Background: RNase R is unstable due to its acetylation and resulting binding of tmRNA-SmpB but is stabilized under stress conditions.
Results: tmRNA-SmpB promotes RNase R proteolysis by stimulating binding of Lon and HslUV.
Conclusion: Acetylation initiates a series of events ultimately leading to RNase R proteolysis.
Significance: This work elucidates a novel regulatory mechanism previously unknown in bacteria.
Keywords: Post-translational Modification, Protein Degradation, Protein Stability, Ribonuclease, RNA
Abstract
RNase R, an important exoribonuclease involved in degradation of structured RNA, is subject to a novel mechanism of regulation. The enzyme is extremely unstable in rapidly growing cells but becomes stabilized under conditions of stress, such as stationary phase or cold shock. RNase R instability results from acetylation which promotes binding of tmRNA-SmpB, two trans-translation factors, to its C-terminal region. Here, we examine how binding of tmRNA-SmpB leads to proteolysis of RNase R. We show that RNase R degradation is due to two proteases, HslUV and Lon. In their absence, RNase R is stable. We also show, using an in vitro system that accurately replicates the in vivo process, that tmRNA-SmpB is not essential, but it stimulates binding of the protease to the N-terminal region of RNase R and that it does so by a direct interaction between the protease and SmpB which stabilizes protease binding. Thus, a sequence of events, initiated by acetylation of a single Lys residue, results in proteolysis of RNase R in exponential phase cells. RNase R in stationary phase or in cold-shocked cells is not acetylated, and thereby remains stable. Such a regulatory mechanism, dependent on protein acetylation, has not been observed previously in bacterial cells.
Introduction
Ribonucleases (RNases) play important roles in all aspects of RNA metabolism including maturation of RNA precursors, turnover of mRNA, and degradation of stable RNAs (1–5). One of these enzymes, RNase R, a processive, 3′ to 5′ Escherichia coli exoribonuclease (6, 7) is unusual in that, by itself, it is able to digest structured RNAs (8). RNase R and polynucleotide phosphorylase, as part of the degradosome, are the primary RNases responsible for degradation of structured RNAs in E. coli (8–12). In fact, cells lacking both RNase R and polynucleotide phosphorylase are inviable, and fragments of rRNA and structured mRNAs accumulate in their absence (9). Interestingly, the amount of RNase R is regulated, increasing 3–10-fold during certain stress conditions such as cold shock (13) and stationary phase (13, 14), and how this is accomplished is of considerable interest.
Recent work from our laboratory has greatly increased our understanding of the mechanism of this regulation (15–18). RNase R is an extremely unstable protein in exponential phase cells with a half-life of ∼10 min (15), whereas it is stabilized under stress conditions leading to its relative elevation (13–15). The instability of RNase R in the exponential phase results from the binding of tmRNA2 and SmpB, two components of the trans-translation system, to its C-terminal region (16). Elimination of either of these factors or deletion of the C-terminal region stabilizes RNase R in exponential phase cells (16). Mass spectrometric analysis of purified RNase R demonstrated that exponential phase RNase R is post-translationally modified by acetylation of a single residue, Lys544 (17), a reaction catalyzed by protein lysine acetyltransferase (17, 18). In contrast, RNase R is unmodified in stationary phase cells (17). This post-translational modification leads to tighter binding of tmRNA and SmpB and subsequent proteolytic degradation of exponential phase RNase R, whereas its counterpart in stationary phase binds tmRNA and SmpB weakly and remains stable (17). Acetylation of RNase R is limited to exponential phase because the acetylating activity decreases in late exponential phase and is absent in stationary phase cells (18). What is not understood, however, is how binding of tmRNA-SmpB ultimately leads to enhanced degradation of RNase R by proteases.
In this paper, we show that Lon and the two-component protease, HslUV, are responsible for the degradation of RNase R in vivo. To examine why these proteases degrade exponential phase, but not stationary phase RNase R, we have developed an in vitro system that accurately mimics the in vivo degradation process. Using this system, we find that proteolysis of RNase R can occur in the absence of tmRNA and SmpB but that the presence of these two factors dramatically increases the process. Interestingly, tmRNA is not essential, but only stimulates the action of SmpB. We also show that the role of tmRNA and SmpB is to stimulate binding of Lon or HslUV to RNase R. Protease binding is to the N-terminal region of RNase R, and deletion of the first 83 amino acids of its N-terminal region completely stabilizes RNase R. In addition, the proteases bind to SmpB, and this interaction is stimulated by RNase R, indicating the presence of a ternary complex. Based on this information, we provide an explanation for the degradation of exponential phase RNase R and the stability of the stationary phase protein by a novel mechanism not previously observed in bacterial cells.
EXPERIMENTAL PROCEDURES
Materials
Antibody against RNase R was prepared and purified as described previously (7, 19). Antibodies against HslU and Lon were provided by Dr. Susan Gottesman, National Institutes of Health. Anti-GST mAb and anti-rabbit and anti-mouse IgG HRP conjugate were obtained from Santa Cruz Biotechnology. Plasmid pET21d and nickel-nitrilotriacetic acid His-bind resin were from Novagen. Plasmids pET-HslU and pET-HslV for purification of HslU and HslV were kindly provided by Dr. Robert T. Sauer, Massachusetts Institute of Technology. Protease inhibitor mixture was purchased from Calbiochem. Purified tmRNA, His- and GST-tagged SmpB and RNase R were described previously (16, 17). All other materials were reagent grade.
Bacterial Strains and Growth Conditions
E. coli K12 strain MG1655(Seq)rph+ and its derivatives lacking ClpP or Lon were obtained from Dr. Kenneth Rudd, University of Miami (20). The hslU and hslV insertion mutant alleles were provided by Dr. Susan Gottesman (21). These mutant genes were each introduced into strain MG1655(Seq)rph+ or the lon mutant strain by phage P1-mediated transduction using P1vir. The N-terminal region (amino acids 1–83) of chromosomal RNase R was removed by recombineering using primers R1 and R2 (Table 1), following a previously published protocol (22). Recombinants were selected on LB-kanamycin plates, and the deletion was confirmed by PCR using primers R3 and R4. The kanamycin resistance cassette was subsequently removed by plasmid pCP20 (23), and the resulting construct was confirmed by DNA sequencing.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotides | Sequence (5′–3′) |
|---|---|
| R1 | CTGGTGGAGTGACGAAAATCTTCATCAGAGATGACAACGGAGGAACCGAGGTGTAGGCTGGAGCTGCTTC |
| R2 | GCCGTAGCCATCACGGTGGCCAATAACGGTACCTTTCACATGGTGATGATGGTGGTGCATATGAATATCCTCCTTAG |
| R3 | GAACAGACCCAGCGTAACGAC |
| R4 | AGCTCAGACGGCTGTCGTCAG |
| L1 | CAGCCATGGCTAATCCTGAGCGTTCTGAACGCATTG |
| L2 | CAGCTCGAGTTTTGCAGTCACAACCTGCATACCAG |
| L3 | CAGCTCGAGGAAACGCTGAACACCGAGATAGTC |
Cells were grown in liquid culture in YT medium. Antibiotics, when present, were at the following concentrations: kanamycin, 50 μg/ml; ampicillin, 100 μg/ml; chloramphenicol, 34 μg/ml. Exponential phase cells were collected at an A550 of ∼0.3, and cells grown overnight were used as stationary phase samples.
Protein Purification
DNA fragments encoding full-length Lon or the C-terminal truncated Lon containing amino acids 1–585 (ΔLon) lacking its protease domain were amplified by PCR with primers L1, L2 and L1, L3, respectively (Table 1). The PCR products were purified and digested with NcoI and XhoI and then cloned into the corresponding sites on pET21d. Purification of His-tagged HslU, HslV, Lon, and N-terminal truncated RNase R were carried out as described (17, 24, 25).
In Vitro Proteolysis Assay
HslUV-mediated in vitro degradation of RNase R was performed as described (24). Briefly, 0.1 nm RNase R was mixed with different amounts of HslUV, as indicated, in buffer containing 25 mm HEPES-KOH, pH 7.6, 5 mm KCl, 20 mm MgCl2, 0.032% Nonidet P-40, 10% glycerol, 4 mm ATP, 50 mm creatine phosphate, and 80 μg/ml creatine kinase, in the absence or presence of 0.1 nm tmRNA and SmpB. The mixtures (50 μl) were incubated at 37 °C, and samples were taken at various time points for analysis of RNase R remaining.
Proteolysis of RNase R by Lon was carried out using a previously published protocol (25). RNase R proteins (0.1 nm) were mixed with various amounts of Lon, as indicated, in buffer containing 50 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 1 mm DTT, 4 mm ATP, 50 mm creatine phosphate, and 80 μg/ml creatine kinase, in the absence or presence of 0.1 nm tmRNA and SmpB. The mixtures (50 μl) were incubated at 37 °C, and samples were removed at varying times. The amount of RNase R remaining was determined by immunoblotting using RNase R antibody.
Pulldown Assay
GST-SmpB and tmRNA (0.2 nm each) or GST-SmpB alone were mixed with 0.1 nm HslU or ΔLon in the absence or presence of 0.1 nm exponential phase RNase R in buffer A (140 mm NaCl, 8 mm NaH2PO4, 2 mm K2HPO4, 10 mm KCl, 0.1% Nonidet P-40, and 1 mm phenylmethanesulfonyl fluoride (PMSF), pH 7.4). Mixtures were incubated with gentle rocking for 60 min at 4 °C. Following addition of 50 μl of GST resin, samples were incubated for an additional 30 min at 4 °C. The GST resin was recovered by centrifugation and washed five times with buffer A. Bound proteins were then eluted with 50 μl of buffer B (50 mm Tris-HCl, pH 8.0, 10 mm reduced glutathione). Eluted proteins were separated by SDS-PAGE and probed with HslU or Lon antibody and anti-GST mAb.
Western Blot Analysis
Proteins were resolved on either 8% or 12% SDS-PAGE and subjected to immunoblotting. RNase R, HslU, Lon, and recombinant GST-SmpB were detected by purified RNase R antibody (1:10,000 dilution), HslU antibody (1:3000 dilution), Lon antibody (1:3000 dilution), and anti-GST mAbs (1:1000 dilution), respectively. Underexposed films were used for quantitation by Quantity One (Bio-Rad).
Far Western Blot Analysis
Far Western blot analysis was performed using a published protocol (26). RNase R proteins (0.1 nm) were resolved on 8% SDS-PAGE and transferred to PVDF membranes. After denaturing and renaturing with guanidine-HCl, the membranes were blocked and incubated overnight at 4 °C with 0.5 nm HslU or ΔLon in the absence or presence of 0.5 nm tmRNA and SmpB. The HslU and ΔLon bound to the membranes were then detected by standard Western blot analysis using HslU and Lon antibodies, respectively.
Measurement of RNase R Half-life
Cells were grown to an A550 of ∼0.3 or overnight. A portion of cells was collected for the zero time point, and chloramphenicol was added to the remaining culture at 200 μg/ml. Cells were collected at the indicated times, lysed by sonication (14), and assayed by immunoblotting to determine the amount of RNase R.
RNase R Activity Assay
The activity of RNase R was determined as described previously (14). Assays were carried out in 50-μl reaction mixtures containing 20 mm Tris-Cl, pH 8.0, 0.25 mm MgCl2, 300 mm KCl, 40 μg [3H]poly(A) (50–100 cpm/nmol), and 1 ng of purified exponential phase or stationary phase RNase R. Reaction mixtures were incubated at 37 °C for 30 min. After precipitation with trichloroacetic acid, acid-soluble radioactivity was determined by liquid scintillation counting.
RESULTS
Earlier work showed that the instability of RNase R in exponential phase cells is a consequence of the binding of tmRNA-SmpB to its C-terminal region (16) and that this binding is promoted by the acetylation of RNase R on a single lysine residue (17). Here, we explore how binding of tmRNA-SmpB leads to proteolytic degradation of RNase R.
Identification of the Proteases That Degrade RNase R
As a first step to defining the mechanism of RNase R degradation, it was important to identify the proteases that participate in the process. To this end, several mutant strains, each devoid of a specific protease or protease subunit, were examined to determine their level of RNase R in exponential phase cells. Normally, in the MG1655 genetic background of the strains examined here, RNase R increases ∼4-fold in stationary phase compared with exponential phase cells due to its complete stabilization (14, 15). Thus, it would be expected that removal of the proteases(s) responsible for RNase R breakdown would lead to a similar elevation in exponential phase.
The data in Fig. 1A, and the quantitation in B show that removal of the protease subunit, ClpP, has no effect, whereas the absence of Lon or either of the subunits of HslUV leads to RNase R elevation. Maximum elevation, amounting to ∼4.5-fold, is seen when both Lon and HslUV are missing. As in earlier papers (14–17) RNase R activity has been normalized to total protein to demonstrate its enrichment relative to other proteins present in the extract. These data indicate that the ATP-dependent proteases, Lon and HslUV, both participate in the degradation of RNase R in exponential phase cells.
FIGURE 1.
Effect of protease mutations on the amount of exponential phase RNase R. Immunoblotting of cell extracts was carried out as described under “Experimental Procedures.” A, representative Western blot. Five μg of total protein was added to each lane. B, quantitation of three independent experiments as in A. The amount of RNase R in wild-type cells was set at 1. Error bars indicate S.E.
Development of an in Vitro System That Mimics RNase R Proteolysis
To examine the mechanistic details of RNase R proteolysis, we made use of a system containing purified components that accurately reflects the in vivo process. The data in Fig. 2A, determined in the presence of purified HslUV, show that both exponential and stationary phase RNase R are intrinsically resistant to the action of the protease. However, upon addition of purified tmRNA-SmpB, both forms of RNase R are destabilized, although to very different degrees. Proteolysis is much more pronounced with exponential phase RNase R, as would be expected from the 10-fold tighter binding of tmRNA-SmpB to this form of the RNase due to its acetylation (17). In fact, at lower levels of tmRNA-SmpB, stationary phase RNase R is essentially resistant to proteolysis (data not shown). Identical results were obtained in the presence of purified Lon protease (Fig. 2B). Because these data mimic the in vivo process so closely, they strongly suggest that components other than the protease and tmRNA-SmpB are not required for RNase R proteolysis.
FIGURE 2.
Development of an in vitro system to examine degradation of RNase R. A, degradation of RNase R by HslUV protease in the absence or presence of tmRNA-SmpB. B, degradation of RNase R by Lon protease in the absence or presence of tmRNA-SmpB. Exponential (Exp) or stationary (Sta) phase RNase R proteins (0.1 nm) were incubated at 37 °C with 0.01 nm HslU and 0.05 nm HslV, or 0.01 nm Lon, in the absence or presence of 0.1 nm tmRNA-SmpB for the times indicated. RNase R protein levels were determined by immunoblotting and were set at 100 at zero time of incubation. The amount of RNase R present is indicated below each lane.
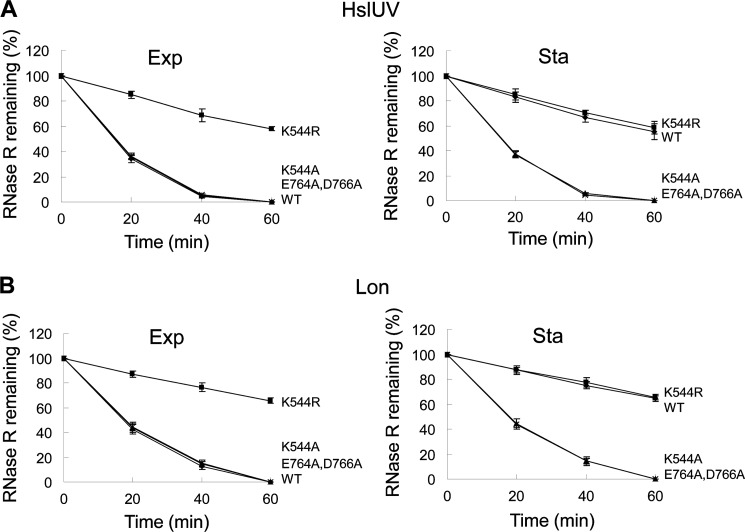
To further assess the similarity of the in vitro system to the process in vivo, we examined degradation of several purified mutant RNase R proteins (Fig. 3). As observed in vivo (17), conversion of Lys544, the residue subject to acetylation, to Arg renders exponential phase RNase R much more resistant to proteolysis by HslUV and Lon, whereas two other mutant proteins, K544A and E764A/D766A, remain sensitive. Moreover, in agreement with in vivo observations (17), the latter two mutations sensitize stationary phase RNase R (Fig. 3). The explanation for the effects of these various mutations has been discussed in detail previously (17), but for the studies presented here, these data confirm that the in vitro system accurately reproduces the in vivo findings, making it ideally suited for analysis of the details of RNase R degradation.
FIGURE 3.
Degradation of mutant RNase R proteins. A, HslUV. B, Lon. Degradation of exponential phase (Exp) or stationary phase (Sta) wild-type (WT) or K544R/K544A and E764A/D766A mutant RNase R proteins carried out as in Fig. 2 in the presence of tmRNA-SmpB. RNase R protein levels at zero time of incubation were set at 100%. WT and RNase R variants were examined in two independent experiments, and the averages ± S.D. (error bars) are presented.
tmRNA Stimulates the Action of SmpB
The data presented in Fig. 2 clearly indicated that the presence of tmRNA-SmpB dramatically stimulates degradation of RNase R by HslUV or Lon, but how this is accomplished is unclear. As a first step to understanding the stimulatory process, we examined the role of tmRNA. The results presented in Fig. 4 indicate that tmRNA is not essential for the degradation of RNase R and that its function can be dispensed with entirely at higher concentrations of SmpB. Thus, the same amount of RNase R decay seen when tmRNA is present can be obtained by increasing the amount of SmpB 10-fold. SmpB is known to bind to a basic domain in the C-terminal region of RNase R (16), and these findings suggest that the only role of tmRNA is to increase the affinity of SmpB for this site. Nevertheless, this role is essential in vivo because at the concentration of SmpB normally present, the absence of tmRNA leads to elimination of RNase R degradation (16). It should be noted that SmpB is completely stable during this process (supplemental Fig. S1).
FIGURE 4.
The role of tmRNA in RNase R degradation. Increasing relative amounts of SmpB or tmRNA added to 1-fold SmpB were incubated with 0.1 nm exponential phase RNase R and 0.01 nm HslU and 0.05 nm HslV, or 0.01 nm Lon, at 37 °C for 40 min. Western blot analysis of RNase R was carried out as in Fig. 2. The amount of RNase R protein in the reaction with 1-fold SmpB was set at 100.
tmRNA-SmpB Promotes Protease Binding to RNase R
To ascertain the role of tmRNA-SmpB in the degradation process, we examined its effect on protease binding to RNase R. To avoid the possibility of protease action during the course of the experiment, we measured the effect of tmRNA-SmpB on the affinity of HslU, the binding subunit of HslUV. The immunoblot presented in Fig. 5A shows that HslU binds poorly to either exponential or stationary phase RNase R. However, addition of tmRNA-SmpB greatly stimulates binding to the exponential phase protein, while having little effect on its binding to stationary phase RNase R. Identical results were obtained with C-terminal truncated, inactive Lon lacking its protease domain (Fig. 5B). The differential stimulation of protease binding to the two forms of RNase R is due to the fact that under these conditions, tmRNA-SmpB binds exclusively to exponential phase RNase R, the acetylated form of the enzyme (17). These findings explain why exponential phase RNase R is much more sensitive to proteolysis.
FIGURE 5.
Effect of tmRNA-SmpB on the binding of protease to full-length and N-terminal-truncated RNase R. A, far Western analysis of HslU binding to RNase R. Equal amounts of purified exponential phase and stationary phase wild-type and ΔN mutant RNase R proteins (0.1 nm) were resolved on 8% SDS-PAGE and transferred onto PVDF membranes. After incubating with 0.5 nm HslU in the presence or absence of 0.5 nm tmRNA-SmpB, HslU bound to the membranes was detected with HslU antibody. The amount of HslU bound to exponential phase wild-type RNase R in the presence of tmRNA-SmpB was set at 100. B, far Western analysis of C-terminal truncated Lon (ΔLon) binding to RNase R. The assay was carried out as in A, and the amount of ΔLon bound to the membranes was detected with Lon antibody. The amount of ΔLon bound to exponential phase wild-type RNase R in the presence of tmRNA-SmpB was set at 100. C, degradation of ΔN mutant RNase R protein by HslUV. D, degradation of ΔN mutant RNase R protein by Lon. Degradation of exponential phase (Exp) or stationary phase (Sta) ΔN mutant RNase R proteins by HslUV and Lon was carried out as in Fig. 2 in the presence of tmRNA-SmpB. RNase R protein levels at zero time of incubation were set at 100. E, amount of wild-type and ΔN mutant RNase R proteins in exponential phase and stationary phase cells. The amount of RNase R in exponential phase wild-type cells was set at 1. F, stability of ΔN mutant RNase R in exponential and stationary phase cells. Cells were grown in YT medium, treated with chloramphenicol (CM), and assayed for RNase R protein by immunoblotting as described under “Experimental Procedures.” The RNase R protein levels at zero time of chloramphenicol addition were set at 100. For E and F, 5 μg of total protein was added to each lane. Experiments were repeated three times; representative experiments are shown.
Protease Binds to the N-terminal Region of RNase R
ATP-dependent proteases often bind and initiate degradation at the N terminus of their protein substrate (27–29). To examine whether this is also true for RNase R, we deleted its 83 N-terminal amino acids, a largely unstructured domain preceding the cold shock domains, and used this purified, fully active protein (supplemental Table S1) to measure HslU binding. As shown in Fig. 5A, HslU does not bind to this truncated protein. Likewise, N-terminal truncated RNase R does not bind to the catalytically inactive form of Lon (Fig. 5B). As expected from the lack of protease binding, truncated RNase R is resistant to the action of HslUV (Fig. 5C) and Lon (Fig. 5D) in vitro. Finally, N-terminal truncated RNase R accumulates in exponential phase cells to the same level as that in stationary phase (Fig. 5E) because it is no longer sensitive to proteolysis in vivo (Fig. 5F). These data lead us to conclude that binding of tmRNA-SmpB to the C-terminal region of RNase R promotes binding of protease to its N-terminal region enabling proteolysis to take place.
Interestingly, the data in Fig. 6 show that the only role of tmRNA-SmpB is to stimulate protease binding. Thus, addition of a 5-fold higher level of HslUV eliminates the need for tmRNA-SmpB in proteolysis of RNase R, and in this situation, there is no difference in the sensitivity of the exponential and stationary phase proteins (Fig. 6A). Identical results were obtained with Lon protease (Fig. 6B). These findings confirm that the differential sensitivity to proteolysis of the two forms of RNase R is simply due to their different affinities for tmRNA-SmpB which thereby leads to differential binding of the protease.
FIGURE 6.
Elevated protease eliminates the requirement for tmRNA-SmpB. A, exponential (Exp) or stationary (Sta) phase RNase R proteins (0.1 nm) were incubated at 37 °C with 0.01 nm HslU and 0.05 nm HslV (1×), or 0.05 nm HslU and 0.25 nm HslV (5×), in the absence of tmRNA-SmpB for the indicated times. The RNase R protein levels at zero time of incubation were set at 100%. B, exponential or stationary phase RNase R proteins (0.1 nm) were incubated at 37 °C with 0.01 nm Lon (1×), or 0.05 nm Lon (5×), in the absence of tmRNA-SmpB for the indicated times. The RNase R protein levels at zero time of incubation were set at 100%.
Protease Binds Directly to SmpB
The data presented in Fig. 5, A and B, indicated that tmRNA-SmpB promotes the binding of protease to RNase R, but these results did not explain how this is accomplished. For example, binding of tmRNA-SmpB could alter the conformation of RNase R to one that binds protease with higher affinity. Alternatively, SmpB may bind directly to the protease to help stabilize its binding to RNase R. Here, we show that the latter explanation is correct (Fig. 7). Immunoprecipitation of purified GST-SmpB with GST resin pulls down HslU as well, and this is unaffected by the presence of tmRNA (Fig. 7A), indicating direct interaction between the protease and SmpB. Moreover, this interaction is stimulated about 10-fold by the presence of RNase R. On the other hand, there is no stimulation by N-terminal truncated RNase R which does not bind the protease. Identical results were obtained with the truncated, inactive form of Lon (Fig. 7B). Taken together with the findings above that tmRNA-SmpB stimulates binding of protease to RNase R, these data indicate the formation of a ternary complex containing RNase R, SmpB, and protease, each bound directly to the other two components.
FIGURE 7.
Pulldown of HslU and ΔLon with GST-SmpB. A, HslU. B, ΔLon. Purified wild-type RNase R (RNR-WT), N-terminal truncated RNase R (RNR-ΔN), HslU (ΔLon), GST-SmpB, and tmRNA were prepared and used for a pulldown assay as described under “Experimental Procedures.” The components present in each assay are indicated above each lane. The presence of HslU, ΔLon protein, and recombinant GST-SmpB protein was detected by HslU or Lon antibody and anti-GST mAb, respectively. The amount of HslU or ΔLon pulled down in the presence of tmRNA-SmpB or SmpB alone, and in the absence of RNase R, was set at 1.
DISCUSSION
The studies described here provide a detailed explanation for the stress-dependent regulation of RNase R which results in degradation of the protein in fast-growing cells and its stabilization in stationary phase and other stress conditions (13–18). Combining both in vitro and in vivo examination of the process, we found that (i) HslUV and Lon are the proteases responsible for degradation of exponential phase RNase R; (ii) the degradative process can be replicated in vitro entirely with purified components; (iii) tmRNA-SmpB stimulates binding of protease to RNase R; (iv) protease binds to the N-terminal region of RNase R; and (v) protease also interacts directly with SmpB, indicating the formation of a ternary complex of RNase R, SmpB, and protease.
Based on all of this information, we propose a model to explain the differential sensitivity of exponential and stationary phase RNase R to proteolysis (Fig. 8). As reported earlier (17), only RNase R from exponential phase cells is acetylated (at Lys544). As a consequence, only the exponential phase enzyme binds tmRNA-SmpB at the concentrations that these trans-translation components are present in vivo. The presence of tmRNA-SmpB, bound to the C-terminal region of RNase R, promotes binding of either HslUV or Lon protease to the N-terminal region of the protein. This is due to a direct interaction of the protease with SmpB as well as with RNase R. Once the protease is bound, proteolysis can proceed. RNase R from stationary phase cells is unaffected because it is not acetylated (17), and as a consequence, the sequence of events leading to proteolysis is not initiated. Based on the in vitro data, accurate functioning of this regulatory system must be exquisitely sensitive to the concentrations in vivo of the various interacting components of the system. Elevated levels of SmpB obviate the requirement for tmRNA and sensitize stationary phase RNase R, and elevated levels of protease render unnecessary the binding of tmRNA-SmpB and even lead to degradation of stationary phase RNase R in vitro. These considerations suggest that the levels of tmRNA, SmpB, and protease should be tightly controlled in vivo.
FIGURE 8.
Model for degradation of RNase R by proteases. In exponential phase cells, acetylation of Lys544 eliminates its positive charge and, as a consequence, disrupts the interaction between the lysine residue and the C-terminal region of RNase R. This exposes the C-terminal region of RNase R, enabling tight binding of the tmRNA-SmpB complex. The protease binds tightly to the N-terminal region of RNase R through its interaction with SmpB, resulting in RNase instability. In stationary phase cells, Lys544 is not acetylated, maintaining a positive charge on this residue, resulting in interaction between the amino residue and the C-terminal region of RNase R. This interaction prevents effective binding of tmRNA-SmpB to the C-terminal region and subsequent tight binding of protease to the N-terminal region of RNase R. Thus, this enzyme is stable.
Regulation of RNase R is, thus far, the only known example in which the stability of a bacterial protein is determined by acetylation. However, it is not yet understood why this method of regulation is used to control the levels of RNase R or why tmRNA and SmpB, which normally function in trans-translation (30–33), serve as cofactors for RNase R degradation. It is known that RNase R participates in removal of mRNAs subject to trans-translation (34) and that binding of tmRNA-SmpB to the C-terminal region of RNase R is required for its role in the mRNA decay process (35). Moreover, the binding helps to recruit RNase R to stalled ribosomes (35). The similarity of this process to that which leads to RNase R degradation raises the possibility that the two processes may be linked and that ribosomes may influence RNase R degradation. The studies presented here detail how tmRNA-SmpB and proteases function in RNase R degradation, but further work will be necessary to understand whether it is directly related to the trans-translation process and/or to central metabolism via RNase R acetylation.
Supplementary Material
Acknowledgments
We thank Dr. Susan Gottesman, Dr. Kenneth E. Rudd, and Dr. Robert T. Sauer for antibodies, bacterial strains and plasmids. We also thank members of the laboratory and Dr. Arun Malhotra for critical comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM16317.

This article contains supplemental Fig. S1 and Table S1.
- tmRNA
- transfer-messenger RNA.
REFERENCES
- 1. Deutscher M. P. (2003) Degradation of stable RNA in bacteria. J. Biol. Chem. 278, 45041–45044 [DOI] [PubMed] [Google Scholar]
- 2. Deutscher M. P. (2006) Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 34, 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andrade J. M., Pobre V., Silva I. J., Domingues S., Arraiano C. M. (2009) The role of 3′-5′ exoribonucleases in RNA degradation. Prog. Mol. Biol. Transl. Sci. 85, 187–229 [DOI] [PubMed] [Google Scholar]
- 4. Carpousis A. J., Luisi B. F., McDowall K. J. (2009) Endonucleolytic initiation of mRNA decay in Escherichia coli. Prog. Mol. Biol. Transl. Sci. 85, 91–135 [DOI] [PubMed] [Google Scholar]
- 5. Deutscher M. P. (2009) Maturation and degradation of ribosomal RNA in bacteria. Prog. Mol. Biol. Transl. Sci. 85, 369–391 [DOI] [PubMed] [Google Scholar]
- 6. Zuo Y., Deutscher M. P. (2001) Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 29, 1017–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng Z. F., Deutscher M. P. (2002) Purification and characterization of the Escherichia coli exoribonuclease RNase R: comparison with RNase II. J. Biol. Chem. 277, 21624–21629 [DOI] [PubMed] [Google Scholar]
- 8. Cheng Z. F., Zuo Y., Li Z., Rudd K. E., Deutscher M. P. (1998) The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 273, 14077–14080 [DOI] [PubMed] [Google Scholar]
- 9. Cheng Z. F., Deutscher M. P. (2005) An important role for RNase R in mRNA decay. Mol. Cell 17, 313–318 [DOI] [PubMed] [Google Scholar]
- 10. Vincent H. A., Deutscher M. P. (2006) Substrate recognition and catalysis by the exoribonuclease RNase R. J. Biol. Chem. 281, 29769–29775 [DOI] [PubMed] [Google Scholar]
- 11. Vincent H. A., Deutscher M. P. (2009) The roles of individual domains of RNase R in substrate binding and exoribonuclease activity: the nuclease domain is sufficient for digestion of structured RNA. J. Biol. Chem. 284, 486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vincent H. A., Deutscher M. P. (2009) Insights into how RNase R degrades structured RNA: analysis of the nuclease domain. J. Mol. Biol. 387, 570–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andrade J. M., Cairrão F., Arraiano C. M. (2006) RNase R affects gene expression in stationary phase: regulation of ompA. Mol. Microbiol. 60, 219–228 [DOI] [PubMed] [Google Scholar]
- 14. Chen C., Deutscher M. P. (2005) Elevation of RNase R in response to multiple stress conditions. J. Biol. Chem. 280, 34393–34396 [DOI] [PubMed] [Google Scholar]
- 15. Chen C., Deutscher M. P. (2010) RNase R is a highly unstable protein regulated by growth phase and stress. RNA 16, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang W., Deutscher M. P. (2010) A novel mechanism for ribonuclease regulation: transfer-messenger RNA (tmRNA) and its associated protein SmpB regulate the stability of RNase R. J. Biol. Chem. 285, 29054–29058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang W., Malhotra A., Deutscher M. P. (2011) Acetylation regulates the stability of a bacterial protein: growth stage-dependent modification of RNase R. Mol. Cell 44, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liang W., Deutscher M. P. (2012) Post-translational modification of RNase R is regulated by stress-dependent reduction in the acetylating enzyme Pka (YfiQ). RNA 18, 37–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang W., Li C., Liu F., Jiang H., Li S., Sun J., Wu X., Li C. (2009) The Arabidopsis homologs of CCR4-associated factor 1 exhibit mRNA deadenylation activity and play a role in plant defense responses. Cell Res. 19, 307–316 [DOI] [PubMed] [Google Scholar]
- 20. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu W. F., Zhou Y., Gottesman S. (1999) Redundant in vivo proteolytic activities of Escherichia coli Lon and the ClpYQ (HslUV) protease. J. Bacteriol. 181, 3681–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Datsenko K. A., Wanner B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97, 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cherepanov P. P., Wackernagel W. (1995) Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158, 9–14 [DOI] [PubMed] [Google Scholar]
- 24. Burton R. E., Baker T. A., Sauer R. T. (2005) Nucleotide-dependent substrate recognition by the AAA+ HslUV protease. Nat. Struct. Mol. Biol. 12, 245–251 [DOI] [PubMed] [Google Scholar]
- 25. Sundermeier T., Ge Z., Richards J., Dulebohn D., Karzai A. W. (2008) Studying tmRNA-mediated surveillance and nonstop mRNA decay. Methods Enzymol. 447, 329–358 [DOI] [PubMed] [Google Scholar]
- 26. Wu Y., Li Q., Chen X. Z. (2007) Detecting protein–protein interactions by far Western blotting. Nat. Protoc. 2, 3278–3284 [DOI] [PubMed] [Google Scholar]
- 27. Gottesman S. (2003) Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19, 565–587 [DOI] [PubMed] [Google Scholar]
- 28. Mogk A., Schmidt R., Bukau B. (2007) The N-end rule pathway for regulated proteolysis: prokaryotic and eukaryotic strategies. Trends Cell Biol. 17, 165–172 [DOI] [PubMed] [Google Scholar]
- 29. Dougan D. A., Truscott K. N., Zeth K. (2010) The bacterial N-end rule pathway: expect the unexpected. Mol. Microbiol. 76, 545–558 [DOI] [PubMed] [Google Scholar]
- 30. Karzai A. W., Roche E. D., Sauer R. T. (2000) The SsrA-SmpB system for protein tagging, directed degradation and ribosome rescue. Nat. Struct. Biol. 7, 449–455 [DOI] [PubMed] [Google Scholar]
- 31. Withey J. H., Friedman D. I. (2002) The biological roles of trans-translation. Curr. Opin. Microbiol. 5, 154–159 [DOI] [PubMed] [Google Scholar]
- 32. Withey J. H., Friedman D. I. (2003) A salvage pathway for protein structure: tmRNA and trans-translation. Annu. Rev. Microbiol. 57, 101–123 [DOI] [PubMed] [Google Scholar]
- 33. Dulebohn D., Choy J., Sundermeier T., Okan N., Karzai A. W. (2007) Trans-translation: the tmRNA-mediated surveillance mechanism for ribosome rescue, directed protein degradation, and nonstop mRNA decay. Biochemistry 46, 4681–4693 [DOI] [PubMed] [Google Scholar]
- 34. Richards J., Mehta P., Karzai A. W. (2006) RNase R degrades non-stop mRNAs selectively in an SmpB-tmRNA-dependent manner. Mol. Microbiol. 62, 1700–1712 [DOI] [PubMed] [Google Scholar]
- 35. Ge Z., Mehta P., Richards J., Karzai A. W. (2010) Non-stop mRNA decay initiates at the ribosome. Mol. Microbiol. 78, 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.