Background: Tp53-induced glycolysis and apoptosis regulator (TIGAR) is a p53 target gene that has been shown to inhibit glycolysis and activate the pentose phosphate pathway (PPP).
Results: TIGAR regulates mitochondrial respiration and intracellular reactive oxygen species (ROS) levels.
Conclusion: TIGAR improves cellular redox homeostasis.
Significance: TIGAR may be a target for metabolic therapies aiming to enhance tumor cell sensitivity toward hypoxia.
Keywords: Apoptosis, Cell Metabolism, Hypoxia, p53, Pentose Pathway, TIGAR, Glioma
Abstract
Altered metabolism in tumor cells is increasingly recognized as a core component of the neoplastic phenotype. Because p53 has emerged as a master metabolic regulator, we hypothesized that the presence of wild-type p53 in glioblastoma cells could confer a selective advantage to these cells under the adverse conditions of the glioma microenvironment. Here, we report on the effects of the p53-dependent effector Tp53-induced glycolysis and apoptosis regulator (TIGAR) on hypoxia-induced cell death. We demonstrate that TIGAR is overexpressed in glioblastomas and that ectopic expression of TIGAR reduces cell death induced by glucose and oxygen restriction. Metabolic analyses revealed that TIGAR inhibits glycolysis and promotes respiration. Further, generation of reactive oxygen species (ROS) levels was reduced whereas levels of reduced glutathione were elevated in TIGAR-expressing cells. Finally, inhibiting the transketolase isoenzyme transketolase-like 1 (TKTL1) by siRNA reversed theses effects of TIGAR. These findings suggest that glioma cells benefit from TIGAR expression by (i) improving energy yield from glucose via increased respiration and (ii) enhancing defense mechanisms against ROS. Targeting metabolic regulators such as TIGAR may therefore be a valuable strategy to enhance glioma cell sensitivity toward spontaneously occurring or therapy-induced starvation conditions or ROS-inducing therapeutic approaches.
Introduction
Glycolysis and mitochondrial respiration are the two main energy sources in eukaryotic cells to fuel biological functions. During the oxygen-independent glycolytic pathway, glucose is metabolized to pyruvate, which can then either be fermented to lactate to regenerate NAD+ or be metabolized via the citric acid cycle, whereby the generated NADH and succinate can be oxidized through the mitochondrial electron transport chain to provide large amounts of ATP. Unlike normal cells, cancer cells preferentially utilize the glycolytic pathway instead of oxidative phosphorylation even in the presence of oxygen (1, 2). Oxidative phosphorylation is by far more efficient in ATP generation per mol of glucose, but it also leads to raised intracellular ROS2 levels (3, 4). Although ROS can play important roles in regulating cell signaling and homeostasis when present in moderate quantity (5–11), excessive amounts can damage cellular components such as proteins or DNA (12–15). ROS homeostasis is dependent on NADPH generation through the pentose phosphate pathway (PPP) and subsequent production of reduced glutathione. In the last few years, regulation of PPP has been found to be more and more complex and versatile (16–20). For example, it was revealed that the tumor suppressor p53 can inhibit the PPP by binding to glucose-6-phosphate dehydrogenase (G6PD), preventing the formation of the active dimer of the enzyme (20), which ultimately results in decreased NADPH levels. Other results suggest that p53 may also regulate genes that function to lower ROS levels, indicating important functions of p53 in preventing DNA damage and tumor development (21, 22).
Underscoring the influence of p53 on metabolism, TIGAR has recently been discovered as a new p53 target gene (17). Analyses of the structure and functions of TIGAR revealed interesting aspects: TIGAR, which shows similarity to fructose 2,6-bisphosphatase (FBPase-2), was demonstrated to inhibit glycolysis and activate PPP in U2OS cells, correlating with the ability to protect cells from oxidative (17) or metabolic stress-induced cell death (16). Primary or de novo glioblastomas are highly aggressive and hypoxic human tumors (23, 24) that typically retain p53 wild-type (WT) status (24, 25). Oxygen concentrations in these tumors often reach levels of profound hypoxia as low as 0.1% O2 (26, 27). Further, the availability of nutrients, i.e. glucose, is also severely impaired in some regions of solid tumors (28–30). We recently showed that WT p53 can limit glucose demands under tumor microenvironment conditions by inducing expression of synthesis of cytochrome c oxidase 2 (SCO2), ultimately promoting cellular survival (31). Resistance mechanisms toward these hypoxic and nutrient-starved conditions are considered to be important for the survival of tumor cells within a solid tumor and for the resistance to radiotherapy, surgery, and targeted therapy (32).
However, although suppression of p53 sensitized cells to metabolic stress even under severe hypoxia, protection by SCO2 required the presence of sufficient oxygen (e.g. 1–5% O2) consistent with its function at the respiratory chain. Therefore, we speculated that other p53-dependent target genes would be functional even under severe hypoxia. For that reason, we investigated whether the p53 target gene TIGAR could be involved in metabolic regulation in glioma cells. Here, we describe a mechanism implicating TIGAR as a regulator of redox metabolism under hypoxic conditions and an activator of the mitochondrial respiratory chain in the oxygenated tumor fraction. Because (i) TIGAR exhibits an antioxidative function through the PPP (16, 17), (ii) the PPP plays an important role in cancer (33–36), and (iii) transketolase-like 1 (TKTL1), an isoenzyme of the transketolase, has been found to be overexpressed in different tumor types (18, 37–39) and was suggested to be important for PPP function and protection of tumor cells against oxidative stress (40), we also assessed a possible link between TIGAR and TKTL1.
EXPERIMENTAL PROCEDURES
Cell Lines
LNT-229 cells were described previously (41). T98G cells were obtained from the ATCC (Manassas, VA). LNT-229 cells stably expressing a temperature-sensitive murine p53V135A possessing dominant-negative properties at 38.5 °C and hygromycin-resistant control cells transfected with the empty vector (LNT-229hygro) were described previously (42). Cells, if not otherwise specified, were maintained in Dulbecco's modified Eagle's medium (DMEM, GE Healthcare Life Sciences, Coelbe, Germany) containing 10% fetal calf serum (FCS), 2 mm glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin. LNT-229hygro and LNT-229p53V135A cells and derived transfectants were cultivated at 38.5 °C. In experiments requiring defined glucose conditions, Dulbecco's modified Eagle's glucose-free medium (PAA Laboratories) was used without FCS, and glucose was added as required. Cells were seeded at a density of 5.7 × 104 cells/cm2 if not otherwise specified.
Constructs
The hygromycin control vector and p53V135A vector were obtained from M. Clarke. The plasmids pcDNA3.1-TIGAR and pcDNA3.1-TIGAR-TM, which encodes a triple mutant of TIGAR lacking glycolysis inhibitory properties, were generously provided by K. Vousden (16, 17). The control pcDNA3.1 vector was purchased from Invitrogen. The p53-luciferase (p-53 luc) reporter gene vector PathDetect p53 was purchased from Stratagene (Cedar Creek, TX), pRL-CMV Renilla vector was obtained from Promega (Mannheim, Germany). All stable and transient transfections of plasmids were done using METAFECTENE PRO (Biontex Laboratories).
To inhibit TIGAR expression, small interfering RNA (siRNA) of the human TIGAR cDNA sequence published in Ref. 17 was used (matching region 115–133 in exon 3 5′-GCAGCAGCTGCTGGTATAT-3′). To inhibit TKTL1 expression, a small interfering RNA matching region 2175–2195 in the 3′-UTR region (5′-AAGTGTTTCCTTCGTGAATAA-3′ described in Ref. 40) was used. A scrambled siRNA was used as control (AllStars negative siRNA, Qiagen, Hilden, Germany). siRNA was transfected using HiPerFect (Qiagen) according to the manufacturer's protocol (3 μl HiPerFect: 100 nm for TIGARsiRNA or 20 nm for TKTL1siRNA).
Luciferase Assay
Cells were seeded at 10,000 cells/well into 96-well plates, cotransfected using METAFECTENE PRO with the p53-luc reporter gene vector and pRL-CMV (Renilla) vector at a ratio of 7.5:1, and exposed to 0, 170, 250, 345, 500, or 1000 ng/ml adriamycin for 20 h. Experiments were conducted in triplicates. Activities of Renilla luciferase and firefly were determined using a luminometer (Mithras). Background was subtracted from all values, and the counts obtained from the measurement of firefly luciferase were normalized to Renilla luciferase (43, 44).
Immunoblot Analysis
Cells were seeded in 6-well plates and exposed to 0, 170, 250, 345, 500, or 1000 ng/ml for 20 h. Thereafter, cells were washed with cold phosphate-buffered saline (PBS) and lysed in lysis buffer (50 mm Tris-HCl pH 8, 120 mm NaCl, 5 mm EDTA, 0.5% Nonidet P-40) containing protease inhibitors (Roche Applied Science, Mannheim, Germany). Cellular lysates were prepared as described (45) and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Membranes were probed with antibodies to human p53 (sc-263, Santa Cruz Biotechnology, Santa Cruz, CA) and GAPDH (MAB374, Chemicon, Nuernberg, Germany). Secondary antibody was purchased from Santa Cruz Biotechnology. The chemiluminescence solution used for detection was composed of 1 ml of solution A (200 ml of 0.1 m Tris-HCl pH 8.6, 50 mg of luminol), 100 μl of solution B (11 mg of p-hydroxycoumarin acid, 10 ml dimethyl sulfoxide (DMSO)), and 0.3 μl of H2O2 (30%).
RNA Extraction and Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using TRIzol and RNeasy Kit (Invitrogen, Karlsruhe, Germany). First strand cDNA was synthesized using the Vilo cDNA synthesis kit (Invitrogen) for 10 min at 25 °C and 2 h at 42 °C. Subsequently, the enzyme was inactivated at 85 °C for 10 min. To determine changes in gene expression, qRT-PCR was performed in the IQ5 real-time PCR detection system (Bio-Rad, Muenchen, Germany) using Absolute Blue Q-PCR master mix with SYBR Green + fluorescein (Thermo Fisher Scientific, Hamburg, Germany) and the following primer pairs: TIGAR reverse 5′-CCATGTGCAATCCAGAGATG-3′, TIGAR forward 5′-CCTTACCAGCCACTCTGAGC-3′ (recognizes both TIGAR and TIGAR-TM sequences), TKTL1 reverse 5′-CATCCTAACAAGCTTTCGCTG-3′, TKTL1 forward 5′-TAACACCATGACGCCTACTGC-3′, 18 S forward 5′-CGGCTACCACATCCAAGGAA-3′, 18 S reverse 5′-GCTGGAATTACCGCGGCT-3′. Cycle threshold (Ct) values were normalized for amplification of the 18 S ribosomal RNA, and the data were analyzed using the Vandesompele method (46).
Cell Death Analysis
Cell death was assessed by propidium iodide (PI)-FACS (31). Experiments were performed in triplicates and are presented as mean ± S.D.
Induction of Hypoxia
Profound hypoxia (0.1% O2) was induced by incubating cells in GasPak pouches for anaerobic culture (Becton-Dickinson GmbH, Heidelberg, Germany) (45). Moderate hypoxia (5% O2) was induced in a Labotect incubator (Goettingen, Germany).
Measurement of Glucose and Lactate
Cell-free supernatant was collected, and glucose and lactate concentrations were measured using the biochemistry analyzer Hitachi 917.
Oxygen Consumption
Oxygen concentration in the medium was measured using the ABL-80 FLEX blood gas analyzer (Radiometer, Willich, Germany) as described previously (31).
ATP Assay
Cells were treated as indicated. Immediately after treatment, plates were placed on ice, cells were collected by centrifugation, and pellets were lysed in ATP releasing reagent (Sigma). ATP concentrations were determined by luciferase assay with the CLS II kit (Roche Applied Science).
ROS Analysis
ROS levels were determined by H2DCFDA-FACS (31). The membrane-permanent acetate ester form of 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA-AM) passes through the cell membrane. In the cytoplasm, cellular esterases hydrolyze H2DCFDA-AM to form the nonfluorescent moiety H2DCFDA. Oxidation of H2DCFDA by intracellular ROS leads to the formation of 2′,7′-dichlorodihydrofluorescein (DCF), which is highly fluorescent and can be assessed by FACS-analysis. Experiments were performed in triplicates and are presented as mean ± S.D.
GSH Measurement
Cellular GSH levels were measured with GSH-GloTM glutathione assay from Promega according to the supplier protocol. Experiments were performed in triplicates and are presented as mean ± S.D.
Lactate Dehydrogenase Measurement
Lactate dehydrogenase assay (Roche Applied Science) was measured according to the manufacturer's instructions.
RESULTS
p53 Regulates TIGAR Expression in Human Glioma Cells
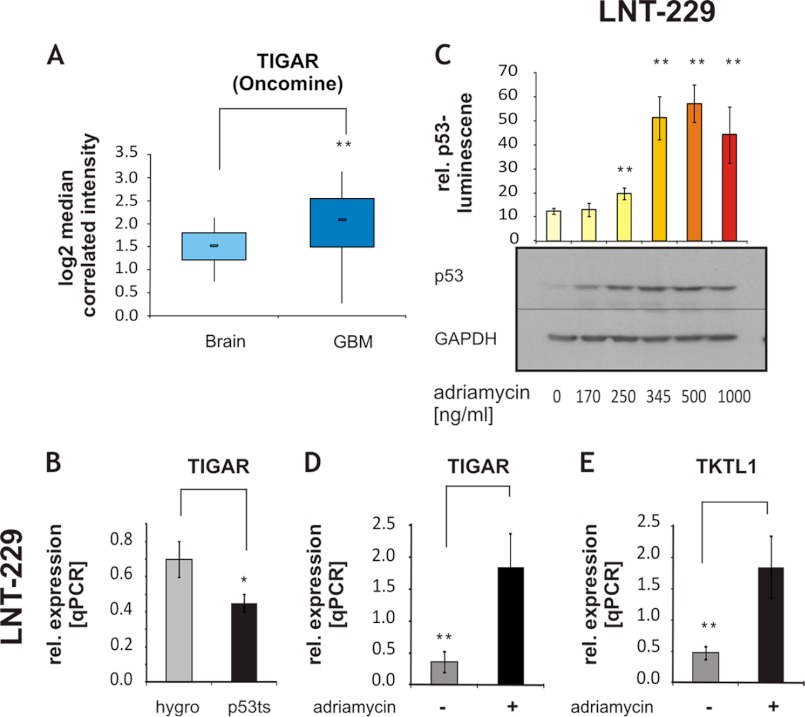
To our knowledge, TIGAR expression in vivo has hitherto only been assessed in invasive breast cancer. A high expression of TIGAR was noted in almost 75% of the examined breast tumors (47). To assess whether TIGAR may be regulated in glioblastoma tumors, an in silico analysis was performed with data from (48), using the Oncomine database, a cancer microarray database allowing gene expression analysis in different tumor types from genome-wide expression analyses of patient material. TIGAR expression was significantly elevated in glioblastomas (n = 81) versus control samples from normal brain (n = 23) (-fold change, 1.407l p value, 1.3 × 10−5, Fig. 1A). As TIGAR was described as a p53 target gene (17), we investigated a possible regulation of TIGAR expression by p53 in glioma cells. TIGAR expression was decreased in LNT-229 cells in which the p53 transactivation activity was inhibited by stable expression of the temperature-sensitive dominant-negative p53V135A mutant (Fig. 1B).
FIGURE 1.
p53 regulates TIGAR expression in human glioma. A, an in silico analysis was performed with the Oncomine database. TIGAR expression in normal brain samples was compared with glioblastoma patient samples (GBM); -fold change, 1.407, p value: 1.3 × 10−5. B, TIGAR expression was assessed by qRT-PCR in LNT-229p53V135A cells carrying the temperature-sensitive dominant-negative mutant p53V135A and hygro control cells (mean ± S.D., *, p < 0.05). rel. expression, relative expression. C, LNT-229 cells were treated with increasing concentrations of adriamycin for 20 h. p53 transactivation activity was determined by luciferase assay (shown is mean ± S.D. of triplicates, one experiment out of two independent experiments with similar results is shown, **, p < 0.01, unpaired Student's t test compared with untreated cells). Expression of endogenous human p53 was confirmed by Western blot. GAPDH expression was employed as loading control. D and E, expression of TIGAR (D) and TKTL1 (E) was determined by qRT-PCR (qPCR) in LNT-229 cells exposed to 345 ng/ml adriamycin for 20 h (shown is mean ± S.D. of triplicates, one experiment out of two independent experiments with similar results is shown, **, p < 0.01, unpaired Student's t test).
To further explore whether expression of TIGAR is regulated by p53, cells were treated by adriamycin (17), a DNA damage-inducing drug, and transcriptional activity of p53 and expression of TIGAR were analyzed. Adriamycin dose-dependently increased p53 accumulation and activity (Fig. 1C). TIGAR expression was up-regulated up to 5-fold in adriamycin-treated cells, supporting the assumption of TIGAR being a p53-dependent gene in the examined glioma cell line (Fig. 1D).
Further, TKTL1 expression, like TIGAR expression, was enhanced by adriamycin (Fig. 1E). TKT expression itself remained unchanged (data not shown).
TIGAR Protects Glioma Cells from Hypoxia-induced Cell Death
In different areas of solid tumors, oxygen concentrations often fluctuate between 5% O2 (49–53) and 0.1% O2 (26, 27). We therefore studied the function of TIGAR under conditions characteristic for the tumor microenvironment in a well established paradigm (0.1% O2 and 2 mm glucose (31)). For this purpose, three models were defined: (i) LNT-229 cells (p53 wild-type) in which TIGAR expression was transiently suppressed by siRNA (Fig. 2A), ii) T98G cells (p53 mutant) stably transfected with TIGAR and corresponding control transfectants (Fig. 2B), and iii) LNT-229p53V135A cells lacking p53 WT transactivational activity in which TIGAR was stably reexpressed (Fig. 1C). We found that suppression of TIGAR in LNT-229 cells enhanced hypoxia-induced cell death (Fig. 2, D and G), whereas TIGAR reexpression protected T98G and LNT-229p53V135A cells against hypoxia-induced cell death (Fig. 2, E, H, F, and I). As expression of TIGAR-TM, a triple mutant of TIGAR that is unable to lower Fru-2,6-P2 levels and to inhibit glycolysis (17), did not lead to protection toward hypoxic cell death (Fig. 2, B and C), TIGAR-mediated rescue seemed to depend on its functionality in glycolysis inhibition and PPP activation.
FIGURE 2.
TIGAR protects glioma cells from hypoxia-induced cell death. A–C, expression of TIGAR was assessed by qRT-PCR (qPCR) in LNT-229 transiently transfected with 100 nm scrambled siRNA (scr si) or TIGARsiRNA (TIGARsi) (A), T98G stably transfected with TIGAR-pcdna3.1 or pcdna3.1 plasmid (neo) (B), and LNT-229p53V135A stably transfected with TIGAR-pcdna3.1 or pcdna3.1 plasmid (neo) and hygro control cells transfected with pcdna3.1 (hygro/neo) (C). rel. expression, relative expression. D–F, these cells were exposed to serum-free medium containing 2 mm glucose and 0.1% O2 for 20 h, and cell death was analyzed by PI-FACS. T98G- and LNT-229-TIGAR-TM-pcdna3.1 cells were used as respective control (shown is mean ± S.D. of triplicates, one experiment out of three or more independent experiments with similar results is shown, *, p < 0.05, **, p < 0.01, unpaired Student's t test). n. s., not significant. G–I, cells were monitored by microscopy (×10) under these conditions.
TIGAR Protects Glioma Cells from ROS-induced Cell Death
As ROS seem to be important for hypoxia-induced cell death in the model described here (31), and TIGAR has previously been shown to confer protection against apoptosis induced by H2O2 in human osteosarcoma (U2OS) and non-small cell lung carcinoma (H1299) cells (17), we analyzed whether TIGAR modulates ROS-induced cell death in glioma cells as well. Suppression of TIGAR expression sensitized LNT-229 cells against hydrogen peroxide-induced cell death (Fig. 3A), whereas reexpression of TIGAR in LNT-229p53V135A or T98G cells efficiently mediated resistance to oxidant stress (Fig. 3, B and C), suggesting that TIGAR enhances ROS detoxification mechanisms. We therefore examined whether TIGAR influenced ROS levels under metabolic stress conditions. Analysis of ROS levels by H2DCFDA-FACS showed decreased levels of ROS in TIGAR-reexpressing cells (Fig. 3, E and F), whereas suppression of TIGAR increased ROS concentrations (Fig. 3D). Again, TIGAR-TM did not recapitulate the effects of TIGAR. One possible mechanism for enhanced ROS detoxification by TIGAR might be an increased flux through the PPP resulting in elevated levels of NADPH and subsequently of reduced glutathione (GSH). In agreement with this hypothesis, luminometric analysis of GSH showed increased levels of GSH in TIGAR-expressing cells (supplemental Fig. 1).
FIGURE 3.
TIGAR protects glioma cells from ROS-induced cell death. A–C, LNT-229 transiently transfected with 100 nm scrambled siRNA (scr si) or TIGARsiRNA (TIGARsi) (A), T98G stably transfected with TIGAR-pcdna3.1 or pcdna3.1 plasmid (neo) (B), and LNT-229p53V135A cells stably transfected with TIGAR-pcdna3.1 or pcdna3.1 plasmid (neo) (C) were exposed to 1 mm H2O2 for 4 h (LNT-229) or 24 h (T98G), and cell death was assessed by PI-FACS (shown is mean ± S.D. of triplicates, one experiment out of three or more independent experiments with similar results is shown, **, p < 0.01, unpaired Student's t test). D–F, similarly, ROS were measured in the same cells by H2DCFDA-FACS and T98G- and LNT-229-TIGAR-TM-pcdna3.1 cells were used as respective control (shown is mean ± S.D. of triplicates, one experiment out of three or more independent experiments with similar results is shown, *, p < 0.05, **, p < 0.01, unpaired Student's t test). n. s., not significant.
Inhibition of TKTL1 Expression Antagonizes TIGAR-mediated Protection
To further characterize the function of the PPP for TIGAR-mediated safeguard functions, it was investigated whether gene suppression of TKTL1 by siRNA would abolish the protective effects of TIGAR in LNT-229p53V135A-TIGAR and T98G-TIGAR cells (Fig. 4A). First, we could confirm that depletion of TKTL1 leads to sensitization of glioma cells toward oxidative stress-induced cell death (data not shown), as reported previously in colon carcinoma cells (40). Analysis of ROS levels by H2DCFDA-FACS showed an increased amount of ROS under hypoxia in TKTL1si cells even in the presence of TIGAR (Fig. 4B). Second, gene suppression of TKTL1 mimicked loss of TIGAR function and enhanced sensitivity toward hypoxic conditions (Fig. 4C). These data indicate that TIGAR function is linked to PPP activation and, at least in the examined cell lines, depends on the presence of TKTL1.
FIGURE 4.
TKTL1 is required for TIGAR-mediated protection against hypoxia. A, TKTL1 expression was suppressed by 20 nm TKTL1siRNA (TKTL1si) in T98G-TIGAR and LNT-229p53V135A-TIGAR cells; scrambled siRNA (scr si) was used as control. rel. expression, relative expression. B, cells were grown in 5 mm glucose in serum-free medium and exposed to 21% or 0.1% O2 for 20 h. ROS levels were determined by H2DCFDA-FACS (shown is mean ± S.D. of triplicates, one experiment out of three or more independent experiments with similar results is shown, *, p < 0.05, unpaired Student's t test). C, T98G and LNT-229p53V135A cells transfected with neomycin (neo) or TIGAR vectors were transiently transfected with scrambled siRNA (100 nm), TKTL1siRNA (20 nm), or TIGARsiRNA (TIGARsi, 100 nm). After 16 h, cells were exposed to 0.1% O2 for 20 h in the presence of serum-free medium containing 2 mm glucose, and cell death was assessed by PI-FACS (shown is mean ± S.D. of triplicates, one experiment out of three or more independent experiments with similar results is shown, *, p < 0.05, **, p < 0.01, unpaired Student's t test).
TIGAR Inhibits Glycolysis and Promotes Cellular Respiration in Glioma Cells
Considering the fluctuant oxygen concentrations in solid tumors, we analyzed the role of TIGAR on cell survival at 5% oxygen, too. In a first step, we evaluated glucose consumption and lactate production in TIGAR-transfected p53 mutant cells (LNT-229p53V135A and T98G) when exposed to limited glucose (5 mm) and physiologic oxygen conditions (5% O2). In TIGAR-expressing cells, decreased glucose consumption and lactate production were observed (Fig. 5, A and E), indicating inhibition of glycolysis by TIGAR as shown previously (17). Because pharmacological inhibition of glycolysis by the glucose analog 2-deoxyglucose has recently been demonstrated to inhibit anaerobic glycolysis by forcing glioma-derived cells into mitochondrial metabolism under low oxygen conditions (54), we analyzed whether reducing glycolytic activity would also modulate respiration in the LNT-229 glioma cell line. Treatment of glioma cells with 2-deoxyglucose similarly conferred a more oxidative phenotype characterized by lower glucose consumption and lactate production accompanied by increased mitochondrial respiration (supplemental Fig. 2, A and B), indicative of at least some metabolic flexibility of glioma cells. We therefore hypothesized that TIGAR, similar to 2-deoxyglucose, could also increase mitochondrial respiration in tumor cells. Expression of TIGAR, but not TIGAR-TM, increased oxygen consumption in LNT-229p53V135A and T98G cells (Fig. 5, B and F), whereas mitochondrial respiration was reduced in TIGARsiRNA cells (data not shown).
FIGURE 5.
TIGAR inhibits glycolysis and promotes cellular respiration. A and E, T98G and LNT-229p53V135A cells carrying TIGAR or empty pcdna3.1 vector (neo) were exposed to serum-free medium containing 5 mm glucose and 5% O2 for 20 h. Glucose consumption and lactate production were determined (shown is mean ± S.D. of triplicates, one experiment out of two independent experiments with similar results is shown, *, p < 0.05, unpaired Student's t test). B and F, oxygen consumption was analyzed in T98G and LNT-229p53V135A neomycin control (neo), TIGAR, or TIGAR-TM cells. A representative experiment out of three independent experiments with similar results is shown. C and G, cell death induced by moderate hypoxia (5% O2) was analyzed by PI-FACS (shown is mean ± S.D. of triplicates, one experiment out of three independent experiments with similar results is shown, *, p < 0.05, **, p < 0.01, unpaired Student's t test). D and H, phase contrast microscopy (×10) under the same conditions as in C and G.
Together, these data indicate that TIGAR shifts cellular metabolism toward oxidative phosphorylation. We therefore wished to define this phenotype in more detail by pharmacologically perturbing oxidative phosphorylation (OXPHOS) in T98G-neo and -TIGAR cells. Inhibition of ATP synthase by oligomycin (31) did not significantly decrease ATP content of neomycin cells, whereas it strongly suppressed ATP levels in TIGAR-reexpressing cells (supplemental Fig. 3A). Similarly, the effect of oligomycin on oxygen consumption was more pronounced in T98G-TIGAR cells (supplemental Fig. 3B). Oligomycin also abolished the protective effect of TIGAR on cell death (supplemental Fig. 3C). These data confirm that TIGAR-reexpressing cells employ OXPHOS to enhance energy homeostasis and to resist cell death under conditions of moderate starvation. Interestingly, the mitochondrial substrate methyl pyruvate mimicked the effect of TIGAR in neomycin cells, enhancing oxygen consumption, increasing ATP levels, and conferring protection from cell death (supplemental Fig. 3C).
DISCUSSION
Solid tumors are characterized by areas of heterogeneous blood supply resulting in regions with varying supply of oxygen and nutrients, thus forcing tumor cells to survive under different metabolic conditions (28–30). Although increased glucose consumption is a hallmark of tumors, metabolic versatility therefore could be useful for tumor cells. Indeed, the assumption of Warburg that the increased aerobic glycolysis is a direct consequence of defects in cellular respiration does not seem to adequately reflect tumor cell metabolism. It is now clear that mitochondria of tumor cells are still capable of oxidative phosphorylation but that oncogenic alterations redirect metabolism away from cellular respiration toward metabolic pathways important for anabolic processes such as glycolysis and the PPP (55). For example, we and others could previously show that the p53 target gene SCO2 is capable of activating oxidative phosphorylation in tumor cells, leading to a more energy-efficient metabolism of glucose, thus delaying glucose depletion and prolonging tumor cell survival under starvation conditions (31, 56). In accordance with the assumption that SCO2-mediated alterations in metabolism involve oxidative phosphorylation, this protective effect was no longer present under severe hypoxia (0.1% O2). We however still observed increased sensitivity of p53 mutant cells toward starvation under severe hypoxia and therefore hypothesized that another metabolic target of p53, TIGAR, could be responsible for the observed phenotype.
Indeed, we were able to demonstrate that under severe hypoxia, expression of TIGAR protected p53 mutant cells against cell death (Fig. 2). Considering the elevated levels of ROS during hypoxia (31), we speculate that this effect is possibly mediated by increased defense mechanisms toward ROS by activation of the PPP and subsequent generation of NADPH and GSH. It has been previously shown that TIGAR activates the PPP by its fructose 2,6-bisphosphatase activity (17). These results are confirmed in our experiments by the demonstration of lower levels of ROS in TIGAR-expressing cells (Fig. 3), the protective effect of TIGAR toward exogenous ROS (Fig. 3), and increased levels of GSH in TIGAR-proficient cells (supplemental Fig. 1).
Further, suppressing the expression of TKTL1 antagonized the protective effects of TIGAR toward ROS and starvation (Fig. 4C). Due to its homology to transketolase, a role for TKTL1 in the PPP is likely, and the loss of the protective effect of TIGAR by suppression of TKTL1 would therefore be compatible with the importance of the PPP for the protection by TIGAR. Important functions of TKTL1 in cancer biology have been proposed based on different observations. First, TKTL1 was shown to be expressed in a variety of tumor cells including glioma (37, 38, 57, 58) and to be associated with malignant grade, presence of metastasis, and prognosis (57, 59, 60). Second, as putative mechanisms for these associations, TKTL1 has been demonstrated to increase glycolysis and hypoxia-inducible factor-1α (HIF-1α) expression, proliferation (61, 62), the generation of NADPH, and resistance toward ROS (40). The presented results of increased sensitivity of TKTL1-suppressed cells against ROS and hypoxia (Fig. 4) are in agreement with these observations.
In addition to the function of TIGAR at severe hypoxia, protective effects of TIGAR in the presence of oxygen were also detected. These were associated with reduced glucose consumption and lactate generation, elevated oxygen consumption, enhanced ATP levels, and increased vulnerability toward OXPHOS inhibition, indicative of a less glycolytic and more oxidative phenotype (Fig. 5 and supplemental Fig. 3).
The way TIGAR acts on respiration remains unclear. It appears possible that metabolites from the PPP are redirected to the tricarboxylic acid cycle and subsequently to OXPHOS. Our finding that the mitochondrial substrate methyl pyruvate mimics the effect of TIGAR on OXPHOS (supplemental Fig. 3C) suggests that indeed the supply with mitochondrial substrates is the critical regulator for OXPHOS.
Despite increased respiration, however, endogenous ROS levels were not elevated (data not shown). Grüning et al. (19) recently revealed new insights into the function of the glycolysis regulator pyruvate kinase in yeast. They showed that lowering pyruvate kinase expression, on the one side, activates mitochondrial energy metabolism. Surprisingly, however, this was not accompanied by increased ROS levels, and the authors identified activation of the PPP by phosphoenolpyruvate-induced inhibition of triosephosphate isomerase as responsible for increased NADPH generation, leading to suppression of ROS formation (19). Our results indicate that inhibition of glycolysis and activation of the PPP by TIGAR have similar effects in tumor cells. Inhibition of glycolysis by TIGAR could impair anabolic metabolism by limiting availability of substrates necessary for macromolecular synthesis. However, under conditions of limited glucose availability, as present in significant areas of gliomas (64–67), the switch to a more oxidative phenotype, as characteristic for quiescent cells (55), could result in a more efficient energy production and prolonged tumor cell survival (Fig. 6). Recently, this assumption has been supported by the observation that glucose oxidation is used in glioblastoma cells to meet energetic and biosynthetic demands (63). Intriguingly, modulating glucose metabolism indeed may alter the fundamental characteristics of tumor cells as suggested by the results of Pistollato et al. (54), who observed that antagonizing glycolysis in glioma cells by 2-deoxyglucose at 2% O2 not only forces cancer cells into a mitochondrial metabolism but also induces cellular differentiation. An additional advantage tumor cells could derive from TIGAR consists of increased defense mechanisms against potentially toxic levels of ROS that occur during hypoxia or anticancer therapies, e.g. radiotherapy (68). Another important mechanism by which TIGAR could mediate cytoprotection involves inhibition of autophagy through modulation of ROS under nutrient starvation or metabolic stress conditions (16). These effects of TIGAR were not assessed here but certainly could also play a role in our paradigm.
FIGURE 6.
Hypothetical schematic drawing showing the TIGAR-dependent dual regulation of energy homeostasis and antioxidant production. TIGAR induces PPP activation, promoting NADPH production and therefore protection against ROS (e.g. produced in mitochondria). A, in the presence of oxygen, enhanced mitochondrial respiration and ATP production by yet unknown mechanisms might contribute to maintain energy homeostasis and viability. B, under severe hypoxia (0.1% O2), PPP is sustained, whereas ATP production by oxidative phosphorylation is irrelevant due to limited oxygen availability. Enhanced detoxification of intracellular ROS might therefore be more relevant for the capacity of TIGAR to promote cellular survival under hypoxia.
In summary, our results indicate that TIGAR is a major metabolic regulator that serves to increase survival of glioma cells under hypoxia and improves energy yield from glucose by activation of respiration, whereas suppressing formation of ROS possibly by PPP activation. As TKTL1 seems to be indispensable for these effects of TIGAR, strategies targeting the PPP or TKTL1 might reduce tumor viability and increase sensitivity toward hypoxia- and ROS-inducing therapies.
Supplementary Material
Acknowledgments
We thank K. Vousden for providing the pcdna3.1+ FLAG-TIGAR and pcdna3.1+ FLAG-TIGAR-TM expression plasmids and J. Coy for helpful discussions. The Dr. Senckenberg Institute of Neurooncology is supported by the Hertie Foundation and the Dr. Senckenberg Foundation.
This work was supported by Grant GRK 1302/1 from the Deutsche Forschungsgemeinschaft (DFG) (to J. P. S.), DFG grant RI2175/1-1 (to J. P. S. and J. R.), and a Frankfurt Initiative for Neurooncology research grant from the “Präsidium der Goethe-Universität Frankfurt” and the interdisciplinary center for neuroscience Frankfurt (to J. P. S. and J. R.).

This article contains supplemental Figs. 1–3.
- ROS
- reactive oxygen species
- H2DCFDA
- 2′,7′-dichlorodihydrofluorescein diacetate
- hygro
- hygromycin
- OXPHOS
- oxidative phosphorylation
- PI
- propidium iodide
- PPP
- pentose phosphate pathway
- TIGAR
- Tp53 inducer and regulator of glycolysis
- TKTL1
- transketolase-like 1
- qRT-PCR
- quantitative RT-PCR
- H2DCFDA
- 2′,7′-di-dichlorodihydrofluorescein diacetate.
REFERENCES
- 1. Warburg O. (1956) On respiratory impairment in cancer cells. Science 124, 269–270 [PubMed] [Google Scholar]
- 2. Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 3. Murphy M. P. (2009) How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papa S., Skulachev V. P. (1997) Reactive oxygen species, mitochondria, apoptosis, and aging. Mol. Cell. Biochem. 174, 305–319 [PubMed] [Google Scholar]
- 5. Kulisz A., Chen N., Chandel N. S., Shao Z., Schumacker P. T. (2002) Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L1324–1329 [DOI] [PubMed] [Google Scholar]
- 6. Goldschmidt-Clermont P. J., Moldovan L. (1999) Stress, superoxide, and signal transduction. Gene Expr. 7, 255–260 [PMC free article] [PubMed] [Google Scholar]
- 7. Griendling K. K., Harrison D. G. (1999) Dual role of reactive oxygen species in vascular growth. Circ. Res. 85, 562–563 [DOI] [PubMed] [Google Scholar]
- 8. Cai H., Harrison D. G. (2000) Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 87, 840–844 [DOI] [PubMed] [Google Scholar]
- 9. Finkel T. (2000) Redox-dependent signal transduction. FEBS Lett. 476, 52–54 [DOI] [PubMed] [Google Scholar]
- 10. Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 [DOI] [PubMed] [Google Scholar]
- 11. Meng T. C., Fukada T., Tonks N. K. (2002) Reversible oxidation and inactivation of protein-tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 12. Guachalla L. M., Rudolph K. L. (2010) ROS-induced DNA damage and checkpoint responses: influences on aging? Cell Cycle 9, 4058–4060 [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320, 661–664 [DOI] [PubMed] [Google Scholar]
- 14. Olinski R., Nackerdien Z., Dizdaroglu M. (1992) DNA-protein cross-linking between thymine and tyrosine in chromatin of γ-irradiated or H2O2-treated cultured human cells. Arch. Biochem. Biophys. 297, 139–143 [DOI] [PubMed] [Google Scholar]
- 15. Martin D., Salinas M., Fujita N., Tsuruo T., Cuadrado A. (2002) Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J. Biol. Chem. 277, 42943–42952 [DOI] [PubMed] [Google Scholar]
- 16. Bensaad K., Cheung E. C., Vousden K. H. (2009) Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 28, 3015–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bensaad K., Tsuruta A., Selak M. A., Vidal M. N., Nakano K., Bartrons R., Gottlieb E., Vousden K. H. (2006) TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126, 107–120 [DOI] [PubMed] [Google Scholar]
- 18. Coy J. F., Dressler D., Wilde J., Schubert P. (2005) Mutations in the transketolase-like gene TKTL1: clinical implications for neurodegenerative diseases, diabetes and cancer. Clin. Lab. 51, 257–273 [PubMed] [Google Scholar]
- 19. Grüning N. M., Rinnerthaler M., Bluemlein K., Mülleder M., Wamelink M. M., Lehrach H., Jakobs C., Breitenbach M., Ralser M. (2011) Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 14, 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang P., Du W., Wang X., Mancuso A., Gao X., Wu M., Yang X. (2011) p53 regulates biosynthesis through direct inactivation of glucose-6-phosphate dehydrogenase. Nat. Cell Biol. 13, 310–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sablina A. A., Budanov A. V., Ilyinskaya G. V., Agapova L. S., Kravchenko J. E., Chumakov P. M. (2005) The antioxidant function of the p53 tumor suppressor. Nat. Med. 11, 1306–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Budanov A. V., Sablina A. A., Feinstein E., Koonin E. V., Chumakov P. M. (2004) Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 304, 596–600 [DOI] [PubMed] [Google Scholar]
- 23. Tohma Y., Gratas C., Van Meir E. G., Desbaillets I., Tenan M., Tachibana O., Kleihues P., Ohgaki H. (1998) Necrogenesis and Fas/APO-1 (CD95) expression in primary (de novo) and secondary glioblastomas. J. Neuropathol. Exp. Neurol. 57, 239–245 [DOI] [PubMed] [Google Scholar]
- 24. Watanabe K., Tachibana O., Sata K., Yonekawa Y., Kleihues P., Ohgaki H. (1996) Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 6, 217–223; discussion 23–24 [DOI] [PubMed] [Google Scholar]
- 25. Ohgaki H., Kleihues P. (2007) Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 170, 1445–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Höckel M., Vaupel P. (2001) Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Nat. Cancer Inst. 93, 266–276 [DOI] [PubMed] [Google Scholar]
- 27. Vaupel P., Harrison L. (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist 9, Suppl. 5, 4–9 [DOI] [PubMed] [Google Scholar]
- 28. Sattler U. G., Meyer S. S., Quennet V., Hoerner C., Knoerzer H., Fabian C., Yaromina A., Zips D., Walenta S., Baumann M., Mueller-Klieser W. (2010) Glycolytic metabolism and tumor response to fractionated irradiation. Radiother. Oncol. 94, 102–109 [DOI] [PubMed] [Google Scholar]
- 29. Yaromina A., Quennet V., Zips D., Meyer S., Shakirin G., Walenta S., Mueller-Klieser W., Baumann M. (2009) Co-localization of hypoxia and perfusion markers with parameters of glucose metabolism in human squamous cell carcinoma (hSCC) xenografts. Int. J. Radiat. Biol. 85, 972–980 [DOI] [PubMed] [Google Scholar]
- 30. Walenta S., Schroeder T., Mueller-Klieser W. (2002) Metabolic mapping with bioluminescence: basic and clinical relevance. Biomol. Eng. 18, 249–262 [DOI] [PubMed] [Google Scholar]
- 31. Wanka C., Brucker D. P., Bähr O., Ronellenfitsch M., Weller M., Steinbach J. P., Rieger J. (2012) Synthesis of cytochrome c oxidase 2: a p53-dependent metabolic regulator that promotes respiratory function and protects glioma and colon cancer cells from hypoxia-induced cell death. Oncogene 31, 3764–3776 [DOI] [PubMed] [Google Scholar]
- 32. Steinbach J. P., Klumpp A., Wolburg H., Weller M. (2004) Inhibition of epidermal growth factor receptor signaling protects human malignant glioma cells from hypoxia-induced cell death. Cancer Res. 64, 1575–1578 [DOI] [PubMed] [Google Scholar]
- 33. Comín-Anduix B., Boren J., Martinez S., Moro C., Centelles J. J., Trebukhina R., Petushok N., Lee W. N., Boros L. G., Cascante M. (2001) The effect of thiamine supplementation on tumor proliferation: a metabolic control analysis study. Eur. J. Biochem. 268, 4177–4182 [DOI] [PubMed] [Google Scholar]
- 34. Boada J., Roig T., Perez X., Gamez A., Bartrons R., Cascante M., Bermúdez J. (2000) Cells overexpressing fructose 2,6-bisphosphatase showed enhanced pentose phosphate pathway flux and resistance to oxidative stress. FEBS Lett. 480, 261–264 [DOI] [PubMed] [Google Scholar]
- 35. Boros L. G., Lee P. W., Brandes J. L., Cascante M., Muscarella P., Schirmer W. J., Melvin W. S., Ellison E. C. (1998) Nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors: is cancer a disease of cellular glucose metabolism? Med. Hypotheses 50, 55–59 [DOI] [PubMed] [Google Scholar]
- 36. Ramos-Montoya A., Lee W. N., Bassilian S., Lim S., Trebukhina R. V., Kazhyna M. V., Ciudad C. J., Noé V., Centelles J. J., Cascante M. (2006) Pentose phosphate cycle oxidative and nonoxidative balance: a new vulnerable target for overcoming drug resistance in cancer. Int. J. Cancer 119, 2733–2741 [DOI] [PubMed] [Google Scholar]
- 37. Földi M., Stickeler E., Bau L., Kretz O., Watermann D., Gitsch G., Kayser G., Zur Hausen A., Coy J. F. (2007) Transketolase protein TKTL1 overexpression: a potential biomarker and therapeutic target in breast cancer. Oncol. Rep. 17, 841–845 [PubMed] [Google Scholar]
- 38. Völker H. U., Hagemann C., Coy J., Wittig R., Sommer S., Stojic J., Haubitz I., Vince G. H., Kämmerer U., Monoranu C. M. (2008) Expression of transketolase-like 1 and activation of Akt in grade IV glioblastomas compared with grades II and III astrocytic gliomas. Am. J. Clin. Pathol. 130, 50–57 [DOI] [PubMed] [Google Scholar]
- 39. Sun W., Liu Y., Glazer C. A., Shao C., Bhan S., Demokan S., Zhao M., Rudek M. A., Ha P. K., Califano J. A. (2010) TKTL1 is activated by promoter hypomethylation and contributes to head and neck squamous cell carcinoma carcinogenesis through increased aerobic glycolysis and HIF1α stabilization. Clin. Cancer Res. 16, 857–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu X., Zur Hausen A., Coy J. F., Löchelt M. (2009) Transketolase-like protein 1 (TKTL1) is required for rapid cell growth and full viability of human tumor cells. Int. J. Cancer 124, 1330–1337 [DOI] [PubMed] [Google Scholar]
- 41. Steinbach J. P., Supra P., Huang H. J., Cavenee W. K., Weller M. (2002) CD95-mediated apoptosis of human glioma cells: modulation by epidermal growth factor receptor activity. Brain Pathol. 12, 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naumann U., Durka S., Weller M. (1998) Dexamethasone-mediated protection from drug cytotoxicity: association with p21WAF1/CIP1 protein accumulation? Oncogene 17, 1567–1575 [DOI] [PubMed] [Google Scholar]
- 43. Dyer B. W., Ferrer F. A., Klinedinst D. K., Rodriguez R. (2000) A noncommercial dual luciferase enzyme assay system for reporter gene analysis. Anal. Biochem. 282, 158–161 [DOI] [PubMed] [Google Scholar]
- 44. Wischhusen J., Melino G., Weller M. (2004) p53 and its family members: reporter genes may not see the difference. Cell Death Differ. 11, 1150–1152 [DOI] [PubMed] [Google Scholar]
- 45. Steinbach J. P., Wolburg H., Klumpp A., Probst H., Weller M. (2003) Hypoxia-induced cell death in human malignant glioma cells: energy deprivation promotes decoupling of mitochondrial cytochrome c release from caspase processing and necrotic cell death. Cell Death Differ. 10, 823–832 [DOI] [PubMed] [Google Scholar]
- 46. Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Won K. Y., Lim S. J., Kim G. Y., Kim Y. W., Han S. A., Song J. Y., Lee D. K. (2012) Regulatory role of p53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum. Pathol. 43, 221–228 [DOI] [PubMed] [Google Scholar]
- 48. Sun L., Hui A. M., Su Q., Vortmeyer A., Kotliarov Y., Pastorino S., Passaniti A., Menon J., Walling J., Bailey R., Rosenblum M., Mikkelsen T., Fine H. A. (2006) Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 9, 287–300 [DOI] [PubMed] [Google Scholar]
- 49. Jamieson D., Vandenbrenk H. A. (1964) Effect of electrode dimensions on tissue PO-2 measurement in vivo. Nature 201, 1227–1228 [DOI] [PubMed] [Google Scholar]
- 50. Sridhar K. S., Plasse T. F., Holland J. F., Shapiro M., Ohnuma T. (1983) Effects of physiological oxygen concentration on human tumor colony growth in soft agar. Cancer Res. 43, 4629–4631 [PubMed] [Google Scholar]
- 51. Campbell J. A. (1925) The influence of O2-tension in the inspired air upon the O2-tension in the tissues. J. Physiol. 60, 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laser H. (1937) Tissue metabolism under the influence of low oxygen tension. Biochem. J. 31, 1671–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caldwell C. C., Kojima H., Lukashev D., Armstrong J., Farber M., Apasov S. G., Sitkovsky M. V. (2001) Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J. Immunol. 167, 6140–6149 [DOI] [PubMed] [Google Scholar]
- 54. Pistollato F., Abbadi S., Rampazzo E., Viola G., Della Puppa A., Cavallini L., Frasson C., Persano L., Panchision D. M., Basso G. (2010) Hypoxia and succinate antagonize 2-deoxyglucose effects on glioblastoma. Biochem. Pharmacol. 80, 1517–1527 [DOI] [PubMed] [Google Scholar]
- 55. Ward P. S., Thompson C. B. (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matoba S., Kang J. G., Patino W. D., Wragg A., Boehm M., Gavrilova O., Hurley P. J., Bunz F., Hwang P. M. (2006) p53 regulates mitochondrial respiration. Science 312, 1650–1653 [DOI] [PubMed] [Google Scholar]
- 57. Langbein S., Zerilli M., Zur Hausen A., Staiger W., Rensch-Boschert K., Lukan N., Popa J., Ternullo M. P., Steidler A., Weiss C., Grobholz R., Willeke F., Alken P., Stassi G., Schubert P., Coy J. F. (2006) Expression of transketolase TKTL1 predicts colon and urothelial cancer patient survival: Warburg effect reinterpreted. Br. J. Cancer 94, 578–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kayser G., Sienel W., Kubitz B., Mattern D., Stickeler E., Passlick B., Werner M., Zur Hausen A. (2011) Poor outcome in primary non-small cell lung cancers is predicted by transketolase TKTL1 expression. Pathology 43, 719–724 [DOI] [PubMed] [Google Scholar]
- 59. Kayser G., Kassem A., Sienel W., Schulte-Uentrop L., Mattern D., Aumann K., Stickeler E., Werner M., Passlick B., zur Hausen A. (2010) Lactate-dehydrogenase 5 is overexpressed in non-small cell lung cancer and correlates with the expression of the transketolase-like protein 1. Diagn. Pathol. 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Krockenberger M., Honig A., Rieger L., Coy J. F., Sutterlin M., Kapp M., Horn E., Dietl J., Kammerer U. (2007) Transketolase-like 1 expression correlates with subtypes of ovarian cancer and the presence of distant metastases. Int. J. Gynecol. Cancer 17, 101–106 [DOI] [PubMed] [Google Scholar]
- 61. Smith I. M., Glazer C. A., Mithani S. K., Ochs M. F., Sun W., Bhan S., Vostrov A., Abdullaev Z., Lobanenkov V., Gray A., Liu C., Chang S. S., Ostrow K. L., Westra W. H., Begum S., Dhara M., Califano J. (2009) Coordinated activation of candidate proto-oncogenes and cancer testes antigens via promoter demethylation in head and neck cancer and lung cancer. PloS One 4, e4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yuan W., Wu S., Guo J., Chen Z., Ge J., Yang P., Hu B., Chen Z. (2010) Silencing of TKTL1 by siRNA inhibits proliferation of human gastric cancer cells in vitro and in vivo. Cancer Biol. Ther. 9, 710–716 [DOI] [PubMed] [Google Scholar]
- 63. Marin-Valencia I., Yang C., Mashimo T., Cho S., Baek H., Yang X. L., Rajagopalan K. N., Maddie M., Vemireddy V., Zhao Z., Cai L., Good L., Tu B. P., Hatanpaa K. J., Mickey B. E., Matés J. M., Pascual J. M., Maher E. A., Malloy C. R., Deberardinis R. J., Bachoo R. M. (2012) Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Marcus H. J., Carpenter K. L., Price S. J., Hutchinson P. J. (2010) In vivo assessment of high-grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors, and cytokines. J. Neurooncol. 97, 11–23 [DOI] [PubMed] [Google Scholar]
- 65. Roslin M., Henriksson R., Bergström P., Ungerstedt U., Bergenheim A. T. (2003) Base-line levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis. J. Neurooncol. 61, 151–160 [DOI] [PubMed] [Google Scholar]
- 66. Tabatabaei P., Bergström P., Henriksson R., Bergenheim A. T. (2008) Glucose metabolites, glutamate and glycerol in malignant glioma tumors during radiotherapy. J. Neurooncol. 90, 35–39 [DOI] [PubMed] [Google Scholar]
- 67. Wibom C., Surowiec I., Mörén L., Bergström P., Johansson M., Antti H., Bergenheim A. T. (2010) Metabolomic patterns in glioblastoma and changes during radiotherapy: a clinical microdialysis study. J. Proteome Res. 9, 2909–2919 [DOI] [PubMed] [Google Scholar]
- 68. Peña-Rico M. A., Calvo-Vidal M. N., Villalonga-Planells R., Martínez-Soler F., Giménez-Bonafé P., Navarro-Sabaté À., Tortosa A., Bartrons R., Manzano A. (2011) TP53-induced glycolysis and apoptosis regulator (TIGAR) knockdown results in radiosensitization of glioma cells. Radiother. Oncol. 101, 132–139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.