mops/Tris, pH 7.0) and mixed with solubilized protein at a phospholipid concentration of 5 mg/ml. The mixture was incubated at 4 °C for 30 min and subsequently added to pre-washed and equilibrated Biobeads (Bio-Rad). Samples were shaken for 30 min at room temperature, following which the Biobeads were replaced and shaken overnight at 4 °C.
Liposome Flotation Assay
Proteoliposomes were floated in a discontinuous Nycodenz® density gradient. The buffer contained 100 mm KCl, 10 mm Mops/Tris, pH 7.0. Nycodenz and proteoliposomes were solubilized in the same buffer and mixed to a Nycodenz concentration of 40%. Subsequent layers of decreasing Nycodenz concentration were added (20, 10, 5, and 2%). Percentages indicated in the figures are the final concentrations of Nycodenz. The gradient was centrifuged at 4 °C for 60 min at 100,000 × g. Subsequently, the gradient was fractionated by taking equal volumes from the top. Fractions were analyzed using SDS-PAGE and Western blotting.
CD Spectroscopy
Purified proteins were reconstituted as described above. Small liposomes were formed as described previously and lysed in a buffer containing 80 mm Mega-9, 10 mm Mops/Tris, pH 7.0. Purified proteins were diluted 10-fold into the mixture. Proteoliposomes were formed by dialysis in a buffer containing 10 mm KCl, 10 mm Mops/Tris, pH 7.0. Samples were adjusted to a protein concentration of 250 μg/ml. CD spectra were recorded with a Jasco J-810 spectropolarimeter after calibration with (+)-10-camphorsulfonic acid. The measurements were performed at room temperature in a quartz cell (0.01-cm optical path length). Scans were performed at a rate of 50 nm/min with a sampling interval of 1 nm and averaged (n = 32) to improve the signal/noise ratio. Spectra were corrected with the corresponding buffer spectra collected under identical conditions. Data sets were converted to mean residue ellipticity and deconvoluted by a neural network approach (18) and the CDPro package (19–21).
Electrophysiological Setup and Measurements
Using the painting technique, we produced planar lipid bilayers. Proteoliposomes were dropped directly below the bilayer in the cis chamber. Buffer conditions were asymmetrical (250 mm KCl, 10 mm Mops/Tris, pH 7.0, in the cis compartment and 20 mm KCl, 10 mm Mops/Tris, pH 7.0, in the trans compartment). This led to osmotically induced fusion of proteoliposomes with the bilayer. Ag/AgCl electrodes were connected by 2 m KCl-agar bridges. The electrode in the trans chamber was connected to the head stage (CV-5–1GU) of a GeneClamp 500 current amplifier (Axon Instruments) and thus was the reference for reported membrane potentials. Current recordings were carried out using a Digidata 1200 A/D converter. Data analysis was performed by a self-written Windows-based single-channel investigation program (SCIP) in combination with Origin 7.0 (Microcal Software). After incorporation of Oxa1 into the bilayer, currents in response to varying applied voltages were recorded. Subsequently, antibodies or Cox2 peptides were added either to proteoliposomes before osmotically induced fusion or directly into the recording chamber after Oxa1 incorporation.
RESULTS
Oxa1 Purified from Yeast Mitochondria Displays Ion Channel Activity
Oxa1 mediates the export of mitochondrion-encoded proteins across the inner mitochondrial membrane and the membrane insertion of protein domains following their matrix import via the TIM23 translocase in a process referred to as conservative sorting (22–24). It is controversially debated how Oxa1 or its homologs mediate the transfer of polypeptide chains across a lipid bilayer. Thus, it has been proposed that Oxa1 may act as a protein-conducting channel in the inner membrane of mitochondria. To address the possible channel activity of Oxa1 directly, we purified Oxa1 from S. cerevisiae mitochondria, in which Oxa1 was expressed with a C-terminal double affinity ZZ/His10 tag (Oxa1ZZ/His) from the chromosomal locus (Fig. 1A) (25). Purified mitochondria were lysed under denaturing conditions. After dilution, Oxa1ZZ/His was purified in Triton X-100-containing buffer and subjected to sequential affinity chromatographic steps utilizing first Ni-NTA-agarose and then IgG-Sepharose. Oxa1 was released from IgG-Sepharose by TEV-protease cleavage. Because of the low amount of purified protein, purity was monitored by Western blotting (Fig. 1A). Next, purified Oxa1 was refolded into liposomes by a detergent-mediated reconstitution. It is important to note that successful reconstitution of the Oxa1 channel required the lipid composition of pre-formed proteoliposomes resembling the composition of the inner mitochondrial membrane (26).
FIGURE 1.
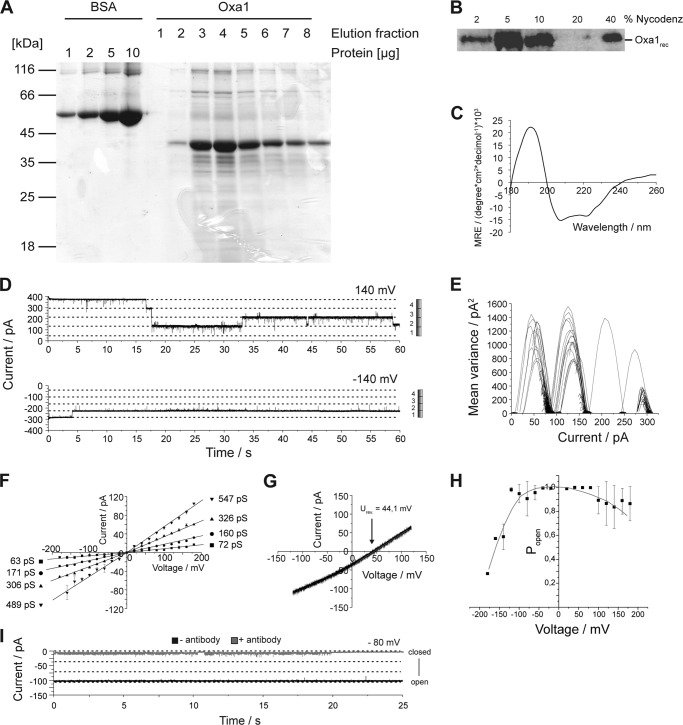
Oxa1 purified from S. cerevisiae constitutes an ion channel. A, three-step isolation of Oxa1 from yeast mitochondria. Upper panel, schematic of Oxa1 affinity tags; lower panels, Western blot analysis of solubilized extracts from wild type mitochondria (strain with authentic nontagged Oxa1) and mitochondria containing Oxa1ZZ/His-10, subjected to Ni-NTA purification (Eluate I) followed by IgG chromatography and TEV cleavage (Eluate II). Cleavage of Oxa1 results in a faster migrating Oxa1 cleavage product indicated by the arrow. (Total = 0.5%, Eluate I = 0.5%, and Eluate II = 5%.) Pam17, Rip1, and Cox1 are mitochondrial proteins serving as controls. Asterisk indicates proteolytic cleavage product. B, current recordings of a bilayer after fusion of an Oxa1-containing proteoliposome (membrane potential as indicated) under symmetrical buffer conditions (250 mm KCl, 20 mm Mops/Tris, pH 7.0). C, current voltage relationship calculated from >10,000 single gating events. Conductance states are indicated. Buffer conditions as in B. D, current-voltage ramp recorded under asymmetrical buffer conditions (cis, 250 mm KCl, 20 mm Mops/Tris, pH 7.0; trans, 20 mm KCl, 20 mm Mops/Tris, pH 7.0). Reversal potential is as indicated. E, current recordings of an Oxa1 containing bilayer before (black) and after the addition of anti-Oxa1 antibodies (red) and the statistical analysis of the mean Oxa1 channel current (n = 3).
When Oxa1 containing proteoliposomes were fused to a planar lipid bilayer by osmotic fusion (27, 28), ion channel activity could readily be detected (Figs. 1B and 2A). As a control, mock samples from yeast cells expressing untagged Oxa1, which had been subjected to the same purification and reconstitution routine, showed no ion channel activity (Fig. 2C).
FIGURE 2.
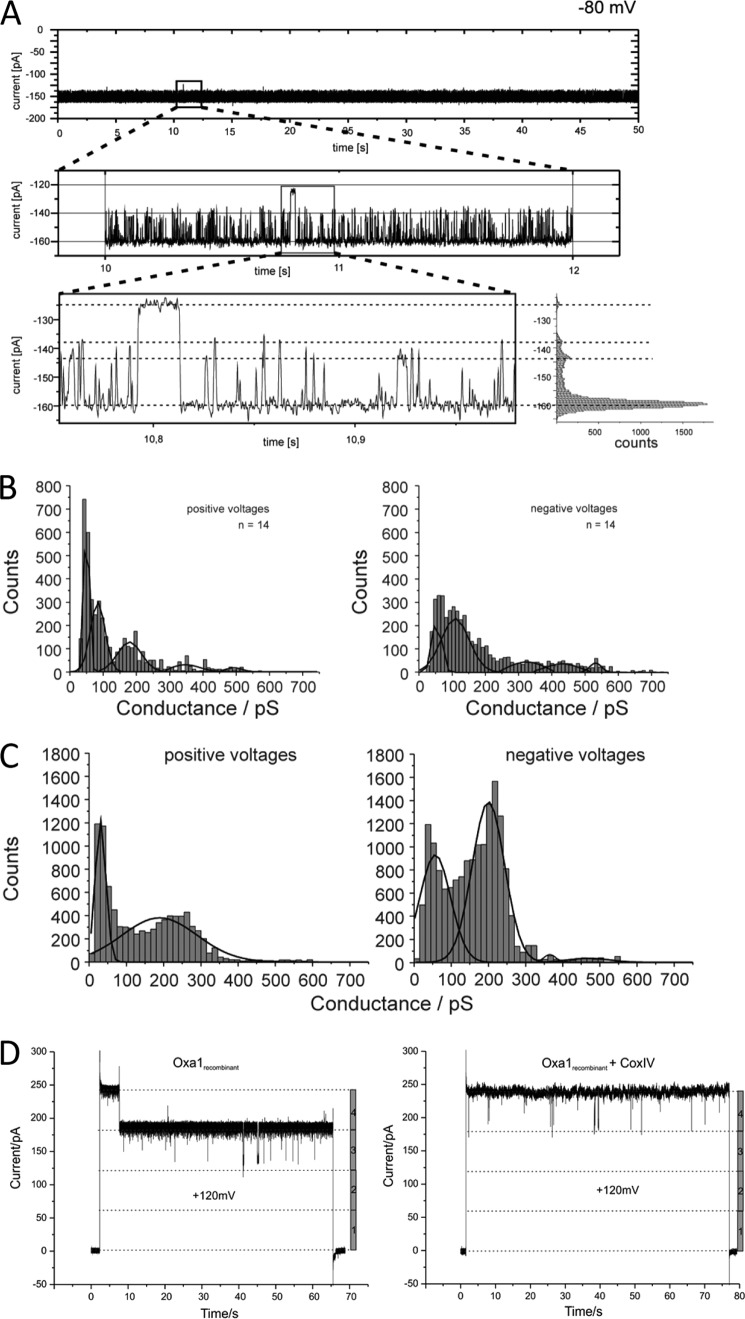
A, current recordings of a bilayer after fusion of an Oxa1-containing proteoliposome (membrane potential as indicated). Zoom plots show single gating events in main and subconductance states. B, conductance state histograms of Oxa1 at positive and negative applied holding potentials. C, current recordings of a bilayer after fusion of an Oxa1rec-containing proteoliposome (left) and from a bilayer fused with mock-treated sample liposomes obtained from cells expressing nontagged Oxa1rec that were added to the cis compartment (membrane potential of the voltage gate as indicated). D, current recordings of a bilayer after fusion of an Oxa1rec-containing proteoliposome in the absence (left) and presence of Pam17 antiserum (membrane potential of the voltage gate as indicated).
Oxa1 channels displayed distinct gating events with a mean maximal conductance of Gmax ≅500 pS and with a minimal subconductance of Gmin ≅75 pS at positive membrane potentials (Vm) and 250 mm KCl on both sides of the membrane. Conductance states varied slightly between negative and positive membrane potentials, and several subconductance states could be detected. This resulted in complex gating behavior of the ion channel (Figs. 1, B and C, and 2, A and B). Under asymmetrical buffer conditions (250/20 mm KCl, cis/trans) a reversal potential of Urev = 44 mV could be detected. Using the Goldman-Hodgkin-Katz approach, a cation-selective pore with a ratio for PK+/PCl− of 10:1 (Fig. 1D) could be calculated.
To assess the specificity of the aforementioned channel activity, we utilized antiserum directed against the intermembrane space domain of Oxa1. After incubation of Oxa1-containing proteoliposomes with the antiserum prior to liposome osmotic fusion with the planar lipid bilayer, ion channel incorporation into the membrane was drastically decreased. In addition, the remaining channel activity was limited to a very low conductance state, comprising only 10% of the main conductance in the absence of antisera (Fig. 1E). As a control, incubation with an antiserum directed against an unrelated inner mitochondrial membrane protein, Pam17, did not lead to any changes in either the fusion rate or ion channel characteristics (Fig. 2D). Thus, we concluded that the channel activity of the purified protein was specific for the reconstituted yeast Oxa1 channel.
Oxa1 Forms a Voltage-gated Ion Channel
Despite the fact that we isolated Oxa1 from mitochondria under stringent and denaturing conditions, we wanted to rule out that a contaminating yeast protein was responsible for the pore forming activity. Therefore, we expressed S. cerevisiae Oxa1 as a His10-tagged version in E. coli. Oxa1 (Oxa1rec) was purified by affinity chromatography from E. coli membranes (Fig. 3A) and reconstituted in the presence of detergent into liposomes (29, 30). Reconstitution efficiency was assessed by flotation of liposomes, which revealed that Oxa1rec was efficiently incorporated into liposomes (Fig. 3B). In the absence of liposomes, Oxa1rec did not float in a density gradient and could be found at the bottom of the gradient after centrifugation (Fig. 4C). In addition, we followed refolding by assessing the secondary structure of Oxa1rec in dodecylmaltoside micelles using circular dichroism (CD) spectroscopy (Fig. 3C). The Oxa1 CD spectrum revealed a shape characteristic of a predominantly α-helical protein (Table 1).
FIGURE 3.
Recombinantly expressed Oxa1 displays ion channel activity with similar characteristics as the yeast protein. A, SDS-PAGE of purified Oxa1rec. A BSA loading control for the estimation of protein concentrations is shown on the same gel. B, Western blot of a flotation assay of Oxa1rec-containing proteoliposomes. 40% represents the bottom of the gradient after centrifugation where aggregated or not incorporated protein is found. The majority of proteoliposomes floats into the 5% fraction of the gradient. C, CD spectrum of Oxa1rec in DDM. D, current recordings of a bilayer after the fusion of Oxa1rec (membrane potential as indicated) under symmetrical buffer conditions (see Fig. 1B). Diagram to the right shows that full channel closure occurs in four main conductance state gating events. E, mean variance analysis of D, with upper current trace showing that four gating events with the main conductance state led to complete channel closure. F, current voltage relationship calculated from over 5,000 single gating events. Conductance states are indicated. Buffer conditions are as in Fig. 1B. G, current-voltage ramp recorded under asymmetrical buffer conditions (as in Fig. 1D). Reversal potential as indicated. H, voltage-dependent open probability of Oxa1rec. Quantification was performed by comparing the mean current determined over a range of 1 min with the maximum current at a constant holding potential. I, current recordings of an Oxa1rec-containing bilayer before (black) and after the addition of anti-Oxa1 antibodies (upper gray).
FIGURE 4.
A, conductance state histograms of Oxa1complex at positive and negative applied holding potentials. Gating frequency of the complexes as indicated (LF, low gating frequency; HF, high gating frequency). B, current voltage ramp of an Oxa1complex with low or high gating frequency. C, His-tagged Oxa1 was expressed in E. coli and purified. The protein was incubated in the presence or absence of E. coli lipids. Liposomes were generated by removal of the detergent using BioBeads. The sample was treated with 0.1 m Na2CO3, adjusted to 1.6 m sucrose, placed on the bottom of a centrifugation tube, overlaid by 1.4 m sucrose, and centrifuged for 2 h at 485,000 × g. The samples were analyzed by Western blotting.
TABLE 1.
Oxa1 secondary structure
| Secondary structure | Relative content |
|---|---|
| % | |
| 190–260 nm | |
| α-Helix | 73 |
| β-Sheet (antiparallel) | 0 |
| β-Sheet (parallel) | 3 |
| β-Turn | 12 |
| Random coil | 12 |
| Total | 100 |
When Oxa1rec proteoliposomes were fused with planar lipid bilayers, ion channel activity could be detected (Figs. 3D and 5A). The basic characteristics resembled those of Oxa1 channels purified from yeast with a mean maximal conductance state for Oxa1rec of Gmax = 530 pS at positive Vm and 250 mm KCl (cis/trans), channel asymmetry in conductance states between negative and positive membrane potentials, and multiple subconductance states (Figs. 3F and 5B). Based on a conductance state of about 500 pS, we calculated a pore diameter of ∼1.9 nm (28, 31, 32). The calculated pore size would accommodate polypeptide chains with secondary or unfolded structure, as assessed by a typical α-helix diameter being roughly 0.5 nm (33, 34).
FIGURE 5.
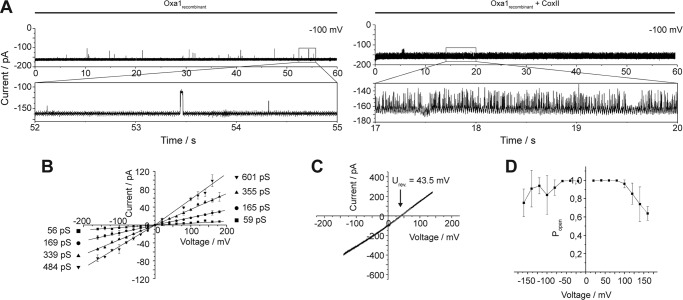
A, current recordings of a bilayer after fusion of a recombinant Oxa1 channel (membrane potential as indicated). Zoom plots show single gating events in main and subconductance states. B, conductance state histograms of Oxa1 at positive and negative applied holding potentials. C, conductance state histograms of Oxa1 at positive and negative applied holding potentials. Oxa1rec-containing proteoliposomes were incubated with Cox2 peptides prior to fusion. D, current recordings of a bilayer after fusion of an Oxa1rec-containing proteoliposome in the absence (left panel) and presence of 1 μm Cox IV1–23 (membrane potential of the voltage gate as indicated).
Remarkably, the minimal unit incorporated into the bilayer by a single fusion event always displayed a maximal current corresponding to four single channel (Gmax) currents (Fig. 3E). As observed in Oxa1 purified from yeast, the Oxa1rec channel was cation-selective with a reversal potential of Urev = 44 mV under asymmetrical salt conditions (250/20 mm KCl, cis/trans) corresponding to a permeability ratio of PK+/PCl− = 10:1 (Fig. 3G).
The observed voltage-dependent open probability of a four-pore-containing Oxa1 channel unit showed clear asymmetric behavior, indicating that the channel incorporated unidirectionally into the membrane (Fig. 3H). Considering the polarization of the membrane potential across the inner mitochondrial membrane, it is tempting to speculate that the cis side of the channel in vitro corresponds to the intermembrane space side in vivo, implying that at a negative Vm of >120 mV the pore will close. Thus, similar to what has been observed for the Tim23 channel of the presequence translocase and the Tim22 channel of the carrier translocase, at physiological inner membrane potentials of >150 mV, the Oxa1 channel will reside in the closed state thereby preventing deleterious ion leakage across this energy-coupling membrane.
As seen with Oxa1 purified from yeast, incubation of Oxa1rec proteoliposomes with anti-Oxa1 antibodies led to a reduction of proteoliposome fusion rates as well as to an almost complete block of channel conductance (Fig. 3I). Accordingly, these results, together with the ones obtained for Oxa1 purified from yeast, show that Oxa1 forms an ion channel in a lipid membrane.
Oxa1 Channels Are Activated by Substrate Peptides
Oxa1 mediates export of mitochondrion-encoded proteins across the inner membrane. A well known Oxa1 substrate is the precursor of Cox2 (pCox2) (22, 24). After processing by the IMP protease, mature Cox2 assembles into the core of the cytochrome c oxidase. To directly test if Oxa1 specifically recognizes transport substrates, we generated a synthetic peptide corresponding to the targeting signal of Cox2, comprising of the 19 N-terminal amino acids (MLDLLRLQLTTFIMNDVPT). Incubation of Oxa1rec proteoliposomes with Cox2 peptides prior to osmotic fusion led to a drastic increase in the gating frequency (flickering) of the ion channel (Fig. 6A). For example, the analysis of a 60-s current sample at a holding potential of V-h = +100 mV (Fig. 6A) yielded an average value fgating of 0.5 Hz, whereas Cox2 incubated channels showed a gating frequency of roughly 60 Hz. This increase in gating frequency was accompanied by changes in the conductance state distribution. Although the main conductance state could still be detected (Fig. 6B), channel flickering occurred not between the closed and the fully open state, but rather between the varieties of subconductance states (compare Fig. 5C with A and B, which are without Cox2)). Although no change in the reversal potential was observed (Fig. 6C), the peptide-induced activation of gating leads to a decreased voltage sensitivity of (Po) (Fig. 6D). One might speculate that the interaction of the channel with the peptide shifts the channel from an idle nonactive state into an active state with high gating frequency, representing a highly flexible pore state. Control measurements in which a presequence peptide of Cox4, a substrate of the Tim23 channel, was used instead of Cox2 showed no significant changes of the Oxa1 channel activity (Fig. 5D).
FIGURE 6.
Activation of Oxa1rec by a substrate peptide. A, current recordings of Oxa1rec containing bilayers in the absence (left panel) and in the presence (right panel) of Cox2 peptide. Oxa1 containing proteoliposomes were incubated prior to fusion with 500 μm Cox2 peptide. B, current voltage relationship of Oxa1rec in the presence of Cox2, calculated from >5,000 single gating events. Conductance states are indicated and show no significant change to Oxa1rec in the absence of Cox2 (Fig. 3F). C, current-voltage ramp of Oxa1rec in the presence of Cox2 recorded under asymmetrical buffer conditions (as in Fig. 1D). Reversal potential as indicated shows no alteration to Oxa1rec in the absence of Cox2. D, voltage-dependent open probability of Oxa1rec in the presence of Cox2.
Oxa1 Complex Displays Two Distinct Activity States
So far our analyses assessed the channel activity of Oxa1 after purification under denaturing conditions, thereby excluding unspecific channel activities. We reasoned that in the inner membrane of mitochondria, Oxa1 likely exists in a different oligomeric state then after reconstitution of an unfolded polypeptide. In previous analyses, we successfully isolated protein complexes from solubilized membranes and reconstituted these for electrophysiological studies (35, 36). Therefore, we purified Oxa1 under native conditions from mitochondria after solubilization in digitonin-containing buffer. Oxa1 containing a C-terminal ZZ-tag has been previously shown to be defective in association with mitochondrial ribosomes (25). After purification of Oxa1 complexes by IgG chromatography, native complexes were released from the resin by TEV protease cleavage of the tag. We confirmed efficient isolation of Oxa1 and purity of the sample by SDS-PAGE (Fig. 7A). When purified Oxa1 complexes were analyzed by blue native-PAGE, two major complexes were detected, one that migrated at ∼70 kDa and a larger one that migrated at 180 kDa (Fig. 7B). Although the smaller complex could represent a dimeric form of Oxa1, the larger complex was reminiscent of the tetrameric form of the protein that has been previously suggested for Oxa1 from S. cerevisiae and Neurospora crassa (37, 38). The ratio of dimeric to tetrameric Oxa1 that is visible on the blue native-PAGE does not necessarily reflect the ratio of complexes in the lipid membrane due to the presence of detergent and Coomassie. To assess the channel properties of native Oxa1, we purified Oxa1 complexes in preparative scale from mitochondria. Oxa1 complexes (Oxa1complex) were reconstituted into liposomes using mixed detergents with subsequent dialysis. Incorporation success was monitored by flotation of Oxa1-containing proteoliposomes (Fig. 7C). After fusion of these proteoliposomes with planar lipid bilayers, ion channel activity could be observed. The basic characteristics resembled those of the reconstituted Oxa1yeast and Oxa1rec channel properties. Strikingly, the OXA1complex channel appeared in two distinguishable forms, a low frequency and a high frequency gating state (Fig. 7, D and E). Both states showed a mean maximal conductance of Gmax ≅500 pS at positive Vm as well as multiple subconductance states (Figs. 7, F and G, and 9A). An asymmetric conductance state distribution, as observed with both channels described above, could also be seen (Figs. 7, F and G, and 9A). The reversal potential (250/20 mm KCl, cis/trans) was determined to be Urev = 45 mV in either activity state corresponding to a cation-selective channel with a permeability ratio of PK+/PCl− = 10:1 (Fig. 7, H and J). Interestingly, the voltage-dependent open probability showed remarkable differences for the two states. The low frequency gating activity showed steep voltage-dependent channel closure above threshold potentials of Vm > −100 mV. This is in line with the voltage dependence observed for Oxa1rec and indicates that the channel is closed under physiological membrane potentials (Fig. 7, I and K). Interestingly, the channel showed a rectifying current voltage relationship with reduced conductance at negative membrane potentials (Fig. 4B). Thus, if the cis compartment was made equivalent to the intermembrane space side of the channel, this would counteract ion leakage in vivo under conditions where the pore is in the open state.
FIGURE 7.
Oxa1 complex displays two activity states. A, purification of Oxa1 complex from S. cerevisiae mitochondria. Asterisk indicates proteolytic cleavage product. Arrow indicates faster migrating cleavage product. B, blue-native PAGE of purified Oxa1 complexes. C, Western blot of a flotation assay of the Oxa1 complex containing proteoliposomes. D and E, current recordings of a bilayer after fusion of the Oxa1 complex containing liposomes. D shows ion channel activity with low gating frequency, whereas the channel depicted in E shows high gating frequency. F, current-voltage relationship of channels with low gating frequency (D). Conductance states are indicated. G, current-voltage relationship of channels with high gating frequency (E). Conductance states are indicated. H, current-voltage ramp of the Oxa1 complex with low gating frequency. Reversal potentials are indicated. I, voltage-dependent open probability of Oxa1 complexes with low gating frequency. J, current-voltage ramp of the Oxa1 complex with high gating frequency. Reversal potentials are indicated. K, voltage-dependent open probability of Oxa1 complexes with high gating frequency.
FIGURE 9.
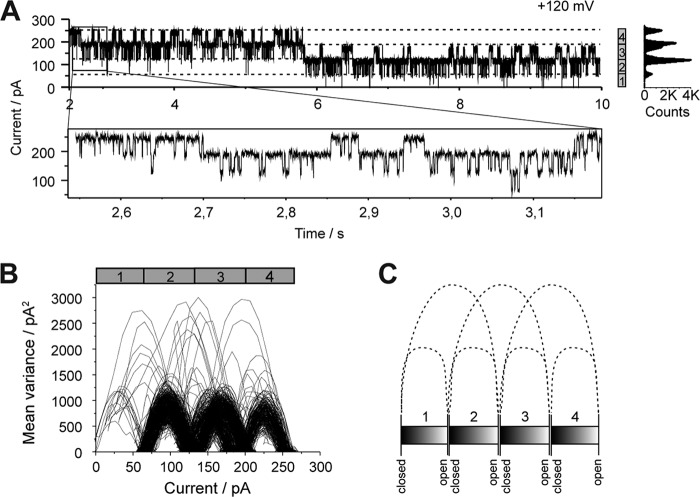
Oxa1 complex gating shows four pores of which two are coupled in each case. A, current recording of an Oxa1 complex containing bilayer at +120 mV. B, mean variance analysis of the current trace depicted in A (zoom plot). C, schematic representation of the gating transitions shown in B. B and C mainly show gating events of the main conductance state (4 to 3, 3 to 2, etc, but also gating events over two main conductance states (i.e. 4 to 2, 3 to 1, and 2 to fully closed) indicating two of the four pores are coupled in each case.
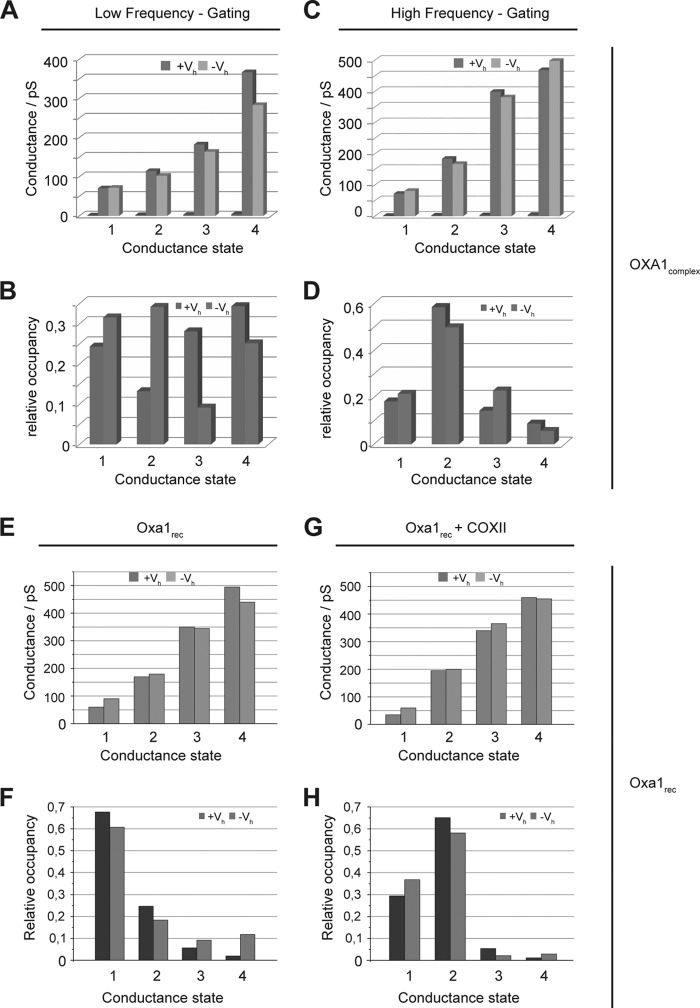
The Oxa1complex channel in the high frequency gating state was less sensitive to high voltage-induced channel closure and therefore resembles Oxa1rec in the presence of the Cox2 substrate. However, Oxa1complex in the low frequency state and Oxa1rec displayed a similar gating frequency. Furthermore, when we analyzed the relative occupancies of the different conductance states, we found remarkable similarities between the Oxa1 complex in its high frequency gating state and the recombinant Oxa1 channel in the presence of the Cox2 peptide (Fig. 8). A comparison of all the conductance occupancies of the two channels in their different activation states reveals that the increase in gating frequency mainly effects conductance state 2 (Fig. 8, D and H).
FIGURE 8.
Occupancies of conductance states in the Oxa1 complex with a high gating frequency resembles Oxa1rec in the presence of substrate. A, conductance states of Oxa1 complexes with low gating frequency at negative (dark gray) and positive (light gray) holding potentials. B, relative occupancies of conductance states shown in A. C and D, conductance states and relative occupancies of conductance states of Oxa1 complexes with high gating frequency. E and F, conductance states and relative occupancies of conductance states of recombinant Oxa1. G and H, conductance states and relative occupancies of conductance states of recombinant Oxa1 that was incubated with Cox2 peptides prior to fusion. Comparison of D and H shows a clear correlation of the relative occupancies of conductance state 2 of Oxa1 complexes with a high gating frequency and Oxa1rec in the presence of substrate.
In addition, we found that a single fusion event of an Oxa1complex proteoliposome yielded a maximal bilayer current in a multiple of four with respect to the mean maximal conductance state (Gmax) of the single Oxa1rec channel (Fig. 9A). At a holding potential of V-h = +120 mV, the maximal observed current was Imax ≅250 pA, which at a bilayer resistance of Rbilayer = 10 gigaohms corresponds to four times Gmax ≅480 pS, the main single channel conductance. As this behavior was also observed with the Oxa1rec channel activity, we analyzed the time course of gating events of Oxa1complex in the high frequency state. Remarkably, the analysis showed statistically significant numbers of direct gating transitions between either two of the four conductance levels of the pore (for example, see Equation 1) (Fig. 9, B and C).
We therefore conclude the pores could be coupled pairwise in their gating activity, presumably forming a common pore unit implying the Oxa1 channel assembles into a tetramer of two functionally coupled dimers (a dimer of dimers).
DISCUSSION
The above-described experiments show that Oxa1 reconstituted into a lipid environment resembling the lipid composition of the inner mitochondrial membrane is able to form a hydrophilic pore. The channel shows selectivity for cations and can be activated by peptides corresponding to a physiological substrate. The dimensions of the pore and its overall properties are similar to that of other protein translocation pores in the inner mitochondrial membrane, namely Tim23 and Tim22. The Oxa1 channel displays sufficient diameter to accommodate secondary structured polypeptides. Importantly, the channel closes in a voltage-dependent manner suggesting that the membrane potential across the inner membrane is maintained with the channel in a closed state in the absence of substrates. In particular, the substrate and voltage dependence of the Oxa1 channel properties resemble those of the previously extensively characterized Tim22 channel, implying similar inner mitochondrial membrane protein insertion mechanisms might be at work in both pathways (39, 40). Moreover, it has been shown for Tom40, Tim22, and Tim23 that the specific interaction of these protein translocation pores with signal peptides represent initial translocation steps leading to fast channel gating (flickering) (37, 39–41). Thus, the high frequency gating state of Oxa1 may be envisaged as a transport initiation state. Because Oxa1 directly binds to ribosomes mediating co-translational precursor protein transport across the inner membrane, it is tempting to speculate that the membrane potential-dependent closure observed here for the ribosome-free Oxa1 reflects its ability to prevent ion leakage in the absence of translocating substrates.
Acknowledgments
We thank Inge Perschil for expert technical assistance and Jonathan Melin for careful reading of the manuscript.
This work was supported by Human Frontiers in Science Program (to M. M.), Deutsche Forschungsgemeinschaft Grant FOR967 (to P. R. and R. W.), the Max Planck Society (to P. R.).
- Ni-NTA
- as nickel-nitrilotriacetic acid
- TEV
- tobacco etch virus
- pS
- picosiemens
- DDM
- n-dodecyl β-d-maltoside.
REFERENCES
- 1. Mandon E. C., Trueman S. F., Gilmore R. (2009) Translocation of proteins through the Sec61 and SecYEG channels. Curr. Opin. Cell Biol. 21, 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapoport T. A. (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669 [DOI] [PubMed] [Google Scholar]
- 3. Samuelson J. C., Chen M., Jiang F., Möller I., Wiedmann M., Kuhn A., Phillips G. J., Dalbey R. E. (2000) YidC mediates membrane protein insertion in bacteria. Nature 406, 637–641 [DOI] [PubMed] [Google Scholar]
- 4. Scotti P. A., Urbanus M. L., Brunner J., de Gier J. W., von Heijne G., van der Does C., Driessen A. J., Oudega B., Luirink J. (2000) YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 19, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Urbanus M. L., Fröderberg L., Drew D., Björk P., de Gier J. W., Brunner J., Oudega B., Luirink J. (2002) Targeting, insertion, and localization of Escherichia coli YidC. J. Biol. Chem. 277, 12718–12723 [DOI] [PubMed] [Google Scholar]
- 6. Serek J., Bauer-Manz G., Struhalla G., van den Berg L., Kiefer D., Dalbey R., Kuhn A. (2004) Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 23, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Laan M., Bechtluft P., Kol S., Nouwen N., Driessen A. J. (2004) F1F0-ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J. Cell Biol. 165, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagamori S., Smirnova I. N., Kaback H. R. (2004) Role of YidC in folding of polytopic membrane proteins. J. Cell Biol. 165, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wagner S., Pop O. I., Pop O., Haan G. J., Baars L., Koningstein G., Klepsch M. M., Genevaux P., Luirink J., de Gier J. W. (2008) Biogenesis of MalF and the MalFGK(2) maltose transport complex in Escherichia coli requires YidC. J. Biol. Chem. 283, 17881–17890 [DOI] [PubMed] [Google Scholar]
- 10. Glick B. S., Von Heijne G. (1996) Saccharomyces cerevisiae mitochondria lack a bacterial type sec machinery. Protein Sci. 5, 2651–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. He S., Fox T. D. (1997) Membrane translocation of mitochondrially coded Cox2p. Distinct requirements for export of N and C termini and dependence on the conserved protein Oxa1p. Mol. Biol. Cell 8, 1449–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hell K., Neupert W., Stuart R. A. (2001) Oxa1p acts as a general membrane insertion machinery for proteins encoded by mitochondrial DNA. EMBO J. 20, 1281–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jia L., Dienhart M., Schramp M., McCauley M., Hell K., Stuart R. A. (2003) Yeast Oxa1 interacts with mitochondrial ribosomes. The importance of the C-terminal region of Oxa1. EMBO J. 22, 6438–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohler R., Boehringer D., Greber B., Bingel-Erlenmeyer R., Collinson I., Schaffitzel C., Ban N. (2009) YidC and Oxa1 form dimeric insertion pores on the translating ribosome. Mol. Cell 34, 344–353 [DOI] [PubMed] [Google Scholar]
- 15. Szyrach G., Ott M., Bonnefoy N., Neupert W., Herrmann J. M. (2003) Ribosome binding to the Oxa1 complex facilitates co-translational protein insertion in mitochondria. EMBO J. 22, 6448–6457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herrmann J. M., Koll H., Cook R. A., Neupert W., Stuart R. A. (1995) Topogenesis of cytochrome oxidase subunit II. Mechanisms of protein export from the mitochondrial matrix. J. Biol. Chem. 270, 27079–27086 [DOI] [PubMed] [Google Scholar]
- 17. Nunnari J., Fox T. D., Walter P. (1993) A mitochondrial protease with two catalytic subunits of nonoverlapping specificities. Science 262, 1997–2004 [DOI] [PubMed] [Google Scholar]
- 18. Böhm G., Muhr R., Jaenicke R. (1992) Quantitative analysis of protein far-UV circular dichroism spectra by neural networks. Protein Eng. 5, 191–195 [DOI] [PubMed] [Google Scholar]
- 19. Sreerama N., Woody R. W. (2000) Estimation of protein secondary structure from circular dichroism spectra. Comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 287, 252–260 [DOI] [PubMed] [Google Scholar]
- 20. Sreerama N., Woody R. W. (2003) Structural composition of βI- and βII-proteins. Protein Sci. 12, 384–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sreerama N., Woody R. W. (2004) On the analysis of membrane protein circular dichroism spectra. Protein Sci. 13, 100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herrmann J. M., Neupert W., Stuart R. A. (1997) Insertion into the mitochondrial inner membrane of a polytopic protein, the nucleus-encoded Oxa1p. EMBO J. 16, 2217–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hell K., Herrmann J. M., Pratje E., Neupert W., Stuart R. A. (1998) Oxa1p, an essential component of the N-tail protein export machinery in mitochondria. Proc. Natl. Acad. Sci. U.S.A. 95, 2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bohnert M., Rehling P., Guiard B., Herrmann J. M., Pfanner N., van der Laan M. (2010) Cooperation of stop-transfer and conservative sorting mechanisms in mitochondrial protein transport. Curr. Biol. 20, 1227–1232 [DOI] [PubMed] [Google Scholar]
- 25. Frazier A. E., Taylor R. D., Mick D. U., Warscheid B., Stoepel N., Meyer H. E., Ryan M. T., Guiard B., Rehling P. (2006) Mdm38 interacts with ribosomes and is a component of the mitochondrial protein export machinery. J. Cell Biol. 172, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Meer G., Voelker D. R., Feigenson G. W. (2008) Membrane lipids. Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen F. S., Zimmerberg J., Finkelstein A. (1980) Fusion of phospholipid vesicles with planar phospholipid bilayer membranes. II. Incorporation of a vesicular membrane marker into the planar membrane. J. Gen. Physiol. 75, 251–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hinnah S. C., Wagner R., Sveshnikova N., Harrer R., Soll J. (2002) The chloroplast protein import channel Toc75. Pore properties and interaction with transit peptides. Biophys. J. 83, 899–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meinecke M., Wagner R., Kovermann P., Guiard B., Mick D. U., Hutu D. P., Voos W., Truscott K. N., Chacinska A., Pfanner N., Rehling P. (2006) Tim50 maintains the permeability barrier of the mitochondrial inner membrane. Science 312, 1523–1526 [DOI] [PubMed] [Google Scholar]
- 30. Truscott K. N., Kovermann P., Geissler A., Merlin A., Meijer M., Driessen A. J., Rassow J., Pfanner N., Wagner R. (2001) A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 8, 1074–1082 [DOI] [PubMed] [Google Scholar]
- 31. Hille B. (2001) Ionic Channels of Excitable Membranes, pp. 347–375, Sinauer Associates, Inc., Sunderland, MA [Google Scholar]
- 32. Smart O. S., Breed J., Smith G. R., Sansom M. S. (1997) A novel method for structure-based prediction of ion channel conductance properties. Biophys. J. 72, 1109–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schwartz M. P., Matouschek A. (1999) The dimensions of the protein import channels in the outer and inner mitochondrial membranes. Proc. Natl. Acad. Sci. U.S.A. 96, 13086–13090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz M. P., Huang S., Matouschek A. (1999) The structure of precursor proteins during import into mitochondria. J. Biol. Chem. 274, 12759–12764 [DOI] [PubMed] [Google Scholar]
- 35. Meinecke M., Cizmowski C., Schliebs W., Krüger V., Beck S., Wagner R., Erdmann R. (2010) The peroxisomal importomer constitutes a large and highly dynamic pore. Nat. Cell Biol. 12, 273–277 [DOI] [PubMed] [Google Scholar]
- 36. van der Laan M., Meinecke M., Dudek J., Hutu D. P., Lind M., Perschil I., Guiard B., Wagner R., Pfanner N., Rehling P. (2007) Motor-free mitochondrial presequence translocase drives membrane integration of preproteins. Nat. Cell Biol. 9, 1152–1159 [DOI] [PubMed] [Google Scholar]
- 37. Nargang F. E., Preuss M., Neupert W., Herrmann J. M. (2002) The Oxa1 protein forms a homooligomeric complex and is an essential part of the mitochondrial export translocase in Neurospora crassa. J. Biol. Chem. 277, 12846–12853 [DOI] [PubMed] [Google Scholar]
- 38. Reif S., Randelj O., Domanska G., Dian E. A., Krimmer T., Motz C., Rassow J. (2005) Conserved mechanism of Oxa1 insertion into the mitochondrial inner membrane. J. Mol. Biol. 354, 520–528 [DOI] [PubMed] [Google Scholar]
- 39. Rehling P., Model K., Brandner K., Kovermann P., Sickmann A., Meyer H. E., Kühlbrandt W., Wagner R., Truscott K. N., Pfanner N. (2003) Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science 299, 1747–1751 [DOI] [PubMed] [Google Scholar]
- 40. Smallridge R. (2003) Doing the three-step. Nat. Rev. Mol. Cell Biol. 4, 343 [Google Scholar]
- 41. Harsman A., Krüger V., Bartsch P., Honigmann A., Schmidt O., Meisinger C., Wagner R. (2010) Protein conducting nanopores. J. Phys. Condensed Matter 22, 454102–454123 [DOI] [PubMed] [Google Scholar]