Background: HIV utilizes its fusion peptide (FP) to both fuse and immunosuppress T-cells.
Results: A conserved GxxxG-like motif within FP is critical for its function and structure.
Conclusion: The GxxxG-like motif exerts its role via interaction with the transmembrane domain of TCRα.
Significance: We shed light on the molecular mechanism of FP immunosuppressant activity and on the molecular recognition within the membrane milieu.
Keywords: AIDS, Biophysics, HIV-1, Immunosuppression, Membrane Fusion, Membrane Proteins, Peptide Interactions, Protein-Protein Interactions
Abstract
To thrive in the human body, HIV fuses to its target cell and evades the immune response via several mechanisms. The fusion cascade is initiated by the fusion peptide (FP), which is located at the N-terminal of gp41, the transmembrane protein of HIV. Recently, it has been shown that the HIV-1 FP, particularly its 5–13 amino acid region (FP5–13), suppresses T-cell activation and interacts with the transmembrane domain (TMD) of the T-cell receptor (TCR) complex. Specific amino acid motifs often contribute to such interactions in TMDs of membrane proteins. Using bioinformatics and experimental studies, we report on a GxxxG-like motif (AxxxG), which is conserved in the FP throughout different clades and strains of HIV-1. Biological activity studies and FTIR spectroscopy revealed that HIV FP5–13-derived peptides, in which the motif was altered either by randomization or by a single amino acid shift, lost their immunosuppressive activity concomitant with a loss of the β-sheet structure in a membranous environment. Furthermore, fluorescence studies revealed that the inactive mutants lost their ability to interact with their target site, namely, the TMD of TCRα, designated CP. Importantly, lipotechoic acid activated macrophages (lacking TCR) were not affected by FP, further demonstrating the specificity of the immunosuppressant activity of CP. Finally, although the AxxxG WT and the GxxxG analog both associated with the CP and immunosuppressed T-cells, the AxxxG WT but not the GxxxG analog induced lipid mixing. Overall, the data support an important role for the AxxxG motif in the function of FP and might explain the natural selection of the AxxxG motif rather than the classical GxxxG motif in FP.
Introduction
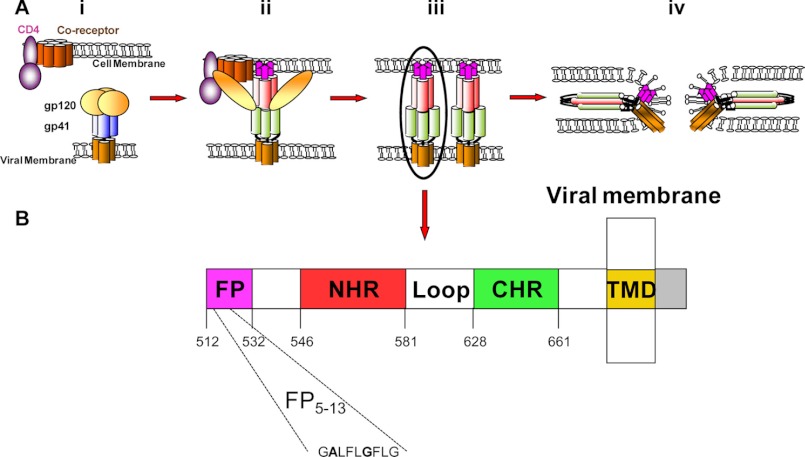
Human immunodeficiency virus (HIV) is the etiological agent for acquired immunodeficiency syndrome (AIDS). Since it was first reported, ∼60 million people have been infected with HIV (1–3). Effective infection of HIV is accomplished by successful fusion between its membrane and the membrane of the T-cell, as well as by its ability to evade the immune response against it. To fuse with the target cell, HIV virions express the envelope glycoprotein gp160, which is composed of gp120-gp41 subunits. The gp120 glycoprotein binds its cell receptor (CD4) and a co-receptor (CXCR4 or CCR5) (4, 5). Following the attachment of gp120, gp41 undergoes conformational changes that enable it to catalyze the fusion between the viral and the cellular membranes or between infected and native cells (6–8). Gp41 is composed of several functional domains including the fusion peptide (FP),3 the N-terminal heptad repeat, the loop, the C-terminal heptad repeat, and the transmembrane domain (TMD) (see Fig. 1) (9, 10). A proposed role of the FP is to reduce the energy barrier for the fusion by binding and dehydrating the outer bilayer at a localized site (11). The fusion itself starts with the insertion of the FP into the host cell membrane (12–14). Only then can N-terminal heptad repeat and C-terminal heptad repeat form a six-helix bundle that bridges the gap between the opposing membranes, enabling the actual membrane fusion (Fig. 1) (11, 13–16).
FIGURE 1.
A schematic illustrating the HIV-I HXB2 fusion process and denotes the gp41 extracellular domain. A, HIV fusion cascade. i, naïve state in which gp120 shields gp41. ii, prehairpin intermediate state in which gp120 is attached to its receptor and co-receptor, causing a conformational change. iii, extended prehairpin, in which gp41 is released and the FP is inserted into the host membrane. iv, post-fusion; NHP and CHP formed a six-helix bundle and the fusion progressed. (61). B, a scheme showing the functional regions within the extracellular portion of HIV HXB2 gp41 (amino acids 512–856) (9, 62). CHR, C-terminal heptad repeat; NHR, N-terminal heptad repeat.
HIV also suppresses the immune response via several mechanisms. Examples include the following: (i) interfering with the expression of the costimulatory molecules CD40 ligand and CD80 (B71) (17); (ii) inducing anergy in naive T lymphocytes through CD4-independent protein kinase A-mediated signaling (18); (iii) inducing dysfunction of uninfected bystander CD4+ T-cells via interaction with both CD4 and co-receptors (19); (iv) exposing CD4+ T-cells during stimulation to noninfectious HIV expressing functional envelope glycoproteins, which fail to provide activation signals to autologous dendritic cells (20); and (v) impairing the ability of dendritic cells to stimulate T-cell proliferation during HIV-1 infection (21).
In addition, studies have shown that elements in gp41 can inhibit T-cell proliferation. These include the following: (i) a peptide derived from the interface between the N-terminal heptad repeat and the loop regions of gp41 (amino acids 583–599), termed the immunosuppressive unit (22), which interferes with calcium influx and the function of a protein kinase C (PKC) (23, 24); (ii) the FP, which specifically binds the TMD of TCRα (termed CP) and as a result interferes with the assembly of the TCR complex (25–29); and (iii) the TMD of gp41, which shares a nine-amino acid motif with the TMD of the α-subunit of the T-cell receptor (TCRα). As a result, the TMD also interferes with the assembly of the TCR complex (30). Importantly, each of these three regions has a different mode of action. Molecular dynamic simulation was further utilized to identify the TCR/FP interacting region at the molecular level, providing a three-dimensional model of the TCR-FP assembly structure. The simulation data revealed that FP5–13 interacts with CP within the membrane in a β-sheet/α-helix interaction (27). Experimentally, attenuated total reflectance (ATR)-FTIR studies revealed a β-sheet structure of the FP5–13. A synthetic peptide mimicking FP5–13 indeed preserved the potent inhibitory activity of FP both in vitro and in vivo. The FP5–13 was found to colocalize and immunoprecipitate specifically with the TCR (26–28).
Interactions between TMDs are usually driven by amino acid motifs, among which the GxxxG motif, originally identified in the TMD of glycophorin A, is the most studied (31–33). Other than stabilizing interactions within the membrane, glycine residues have also been shown to facilitate conversion to a β-sheet secondary structure and to mediate sheet-to-sheet packing within the membrane (34). The glycine residues in the GxxxG motif can also be replaced by the small residue Ala (32, 35, 36). Furthermore, the GxxxG dimerization is reinforced by the presence of phenylalanines (37).
In this study, we combined bioinformatics with experimental studies and characterized an AxxxG motif within the FP5–13 region (GALFLGFLG) of HIV-1, which is crucial for the immunosuppressant activity of FP. Mode of action studies using several analogs revealed that the motif controls the heteroassociation between FP and TCRα-TMD within the membrane and contributes to the β-sheet secondary structure of FP. Interestingly, the AxxxG WT and the GxxxG analog both associate with the CP and immunosuppress T-cells, whereas the AxxxG WT but not the GxxxG analog induced lipid mixing of zwitterionic membranes. The results are discussed regarding the mechanism of the immunosuppressant activity of FP as well as the possible reason for the natural selection of the AxxxG motif rather than the classical GxxxG motif.
EXPERIMENTAL PROCEDURES
Materials
Rink amide 4-methyl-benzhydrylamine resin and 9-fluorenylmethoxycarbonyl (Fmoc) amino acids were purchased from Calibochem-Novabiochem AG. Other reagents used for peptide synthesis include N,N-diisopropylethylamine (Sigma-Aldrich), dimethylformamide, dicheloromethane, and piperidine. Egg phosphatidylcholine (PC) was purchased from Lipid Products (South Nutfield, UK). 4-Chloro-7-nitrobenz-2-oxa-1,3-diazole fluoride (NBD-F) and rhodamine-N-hydroxysuccinimide (Rho-N) were purchased from Molecular Probes (Junction City, OR). The myelin oligodendrocyte glycoprotein (MOG) p35–55 antigen used for the specific activation of the T-cell line was synthesized using the Fmoc technique with an automatic multiple peptide synthesizer (AMS 422, ABIMED, Langenfeld, Germany). Primary CD4 T-cells specific to MOG p35–55 were a kind gift from Professor Avraham Ben-Nun from the Weizmann Institute of Science.
Peptide Synthesis and Fluorescent Labeling
Peptides were synthesized using the Fmoc solid phase method on a Rink amide resin (0.68 meq/gm), as described previously (38). The synthetic peptides were purified (>98% homogeneity) by reverse phase high performance liquid chromatography on a C4 or C18 column using a linear gradient of 30–70% acetonitrile in 0.1% trifluoroacetic acid (TFA) for 40 min. The peptides were subjected to amino acids and mass spectrometry analysis to confirm their composition. To avoid aggregation of the peptides prior to their use in the cell culture assays, the stock solutions of the concentrated peptides were maintained in dimethyl sulfoxide (DMSO). The final concentration of DMSO in each experiment was <0.25% v/v and had no effect on the system under investigation. For NBD-fluorescent labeling, resin-bound peptides were treated with NBD-F (2-fold excess) dissolved in dimethyl formamide (DMF), leading to the formation of resin-bound N-terminal NBD peptides (39). After 1 h, the resins were washed thoroughly with DMF and then with methylene chloride, dried under nitrogen flow, and then cleaved for 3 h with 95% TFA, 2.5% H2O, and 2.5% triethylsilane. For Rho-N fluorescent labeling, the Fmoc protecting group was removed from the N terminus of the resin-bound peptides by incubation with piperidine (20% in DMF) for 12 min, whereas all of the other reactive amine groups of the attached peptides were kept protected. The resin-bound peptides were washed twice with DMF and then treated with rhodamine-N-hydroxysuccinimide (2-fold excess), in anhydrous DMF containing 2% N,N-diisopropylethylamine, leading to the formation of a resin-bound N-rhodamine peptide. After 24 h, the resin was washed thoroughly with DMF and then with methylene chloride, dried under nitrogen flow, and then cleaved for 3 h with 95% TFA, 2.5% H2O, and 2.5% triethylsilane. The labeled peptides were purified on a reverse phase HPLC C4 or C18 column as described above. Unless stated otherwise, stock solutions of concentrated peptides were maintained in DMSO to avoid aggregation of the peptides prior to use.
Preparation of Large Unilamellar Vesicles (LUV)
Thin films of PC were generated after dissolving the lipids in a 2:1 (v/v) mixture of CHCL3/MeOH and drying them under a stream of nitrogen gas while rotating them. The films were lyophilized overnight, sealed with argon gas to prevent oxidation of the lipids, and stored at −20 °C. Before the experiments, films were suspended in the appropriate buffer and vortexed for 1.5 min. The lipid suspension underwent five cycles of freezing-thawing and extrusion through polycarbonate membranes with 1- and 0.1-μm diameter pores to create large unilamellar vesicles.
Lipid Mixing
Lipid mixing of LUVs was measured using a fluorescence probe dilution assay (40). LUVs were prepared with PBS, as described above, from unlabeled and labeled films combined to give a 9:1 ratio of 100 μm total lipid concentration. The basal fluorescence level was measured initially for a 400-μl vesicle mixture. Then, peptides dissolved in 2 μl of DMSO were added to the mixture. Fluorescence was monitored after the addition of the peptide until a steady state was achieved, as indicated by a plateau. The emission of NBD, the energy donor, was monitored at 530 nm with the excitation set at 467 nm. Fluorescence intensity was compared with 2% (v/v) of Triton X-100.
Macrophage Activation by LTA
RAW264.7 cells (2 × 105 per well) were cultured overnight in a 96-well plate. The following day, the medium was replaced by fresh DMEM, including all supplements. Peptides were dissolved in DMSO and added to the cells to reach a 20 μm final concentration. The final concentration of DMSO was 1% for all groups. Cells were incubated with the peptide for 2 h and then washed and incubated with fresh medium containing 500 ng/ml lipotechoic acid (LTA).
Determination of Secreted TNFα
Cells were incubated with the indicated activators for 5 h at 37 °C, after which samples of the medium from each treatment were collected and stored at −20 °C. TNFα levels in each sample were evaluated using a mouse TNFα enzyme-linked immunosorbent assay kit (BIOSOURCETM ELISA, Invitrogen) according to the manufacturer's protocol. All experiments were done in triplicate.
Fluorescence Energy Transfer (FRET) Measurements
The FRET experiments were performed by using NBD and Rho-labeled peptides as energy donors and energy acceptors, respectively. Fluorescence spectra were obtained at room temperature, with excitation set at 467 nm (10-nm slit) and emission scan at 500–600 nm (10-nm slits). An NBD-labeled peptide was added first from a stock solution in DMSO (final concentration 0.1 μm and a maximum of 0.25% (v/v) DMSO) to a dispersion of PC LUV (200 μm) in PBS. This was followed by the addition of Rho-labeled peptide (stock in DMSO) in several sequential doses, ranging from 1:20 to 1:7 Rho/NBD molar ratios. Fluorescence spectra were obtained before and after addition of the Rho labeled peptide. The fluorescence values were corrected by subtracting the corresponding blank (buffer with the same vesicles concentration).
T-cell Activation and Proliferation
Primary CD4 T-cells specific to MOG p35–55 were plated onto round 96-well plates in medium containing RPMI 1640 supplemented with 2.5% fetal calf serum (FCS), 100 units/ml penicillin, 100 μg/ml streptomycin, 50 μm β-mercaptoethanol, and 2 mm l-glutamine. Each of the 96 wells had a final volume of 200 μl and contained 2 × 104 T-cells, 5 × 105 irradiated (25 gray) antigen presenting cells from spleen, and 5 μg/ml of MOG p35–55. In addition, the relevant peptide was added at a final 50 μg/ml concentration. Each read out was made with minimum of four repeats. To exclude interaction between the examined peptides and the MOG p35–55 antigen, we initially added the MOG p35–55 antigen to the antigen presenting cells in a test tube, and in a second test tube, we added the examined peptides to the T-cells. After 1 h, we mixed the antigen presenting cells with the T-cells and incubated them for 48 h in a 96-well round bottomed plate. The T-cells were pulsed with 1 μCi (H3) thymidine, with a specific activity of 5.0 Ci/mmol for 24 h, and [H3]thymidine incorporation was measured using a 96-well plate β-counter. The mean cpm ± S.D. was calculated for each quadruplicate or more. The results of T-cell proliferation experiments are shown as the percentage of T-cell proliferation inhibition triggered by the antigen in the absence of any peptide.
Mice
C57BL/6J mice were purchased from Harlan Olac (Bicester UK). The mice were maintained in a specific pathogen-free facility and were used according to the guidelines and under the supervision of the animal welfare committee.
ATR-FTIR Spectroscopy
Spectra were obtained with a Bruker equinox 55 FTIR spectrometer, equipped with a deuterated triglyceride sulfate detector, coupled with an ATR device. For each spectrum, 150 scans were collected, with a resolution of 4 cm−1. Samples were prepared as described (41). Briefly, lipids alone or with a peptide were deposited on a ZnSe horizontal ATR prism (80 × 7 mm). Before the sample was prepared, the trifluoroacetate (CF3COO−) counterions, which strongly associate with the peptide, were replaced by chloride ions through several washings in 0.1 m HCl and lyophilization. This eliminated the strong stretching absorption band of the water near 1673 cm−1 (42). Peptides were dissolved in MeOH, which was chosen over DMSO, used in the lipid mixing experiments, as MeOH evaporates readily and is a suitable solvent for lipids. Lipids were dissolved in a 1:2 MeOH/CHCl3 mixture. Lipid-peptide mixtures at a 200:1 molar ratio or lipids alone with the corresponding volume of methanol were spread with a Teflon bar on the ZnSe prism. The solvents were eliminated by drying under vacuum for 15 min. Pure phospholipid spectra were subtracted to yield the different spectra. The background for each spectrum was a clean ZnSe prism. The samples were hydrated by introducing an excess of deuterium oxide (2H2O) into a chamber placed on top of the ZnSe prism in the ATR casting and incubating for 5 min before acquiring the spectra. Hydrogen/deuterium exchange was considered complete if the complete shift of the amide II band was achieved. Any contribution of 2H2O vapor to the absorbance spectra near the amide I peak region was eliminated by subtracting the spectra of pure lipids equilibrated with 2H2O under the same conditions.
ATR-FTIR Data Analysis
To resolve overlapping bands, we processed spectra using PEAKFIT (Jandel Scientific, San Rafael, CA) software. Fourth-derivative spectra were calculated to identify the positions of the component bands in the spectra. These wave numbers were used as initial parameters for curve fitting with Gaussian component peaks. Positions, bandwidths, and amplitudes of the peaks were varied until a good agreement between the calculated sum of all components and the experimental spectra was achieved (r2 > 0.997) under the following constraints: (i) the resulting bands shifted by no more than 2 cm−1 from the initial parameters, and (ii) all of the peaks had reasonable half-widths (<20–25 cm−1). The relative amounts of the different secondary structure elements were estimated by dividing the areas of individual peaks assigned to a particular secondary structure by the whole area of the resulting amide I band (43).
RESULTS
The AxxxG Motif Is Conserved in FP of Various HIV-1 Strains
HIV-1 FP5–13 (GALFLGFLG) adopts a β-sheet conformation and interacts with a nine-amino acid fragment of the TMD of the TCRα to exert its immunosuppressant activity (27, 28). We identified an AxxxG motif within this region, a known amino acid sequence that drives TMDs association (44). Using bioinformatics, we found that it is highly conserved within various HIV-1 clades and strains (Fig. 2). Additionally, we identified this motif in several SIV strains. HIV-1 has a high mutation rate, which is 1 in 104 to 1 in 105 nucleotide mutations per replication cycle or about one mutation per newly produced viral genome (45, 46). Therefore, the conservation of this motif suggests its significance for the various properties of the FP.
FIGURE 2.
Multiple protein sequence alignment by ClustalW2. Fusion peptides found in different HIV-1 envelope proteins were aligned, and the GxxxG-like motif (AxxxG) was identified. The Clustal approach was taken from Thompson (63) and Larkin (64).
The AxxxG Motif Is Important for the Biological Function and the Biophysical Properties of FP5–13
The GxxxG motif is mainly known as a mediator of helix-helix interaction. However, its glycine residues have been shown to facilitate the conversion to β-strand secondary structure and to mediate sheet-to-sheet packing (34). To investigate whether the AxxxG motif contributes to the FP immunosuppressant activity, we synthesized several peptides. The list, shown in Table 1, includes the following: (i) FP5–13,the wild-type peptide, (ii) FP5–13(A1G2), in which the classical GxxxG motif was created by switching the position of the first two amino acids, (iii) FP5–13(L2A3), in which the AxxxG motif was altered by switching the position of the second and third amino acids, (iv) FP5–13 (scrambled), in which the sequence was scrambled, and (v) FP5–13(Rsc) L7G8, in which the GxxxG motif was recreated by exchanging the positions of the seventh and the eighth amino acids.
TABLE 1.
Sequences, designations, and secondary structures of the peptides investigated
| Peptide designation | Sequence | β-Sheet |
|---|---|---|
| % | ||
| FP5–13 WT | GALFLGFLG | 80 |
| FP5–13 A1G2 | AGLFLGFLG | 86 |
| FP5–13 L2A3 | GLAFLGFLG | 15 |
| FP5–13 Scrambled (Scr) | AGFGLLGLF | 7 |
| FP5–13 Rescued L7G8 (Rsc) | AGFGLLLGF | 76 |
| TCRα-TMD (CP) | GLRILLLKV | α-helixa |
a Taken from Ref. 60.
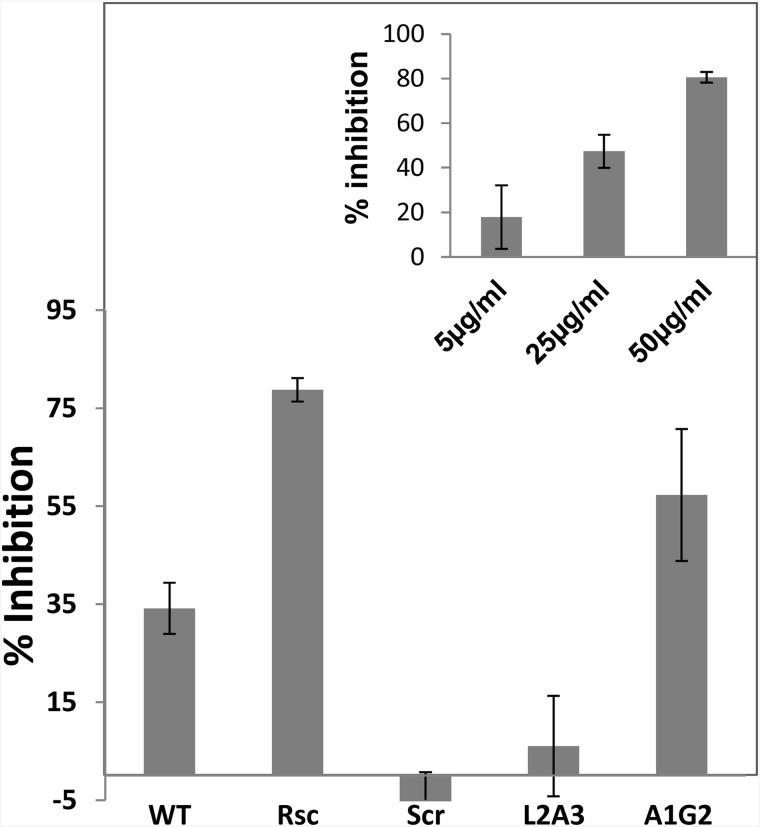
The peptides were then tested for their ability to inhibit T-cell proliferation. The data shown in Fig. 3 reveal, as expected, that the WT peptide is active. In contrast, a peptide analog, FP5–13(L2A3), in which the positions of only two amino acids were replaced to disrupt the GxxxG motif, lost its proliferation inhibitory activity (Fig. 3). Similarly, a scrambled peptide also lost its inhibitory effect, pointing to the specificity of the interaction. To further validate the importance of the GxxxG motif, two more analogs were synthesized. In the first analog, FP5–13 (rescued) L7G8, the glycine at position 7, and leucine at position 8 of the scrambled peptide, replaced positions, thus recreating a GxxxG motif, while leaving the rest of the sequence unchanged. Interestingly, the rescued analog became highly active. In the second analog, FP5–13(A1G2), the AxxxG motif was changed to a GxxxG motif by switching the positions of only Ala and Gly. This analog was ∼2-fold more active than the WT. In addition, we found that the FP of the measles virus had no immunosuppressant activity on T-cells (data not shown), further supporting the specific activity of HIV-1-FP.
FIGURE 3.
Inhibition of T-cell proliferation by HIV FP5–13 WT and its analogs. T-cells were activated with both irradiated antigen presenting cells and the MOG 35–55 peptide in the presence of the WT and its analogs, all at 50 μg/ml. The proliferative responses were assessed by [3H]thymidine incorporation and normalized to the untreated proliferated T-cells. The data are presented as mean inhibitions of three independent assays, each made with four repetitions. Inset, dose response of Rsc shown as an example.
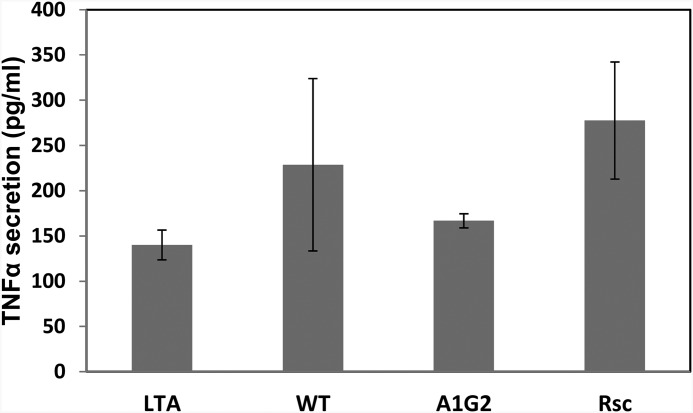
In addition to inhibition of T-cell activation, the ability of the active peptides to inhibit cells completely lacking TCR was also examined. The peptides were added to macrophages which were then activated by LTA. Fig. 4 demonstrates that the peptides had no suppressing effect on macrophages. In fact, a slight increase in activation was observed, perhaps due to the ability of macrophages to recognize foreign antigens. This result, added to previous studies of colocalization and immunoprecipitation (25–29), further demonstrates the specificity of the FP to the TCR. These results might suggest that other peptides having the GxxxG motif would be active as well. However, analogs of FP5–13 in which the motif was preserved but the two phenylalanines were replaced by glycines or leucines were also practically inactive (28). This suggests that the GxxxG motif alone is crucial but insufficient to restore the inhibitory effect of T-cells and consequently, other amino acids are required.
FIGURE 4.
The effect of HIV FP peptides on TNFα secretion from RAW264.7 macrophages. Cells were incubated with 20 μm of the indicated peptides and then washed and stimulated with high LTA concentrations (500 ng/ml). The levels of TNFα in the supernatant were assessed by ELISA after 5 h of stimulation.
Secondary Structure of the Peptides in PC Membranes Determined by FTIR Spectroscopy
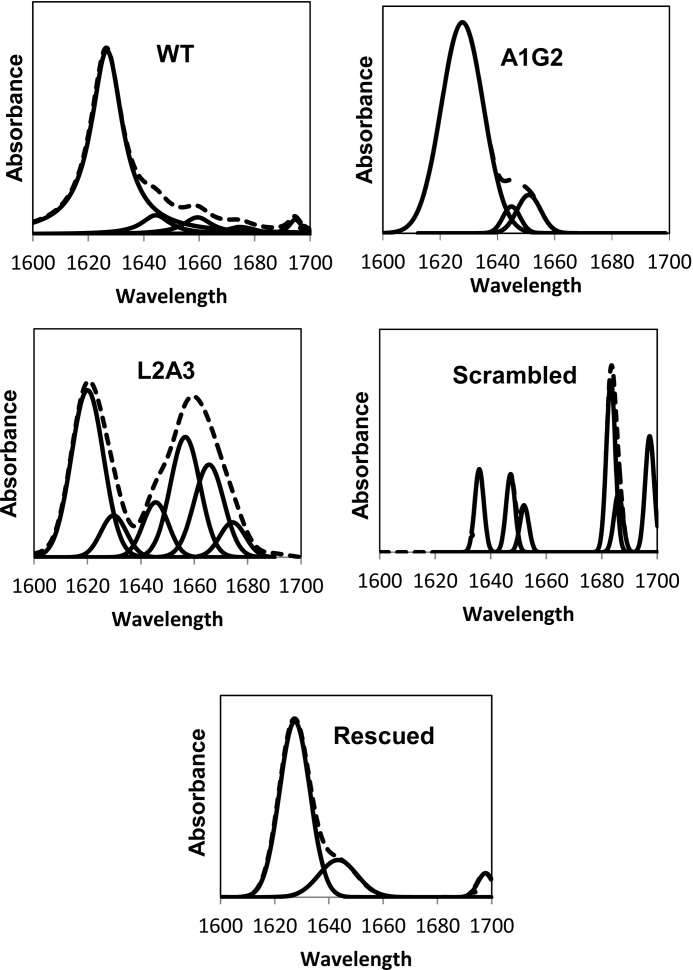
FTIR spectroscopy was used to determine the secondary structure of the peptides within PC membranes after complete deuteration. The amide I region spectra, as well as the fitted band components of the peptides bound to PC multibilayers, were determined and are shown in Fig. 5. Assignment of the different secondary structures to the various amide I regions was calculated according to the values taken from earlier studies (43, 47, 48). The relative areas of the major component peaks are summarized in Table 1. The data reveal a strong band typical for a β-sheet structure at 1620 cm−1. These results are supported by previous studies indicating that FP interacts as a β-sheet within the membrane (27, 28). Here, we show that only the three active peptides: the WT, A1G, and the rescued peptides adopt a β-sheet structure. In comparison, the non-active peptides, L2A, and the scrambled peptide, lost their β-sheet structure.
FIGURE 5.
Secondary structures of WT, A1G2, Scr, L2A3, and Rsc in PC:choline (9:1) membranes. ATR-FTIR spectra of deuterated amide I band (1600–1700) of WT, A1G2, Scr, L2A3, and Rsc peptides. Upper curves represent the experimental FTIR spectra; lower curves represent the fitted components.
The Trade Off of the Motif, Fusion versus immunosuppression
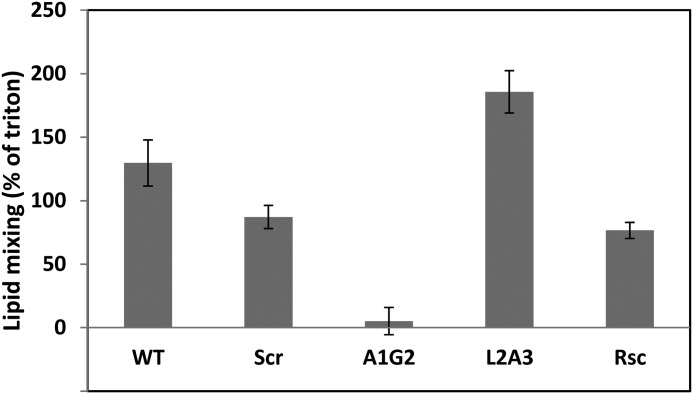
Mutations in HIV-1 are very common (45, 46). Our results indicate that the GxxxG motif is more potent than the AxxxG motif in immunosuppressant activity. This leads to the question why, given natural selection, a mutant such as A1G2 does not thrive in the population. Because the main known purpose of FP is to fuse to the target cell membrane, we examined the ability of all the peptides to induce lipid mixing (Fig. 6). The results clearly demonstrate that the WT peptide, although not the best immunosuppressant, is highly active in lipid mixing compared with the A1G2 analog, which is practically inactive. This result might explain why nature has selected the WT sequence AxxxG over GxxxG. Overall, the lipid mixing data reveal that there is no direct correlation between the immunosuppressant activity of a particular peptide and its ability to induce lipid mixing.
FIGURE 6.
Lipid mixing of PC:choline (9:1) vesicles induced by the peptides. Peptides were added to 200 μm LUV of PC:Choline (9:1) that contained unlabeled and labeled LUV at 9:1 molar ratio, respectively. Lipid mixing was monitored by measuring the increase of NBD fluorescence intensity at 0.06 [peptide]:[lipid] ratio. The ability of the WT and its analogs to induce lipid mixing was measured and compared with 2% Triton which was taken as 100%.
Heteroassembly between FP5–13 and CP Using FRET
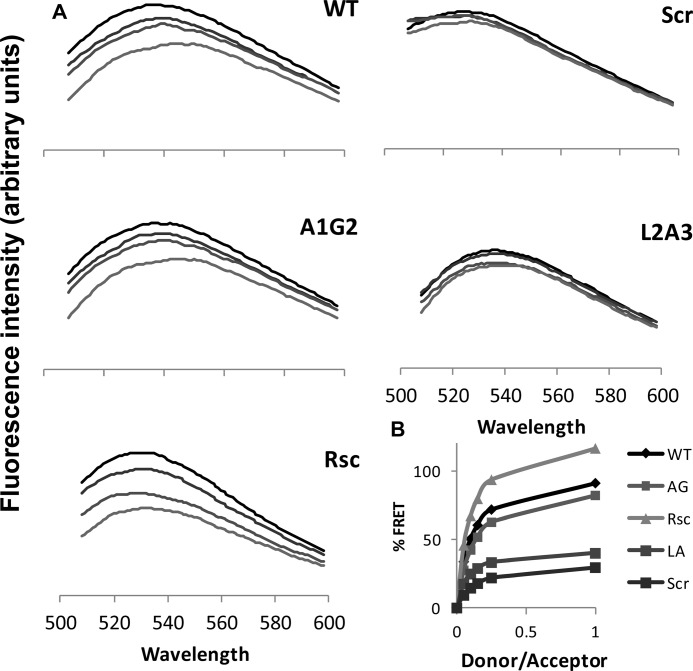
Previous studies using colocalization, immunoprecipitation, and structure-function studies, revealed that HIV-1 FP5–13 binds to CP within the TCRα (26–28). This interaction could explain the ability of FP to interfere with the functional assembly of the TCR complex. Here, we found that in addition to the WT peptide, A1G2 and the rescued peptide are also active, whereas L2A3 and the scrambled peptide lost their ability to inhibit T-cell proliferation. To verify whether the inactivity of the analogs is due to their inability to interact with CP, we performed a FRET assay between NBD-labeled FP5–13 and its analogs (which served as donors), and rhodamine-labeled CP (an acceptor). Specifically, a NBD-labeled peptide was added to PC:cholesterol (9:1) LUVs, followed by the gradual addition of Rho-labeled CP. Fig. 7 shows a reduction in the maxima of NBD fluorescence at 530 nm in different NBD:rhodamine peptide ratios. These data indicate significantly higher levels of FRET with the HIV WT, A1G2, and the rescued peptides compared with the scrambled and L2A3 derivatives (Fig. 7). This result suggests that the inability of the scrambled and L2A3 analogs to inhibit T-cell proliferation is due to lack of interaction between the peptides and their target site, the CP.
FIGURE 7.
FRET experiments using NBD-labeled FP5–13 and its derivatives serving as fluorescent donors, and Rho-labeled CP as an acceptor. A, spectra were obtained at room temperature with excitation set at 467 nm (10-nm slit) and an emission scan at 500–600 nm (10-nm slits). An NBD-labeled peptide was added to PC LUV (200 μm) in PBS to a final concentration of 0.4 μm peptide. This was followed by the addition of Rho-labeled CP peptide in several sequential doses. Fluorescence spectra were obtained first without Rho-peptide (upper curve) followed by four increasing Rho:NBD-labeled peptide ratios of 1:20 (second from top), 1:10 (third from top), and 1:7 (lower curve). B, nonlinear regression plot of FRET efficiency was determined by the relative percentage of emission at 530 nm between the NBD-labeled peptides alone and the peptides at different CP/NBD-labeled ratios.
DISCUSSION
To effectively infect the human CD4 T-cells, HIV-1 must both successfully fuse to the T-cells and escape the immune response. One possible mechanism involves a particular region in gp41, the FP (Fig. 1). The FP is known to initiate the fusion cascade of HIV, and a peptide derived from this region possesses immunosuppressant activity in cell culture and in animal models (26, 27). This activity is primarily due to the HIV-1 FP5–13 region (GALFLGFLG), which was shown to interact with the CP, a nine-amino acid TMD peptide derived from TCRα (27, 49).
In the present study, we identified a GxxxG-like motif (AxxxG) within FP5–13 by aligning FPs from different HIV-1 clades and strains. This region is highly conserved within all of these strains (Fig. 2). Note that the GxxxG-like motif is well known for its ability to drive TMD association (44). Because HIV-1 has about one mutation for each newly produced viral genome (45, 46), the conservation of the motif suggests that it plays a role in the function of the FP. Indeed, this study provides experimental evidence for the contribution of the GxxxG-like motif to the immunosuppressant activity of the HIV FP5–13. For that purpose, we synthesized the HIV FP5–13 and its analogs, in which the GxxxG-like motif was altered (32, 35, 36). The structures of the peptides in the membrane were determined by using FTIR spectroscopy, and their function was examined using the CD4+ T-cell proliferation assay. In addition, the FP of the measles virus was used as a control of an unrelated FP, and LTA-activated macrophages were used as a control of cells that do not have TCR.
Previous studies have shown that the structure of HIV FP is either an α-helix or a β-sheet, depending on its length and the type of the membrane environment used (50). Here, similarly to previous studies of experimental and molecular dynamic simulations (27, 28, 51, 52), the structure of FP5–13 in zwitterionic membranes was found to be a β-sheet. Importantly, the data of the present study reveal that the AxxxG/GxxxG motif contributes to both the immunosuppressant activity of the peptides and their β-sheet structure in membranes. Peptides with altered GxxxG motifs lost the native β-sheet structure within the membrane, whereas peptides containing the motif retained a high-content of β-sheet structure (Fig. 5). Moreover, the ability to interact with CP and to inhibit the proliferation of CD4 T-cells was also abolished in those peptides with altered GxxxG motifs (Figs. 3 and 7). Interestingly, mutating one of these inactive analogs by exchanging the positions of only two adjacent amino acids to create the GxxxG motif restored both the β-sheet structure of the peptide and its ability to suppress T-cell activation. However, the GxxxG analog, while more effective in T-cell immunosuppression, is practically inactive in lipid mixing activity. This is in contrast with the AxxxG WT which has both immunosuppression and lipid mixing functions. These results suggest that natural selection has screened the AxxxG motif, which contains both the lipid mixing and immunosuppression functions.
Although the GxxxG motif is crucial for the function of FP, other amino acids are also required. For example, it has been previously demonstrated that FPs in which the aromatic amino acids were mutated to non-aromatic residues significantly reduced their immunosuppressant activity (28).
Recent studies have shown that FP is not the only region within gp41 that contains a functional GxxxG motif. gp41 TMD (FIMIVGGLVGLRIVFAVLSIV) is also highly conserved within different strains of HIV (53) and was suggested to play several roles during HIV-1 cell fusion, including anchoring the envelope glycoprotein to both viral and cellular membranes (54), assisting in the oligomerization of gp41 (55), inducing phospholipid vesicle fusion (55, 56), promoting cell-cell fusion (57, 58), and immunosuppressing T-cell activation (30). Importantly, experiments performed with the intact virus demonstrated that a mutant in which the GxxxG motif within the TMD in the HXB2 envelope was altered, was defective in fusion (58). Furthermore, the FP and the TMD of HIV-1 heteroassemble in the membrane and synergize in inducing membrane fusion (55). A similar observation was previously demonstrated in the influenza virus, in which TMD-FP interactions were shown to play a key role in the fusion cascade (59). In HIV-1, in addition to their role in membrane fusion, both the TMD and the FP domains possess immunosuppressant activity (26, 30).
In summary, combining bioinformatics and experimental studies, we identified a conserved GxxxG-like motif (AxxxG) within HIV-1 FP, which is crucial for its immunosuppressive activity possibly via interaction with the TMD of TCRα. In contrast, LTA activated macrophages (lacking TCR) were not affected by FP. Furthermore, although the AxxxG WT and the GxxxG analog both associated with the TMD of TCR and immunosuppressed T-cells, the AxxxG WT but not the GxxxG analog induced lipid mixing. This might explain the natural selection of the AxxxG motif rather than the classical GxxxG motif in FP. Besides giving us important mechanistic information, such short peptides can serve as templates for the design of immunosuppressive drugs urgently needed for various autoimmune diseases.
Acknowledgment
The authors thank Debby Abramov for helpful assistance.
This work was supported by the Israel Science Foundation.
- FP
- fusion peptide
- CP
- core peptide
- TMD
- transmembrane domain
- TCR
- T-cell receptor
- LTA
- lipotechoic acid
- ATR
- attenuated total reflectance
- Fmoc
- 9-fluorenylmethoxycarbonyl
- PC
- phosphatidylcholine
- NBD-F
- 4-chloro-7-nitrobenz-2-oxa-1,3-diazole fluoride
- Rho-N
- rhodamine-N-hydroxysuccinimide
- MOG
- myelin oligodendrocyte glycoprotein
- DMSO
- dimethyl sulfoxide
- DMF
- dimethyl formamide
- LUV
- large unilamellar vesicles.
REFERENCES
- 1. Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C., Rozenbaum W., Montagnier L. (1983) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 [DOI] [PubMed] [Google Scholar]
- 2. Gallo R. C., Salahuddin S. Z., Popovic M., Shearer G. M., Kaplan M., Haynes B. F., Palker T. J., Redfield R., Oleske J., Safai B. (1984) Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224, 500–503 [DOI] [PubMed] [Google Scholar]
- 3. Maltêz F., Doroana M., Branco T., Valente C. (2011) Recent advances in antiretroviral treatment and prevention in HIV-infected patients. Curr. Opin. HIV AIDS 6, S21–30 [DOI] [PubMed] [Google Scholar]
- 4. Alkhatib G., Combadiere C., Broder C. C., Feng Y., Kennedy P. E., Murphy P. M., Berger E. A. (1996) CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 [DOI] [PubMed] [Google Scholar]
- 5. Wilkin T. J., Gulick R. M. (2012) CCR5 Antagonism in HIV Infection: Current Concepts and Future Opportunities. Annu. Rev. Med. 63, 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. (1987) Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science 237, 1351–1355 [DOI] [PubMed] [Google Scholar]
- 7. Veronese F. D., DeVico A. L., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. (1985) Characterization of gp41 as the transmembrane protein coded by the HTLV-III/LAV envelope gene. Science 229, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 8. Wyatt R., Kwong P. D., Desjardins E., Sweet R. W., Robinson J., Hendrickson W. A., Sodroski J. G. (1998) The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393, 705–711 [DOI] [PubMed] [Google Scholar]
- 9. Chan D. C., Kim P. S. (1998) HIV entry and its inhibition. Cell 93, 681–684 [DOI] [PubMed] [Google Scholar]
- 10. Weissenhorn W., Dessen A., Calder L. J., Harrison S. C., Skehel J. J., Wiley D. C. (1999) Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16, 3–9 [DOI] [PubMed] [Google Scholar]
- 11. Durell S. R., Martin I., Ruysschaert J. M., Shai Y., Blumenthal R. (1997) What studies of fusion peptides tell us about viral envelope glycoprotein-mediated membrane fusion (review) Mol. Membr. Biol. 14, 97–112 [DOI] [PubMed] [Google Scholar]
- 12. Blumenthal R., Schoch C., Puri A., Clague M. J. (1991) A dissection of steps leading to viral envelope protein-mediated membrane fusion. Ann. N.Y. Acad. Sci. 635, 285–296 [DOI] [PubMed] [Google Scholar]
- 13. Suárez T., Nir S., Goñi F. M., Saéz-Cirión A., Nieva J. L. (2000) The pre-transmembrane region of the human immunodeficiency virus type-1 glycoprotein: A novel fusogenic sequence. FEBS Lett. 477, 145–149 [DOI] [PubMed] [Google Scholar]
- 14. Peisajovich S. G., Shai Y. (2003) Viral fusion proteins: Multiple regions contribute to membrane fusion. Biochim. Biophys. Acta 1614, 122–129 [DOI] [PubMed] [Google Scholar]
- 15. White J. M. (1992) Membrane fusion. Science 258, 917–924 [DOI] [PubMed] [Google Scholar]
- 16. Chan D. C., Fass D., Berger J. M., Kim P. S. (1997) Core structure of gp41 from the HIV envelope glycoprotein. Cell 89, 263–273 [DOI] [PubMed] [Google Scholar]
- 17. Chirmule N., McCloskey T. W., Hu R., Kalyanaraman V. S., Pahwa S. (1995) HIV gp120 inhibits T-cell activation by interfering with expression of costimulatory molecules CD40 ligand and CD80 (B71) J. Immunol. 155, 917–924 [PubMed] [Google Scholar]
- 18. Masci A. M., Galgani M., Cassano S., De Simone S., Gallo A., De Rosa V., Zappacosta S., Racioppi L. (2003) HIV-1 gp120 induces anergy in naive T lymphocytes through CD4-independent protein kinase A-mediated signaling. J. Leukoc. Biol. 74, 1117–1124 [DOI] [PubMed] [Google Scholar]
- 19. Fernando K., Hu H., Ni H., Hoxie J. A., Weissman D. (2007) Vaccine-delivered HIV envelope inhibits CD4+ T-cell activation, a mechanism for poor HIV vaccine responses. Blood 109, 2538–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang R., Lifson J. D., Chougnet C. (2006) Failure of HIV-exposed CD4+ T-cells to activate dendritic cells is reversed by restoration of CD40/CD154 interactions. Blood 107, 1989–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donaghy H., Stebbing J., Patterson S. (2004) Antigen presentation and the role of dendritic cells in HIV. Curr. Opin. Infect. Dis. 17, 1–6 [DOI] [PubMed] [Google Scholar]
- 22. Ruegg C. L., Monell C. R., Strand M. (1989) Inhibition of lymphoproliferation by a synthetic peptide with sequence identity to gp41 of human immunodeficiency virus type 1. J. Virol. 63, 3257–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruegg C. L., Strand M. (1990) Inhibition of protein kinase C and anti-CD3-induced Ca2+ influx in Jurkat T-cells by a synthetic peptide with sequence identity to HIV-1 gp41. J. Immunol. 144, 3928–3935 [PubMed] [Google Scholar]
- 24. Ruegg C. L., Strand M. (1991) A synthetic peptide with sequence identity to the transmembrane protein GP41 of HIV-1 inhibits distinct lymphocyte activation pathways dependent on protein kinase C and intracellular calcium influx. Cell. Immunol. 137, 1–13 [DOI] [PubMed] [Google Scholar]
- 25. Haynes B. F., Arthur L. O., Frost P., Matthews T. J., Langlois A. J., Palker T. J., Hart M. K., Scearce R. M., Jones D. M., McDanal C. (1993) Conversion of an immunogenic human immunodeficiency virus (HIV) envelope synthetic peptide to a tolerogen in chimpanzees by the fusogenic domain of HIV gp41 envelope protein. J. Exp. Med. 177, 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quintana F. J., Gerber D., Kent S. C., Cohen I. R., Shai Y. (2005) HIV-1 fusion peptide targets the TCR and inhibits antigen-specific T-cell activation. J. Clin. Invest. 115, 2149–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloch I., Quintana F. J., Gerber D., Cohen T., Cohen I. R., Shai Y. (2007) T-cell inactivation and immunosuppressive activity induced by HIV gp41 via novel interacting motif. FASEB J. 21, 393–401 [DOI] [PubMed] [Google Scholar]
- 28. Cohen T., Pevsner-Fischer M., Cohen N., Cohen I. R., Shai Y. (2008) Characterization of the interacting domain of the HIV-1 fusion peptide with the transmembrane domain of the T-cell receptor. Biochemistry 47, 4826–4833 [DOI] [PubMed] [Google Scholar]
- 29. Sigalov A. B. (2009) Novel mechanistic insights into viral modulation of immune receptor signaling. PLoS Pathog. 5, e1000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen T., Cohen S. J., Antonovsky N., Cohen I. R., Shai Y. (2010) HIV-1 gp41 and TCRalpha trans-membrane domains share a motif exploited by the HIV virus to modulate T-cell proliferation. PLoS Pathog. 6, e1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Senes A., Ubarretxena-Belandia I., Engelman D. M. (2001) The Cα–HO hydrogen bond: A determinant of stability and specificity in transmembrane helix interactions. Proc. Natl. Acad. Sci. U.S.A. 98, 9056–9061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senes A., Gerstein M., Engelman D. M. (2000) Statistical analysis of amino acid patterns in transmembrane helices: The GxxxG motif occurs frequently and in association with β-branched residues at neighboring positions. J. Mol. Biol. 296, 921–936 [DOI] [PubMed] [Google Scholar]
- 33. Fink A., Sal-Man N., Gerber D., Shai Y. (2012) Transmembrane domains interactions within the membrane milieu: Principles, advances, and challenges. Biochim. Biophys. Acta 1818, 974–983 [DOI] [PubMed] [Google Scholar]
- 34. Liu W., Crocker E., Zhang W., Elliott J. I., Luy B., Li H., Aimoto S., Smith S. O. (2005) Structural role of glycine in amyloid fibrils formed from transmembrane α-helices. Biochemistry 44, 3591–3597 [DOI] [PubMed] [Google Scholar]
- 35. Senes A., Engel D. E., DeGrado W. F. (2004) Folding of helical membrane proteins: The role of polar, GxxxG-like, and proline motifs. Curr. Opin. Struct. Biol. 14, 465–479 [DOI] [PubMed] [Google Scholar]
- 36. Walters R. F., DeGrado W. F. (2006) Helix-packing motifs in membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 103, 13658–13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unterreitmeier S., Fuchs A., Schäffler T., Heym R. G., Frishman D., Langosch D. (2007) Phenylalanine promotes interaction of transmembrane domains via GxxxG motifs. J. Mol. Biol. 374, 705–718 [DOI] [PubMed] [Google Scholar]
- 38. Kliger Y., Aharoni A., Rapaport D., Jones P., Blumenthal R., Shai Y. (1997) Fusion peptides derived from the HIV type 1 glycoprotein 41 associate within phospholipid membranes and inhibit cell-cell fusion. Structure-function study. J. Biol. Chem. 272, 13496–13505 [DOI] [PubMed] [Google Scholar]
- 39. Gerber D., Shai Y. (2000) Insertion and organization within membranes of the δ-endotoxin pore-forming domain, helix 4-loop-helix 5, and inhibition of its activity by a mutant helix 4 peptide. J. Biol. Chem. 275, 23602–23607 [DOI] [PubMed] [Google Scholar]
- 40. Struck D. K., Hoekstra D., Pagano R. E. (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry 20, 4093–4099 [DOI] [PubMed] [Google Scholar]
- 41. Gazit E., Miller I. R., Biggin P. C., Sansom M. S., Shai Y. (1996) Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J. Mol. Biol. 258, 860–870 [DOI] [PubMed] [Google Scholar]
- 42. Surewicz W. K., Mantsch H. H., Chapman D. (1993) Determination of protein secondary structure by Fourier transform infrared spectroscopy: A critical assessment. Biochemistry 32, 389–394 [DOI] [PubMed] [Google Scholar]
- 43. Jackson M., Mantsch H. H. (1995) The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 30, 95–120 [DOI] [PubMed] [Google Scholar]
- 44. Escher C., Cymer F., Schneider D. (2009) Two GxxxG-like motifs facilitate promiscuous interactions of the human ErbB transmembrane domains. J. Mol. Biol. 389, 10–16 [DOI] [PubMed] [Google Scholar]
- 45. Coffin J. M. (1995) HIV population dynamics in vivo: Implications for genetic variation, pathogenesis, and therapy. Science 267, 483–489 [DOI] [PubMed] [Google Scholar]
- 46. Mansky L. M., Temin H. M. (1995) Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 69, 5087–5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frey S., Tamm L. K. (1991) Orientation of melittin in phospholipid bilayers. A polarized attenuated total reflection infrared study. Biophys. J. 60, 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oren Z., Hong J., Shai Y. (1999) A comparative study on the structure and function of a cytolytic α-helical peptide and its antimicrobial β-sheet diastereomer. Eur. J. Biochem. 259, 360–369 [DOI] [PubMed] [Google Scholar]
- 49. Wang X. M., Djordjevic J. T., Bender V., Manolios N. (2002) T-cell antigen receptor (TCR) transmembrane peptides colocalize with TCR, not lipid rafts, in surface membranes. Cell. Immunol. 215, 12–19 [DOI] [PubMed] [Google Scholar]
- 50. Gorry P. R., Ancuta P. (2011) Coreceptors and HIV-1 pathogenesis. Curr. HIV/AIDS Rep. 8, 45–53 [DOI] [PubMed] [Google Scholar]
- 51. Sackett K., Wexler-Cohen Y., Shai Y. (2006) Characterization of the HIV N-terminal fusion peptide-containing region in context of key gp41 fusion conformations. J. Biol. Chem. 281, 21755–21762 [DOI] [PubMed] [Google Scholar]
- 52. Grasnick D., Sternberg U., Strandberg E., Wadhwani P., Ulrich A. S. (2011) Irregular structure of the HIV fusion peptide in membranes demonstrated by solid-state NMR and MD simulations. Eur. Biophys. J. 40, 529–543 [DOI] [PubMed] [Google Scholar]
- 53. Shang L., Yue L., Hunter E. (2008) Role of the membrane-spanning domain of human immunodeficiency virus type 1 envelope glycoprotein in cell-cell fusion and virus infection. J. Virol. 82, 5417–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gabuzda D., Olshevsky U., Bertani P., Haseltine W. A., Sodroski J. (1991) Identification of membrane anchorage domains of the HIV-1 gp160 envelope glycoprotein precursor. J. Acquir. Immune Defic. Syndr. 4, 34–40 [PubMed] [Google Scholar]
- 55. Reuven E. M., Dadon Y., Viard M., Manukovsky N., Blumenthal R., Shai Y. (2012) HIV-1 gp41 transmembrane domain interacts with the fusion peptide: Implication in lipid mixing and inhibition of virus-cell fusion. Biochemistry 51, 2867–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moreno M. R., Giudici M., Villalain J. (2006) The membranotropic regions of the endo and ecto domains of HIV gp41 envelope glycoprotein. Biochim. Biophys. Acta 1758:111–23 [DOI] [PubMed] [Google Scholar]
- 57. Miyauchi K., Komano J., Yokomaku Y., Sugiura W., Yamamoto N., Matsuda Z. (2005) Role of the specific amino acid sequence of the membrane-spanning domain of human immunodeficiency virus type 1 in membrane fusion. J. Virol. 79, 4720–4729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Miyauchi K., Curran A. R., Long Y., Kondo N., Iwamoto A., Engelman D. M., Matsuda Z. (2010) The membrane-spanning domain of gp41 plays a critical role in intracellular trafficking of the HIV envelope protein. Retrovirology 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang D. K., Cheng S. F., Kantchev E. A., Lin C. H., Liu Y. T. (2008) Membrane interaction and structure of the transmembrane domain of influenza hemagglutinin and its fusion peptide complex. BMC Biol. 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manolios N., Ali M., Amon M., Bender V. (2008) Therapeutic application of transmembrane T and natural killer cell receptor peptides. Adv. Exp. Med. Biol. 640, 208–219 [DOI] [PubMed] [Google Scholar]
- 61. Ashkenazi A., Shai Y. (2011) Insights into the mechanism of HIV-1 envelope induced membrane fusion as revealed by its inhibitory peptides. Eur. Biophys. J. 40, 349–357 [DOI] [PubMed] [Google Scholar]
- 62. Buzon V., Natrajan G., Schibli D., Campelo F., Kozlov M. M., Weissenhorn W. (2010) Crystal structure of HIV-1 gp41 including both fusion peptide and membrane proximal external regions. PLoS Pathog. 6, e1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thompson J. D., Gibson T. J., Higgins D. G. (2002) Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinformatics Chapter 2, Unit 2.3 [DOI] [PubMed] [Google Scholar]
- 64. Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., Thompson J. D., Gibson T. J., Higgins D. G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 [DOI] [PubMed] [Google Scholar]