Abstract
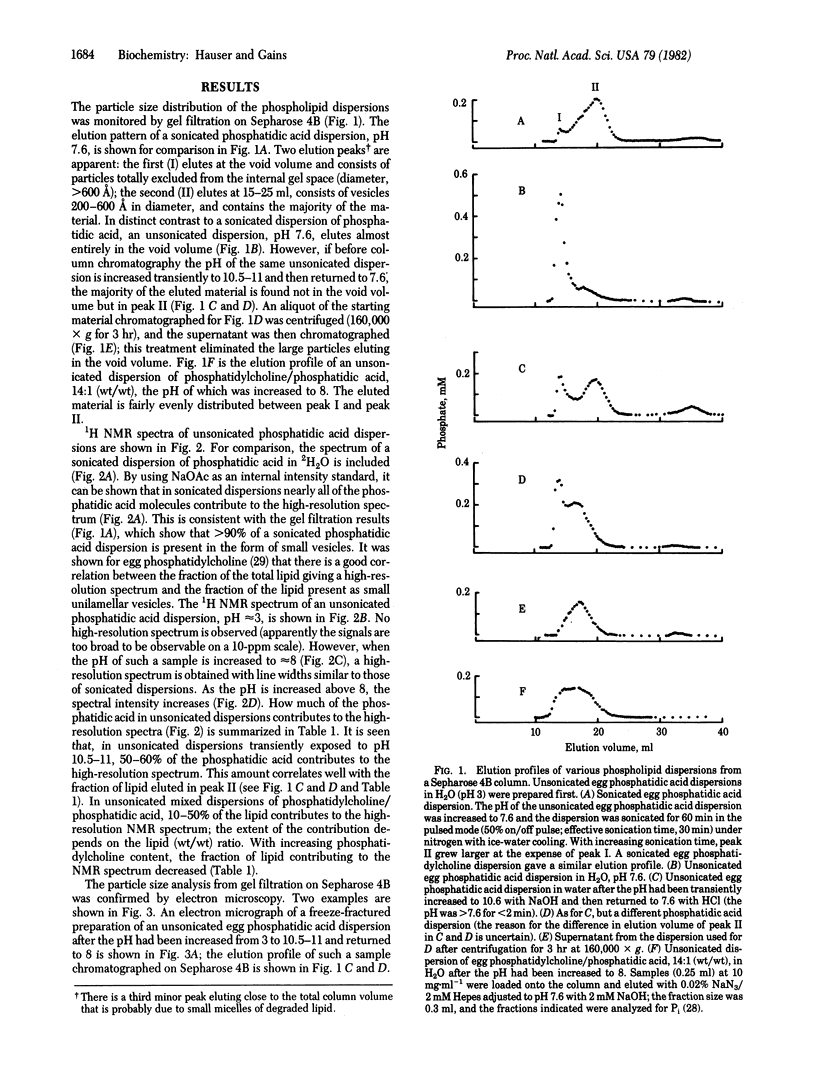
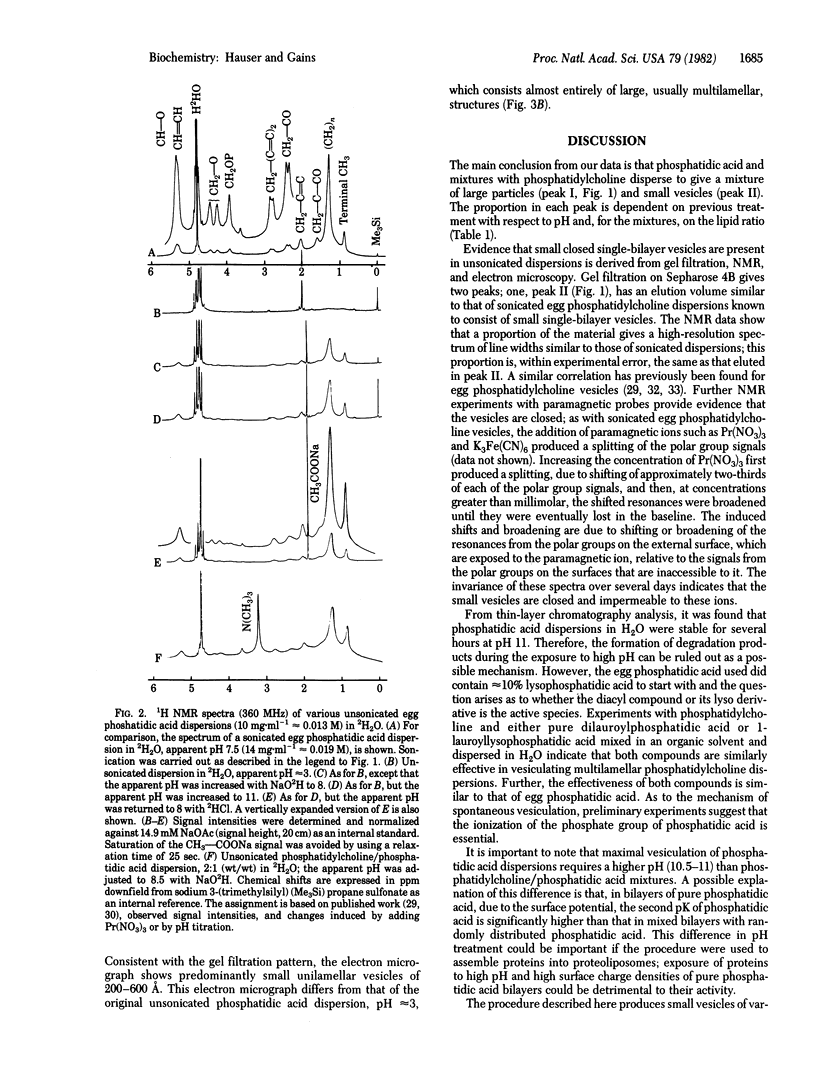
Phosphatidic acid dispersions in H2O vesiculate when the pH is increased transiently (less than 2 min) from approximately 3 to 10.5-11. The same phenomenon is observed in mixed dispersions of phosphatidylcholine and phosphatidic acid in H2O when the pH is increased from approximately 3 to 7 to 8. With phosphatidic acid, this treatment produces small closed unilamellar vesicles (200-600 A) from 50-60% of the total phospholipid, with the remainder present as large unilamellar vesicles and multilamellar structures. The extent of vesiculation depends on the pH that the dispersion is exposed to. With mixed phospholipid dispersions, similar vesicles are obtained; in addition to pH, the extent of vesiculation depends on the phosphatidylcholine/phosphatidic acid (wt/wt) ratio; as this ratio increases, the percentage of small unilamellar vesicles formed decreases. The short exposure of the phospholipids to high pH does not cause lipid degradation, as assessed by thin-layer chromatography; hence, the formation of degradation products can be ruled out as being responsible for the spontaneous vesiculation. Ionization of the phosphate group, resulting in a high surface charge density, may be an important factor in the spontaneous vesiculation. The proportion of phospholipid present as small unilamellar vesicles was determined by gel filtration on Sepharose 4B and by 1H NMR. The small vesicles gave rise to a reasonably well resolved high-resolution NMR spectrum. A good correlation was found between the proportion of phospholipid giving rise to the high resolution spectrum, as derived from spectral intensity measurements, and the proportion present as small unilamellar vesicles, as derived from gel filtration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSON M. B., KATZMAN R., GREGOR H. P. AQUEOUS DISPERSIONS OF PHOSPHATIDYLSERINE. IONIC PROPERTIES. J Biol Chem. 1964 Jan;239:70–76. [PubMed] [Google Scholar]
- Barenholzt Y., Amselem S., Lichtenberg D. A new method for preparation of phospholipid vesicles (liposomes) - French press. FEBS Lett. 1979 Mar 1;99(1):210–214. doi: 10.1016/0014-5793(79)80281-1. [DOI] [PubMed] [Google Scholar]
- Batzri S., Korn E. D. Single bilayer liposomes prepared without sonication. Biochim Biophys Acta. 1973 Apr 16;298(4):1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Semenza G. Single bilayer lipid-protein vesicles formed from phosphatidylcholine and small intestinal sucrase.isomaltase. J Biol Chem. 1978 Oct 25;253(20):7538–7546. [PubMed] [Google Scholar]
- Brunner J., Skrabal P., Hauser H. Single bilayer vesicles prepared without sonication. Physico-chemical properties. Biochim Biophys Acta. 1976 Dec 2;455(2):322–331. doi: 10.1016/0005-2736(76)90308-4. [DOI] [PubMed] [Google Scholar]
- Chowhan Z. U., Yotsuyanagi T., Higuchi W. I. Model transport studies utilizing lecithin spherules. I. Critical evaluations of several physical models in the determination of the permeability coefficient for glucose. Biochim Biophys Acta. 1972 May 9;266(2):320–342. doi: 10.1016/0005-2736(72)90091-0. [DOI] [PubMed] [Google Scholar]
- Deamer D. W. Preparation and properties of ether-injection liposomes. Ann N Y Acad Sci. 1978;308:250–258. doi: 10.1111/j.1749-6632.1978.tb22027.x. [DOI] [PubMed] [Google Scholar]
- Deamer D., Bangham A. D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976 Sep 7;443(3):629–634. doi: 10.1016/0005-2736(76)90483-1. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Strittmatter P. Formation and properties of 1000-A-diameter, single-bilayer phospholipid vesicles. Proc Natl Acad Sci U S A. 1979 Jan;76(1):145–149. doi: 10.1073/pnas.76.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. Mechanism of sonication of aqueous egg yolk lecithin dispersions and nature of the resultant particles. Biochim Biophys Acta. 1972 Jan 27;260(1):49–58. doi: 10.1016/0005-2760(72)90073-2. [DOI] [PubMed] [Google Scholar]
- Finer E. G., Flook A. G., Hauser H. The nature and origin of the NMR spectrum of unsonicated and sonicated aqueous egg yolk lecithin dispersions. Biochim Biophys Acta. 1972 Jan 27;260(1):59–69. doi: 10.1016/0005-2760(72)90074-4. [DOI] [PubMed] [Google Scholar]
- Gerritsen W. J., Verkley A. J., Zwaal R. F., Van Deenen L. L. Freeze-fracture appearance and disposition of band 3 protein from the human erythrocyte membrane in lipid vesicles. Eur J Biochem. 1978 Apr;85(1):255–261. doi: 10.1111/j.1432-1033.1978.tb12234.x. [DOI] [PubMed] [Google Scholar]
- Gregoriadis G. The carrier potential of liposomes in biology and medicine (first of two parts). N Engl J Med. 1976 Sep 23;295(13):704–710. doi: 10.1056/NEJM197609232951305. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Jr, Goerke J., Guo L. S., Williams M. C., Havel R. J. Unilamellar liposomes made with the French pressure cell: a simple preparative and semiquantitative technique. J Lipid Res. 1980 Nov;21(8):981–992. [PubMed] [Google Scholar]
- Hauser H., Howell K., Dawson R. M., Bowyer D. E. Rabbit small intestinal brush border membrane preparation and lipid composition. Biochim Biophys Acta. 1980 Nov 18;602(3):567–577. doi: 10.1016/0005-2736(80)90335-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Phillips M. C., Levine B. A., Williams R. J. Ion-binding to phospholipids. Interaction of calcium and lanthanide ions with phosphatidylcholine (lecithin). Eur J Biochem. 1975 Oct 1;58(1):133–144. doi: 10.1111/j.1432-1033.1975.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Hauser H., Phillips M. C. Structures of aqueous dispersions of phosphatidylserine. J Biol Chem. 1973 Dec 25;248(24):8585–8591. [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Huang C. Studies on phosphatidylcholine vesicles. Formation and physical characteristics. Biochemistry. 1969 Jan;8(1):344–352. doi: 10.1021/bi00829a048. [DOI] [PubMed] [Google Scholar]
- Milsmann M. H., Schwendener R. A., Weder H. G. The preparation of large single bilayer liposomes by a fast and controlled dialysis. Biochim Biophys Acta. 1978 Sep 11;512(1):147–155. doi: 10.1016/0005-2736(78)90225-0. [DOI] [PubMed] [Google Scholar]
- Müller M., Meister N., Moor H. Freezing in a propane jet and its application in freeze-fracturing. Mikroskopie. 1980 Sep;36(5-6):129–140. [PubMed] [Google Scholar]
- Papahadjopoulos D., Miller N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim Biophys Acta. 1967 Sep 9;135(4):624–638. doi: 10.1016/0005-2736(67)90094-6. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Jacobson K., Poste G. Cochleate lipid cylinders: formation by fusion of unilamellar lipid vesicles. Biochim Biophys Acta. 1975 Jul 3;394(3):483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- SAUNDERS L., PERRIN J., GAMMACK D. Ultrasonic irradiation of some phospholipid sols. J Pharm Pharmacol. 1962 Sep;14:567–572. doi: 10.1111/j.2042-7158.1962.tb11141.x. [DOI] [PubMed] [Google Scholar]
- Schieren H., Rudolph S., Finkelstein M., Coleman P., Weissmann G. Comparison of large unilamellar vesicles prepared by a petroleum ether vaporization method with multilamellar vesicles: ESR, diffusion and entrapment analyses. Biochim Biophys Acta. 1978 Aug 3;542(1):137–153. doi: 10.1016/0304-4165(78)90240-4. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- Szoka F., Jr, Papahadjopoulos D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4194–4198. doi: 10.1073/pnas.75.9.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell D. A., Heath T. D., Colley C. M., Ryman B. E. New aspects of liposomes. Biochim Biophys Acta. 1976 Dec 14;457(3-4):259–302. doi: 10.1016/0304-4157(76)90002-2. [DOI] [PubMed] [Google Scholar]
- Zumbuehl O., Weder H. G. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. Biochim Biophys Acta. 1981 Jan 8;640(1):252–262. doi: 10.1016/0005-2736(81)90550-2. [DOI] [PubMed] [Google Scholar]




