Abstract
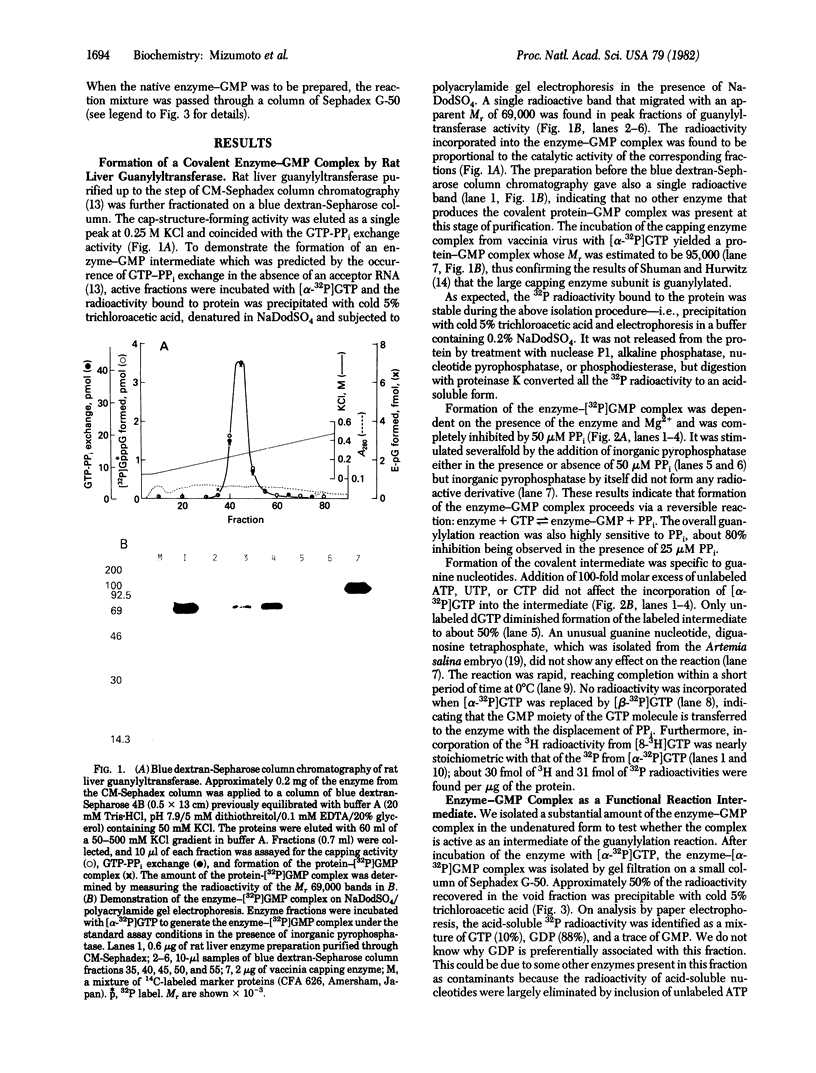
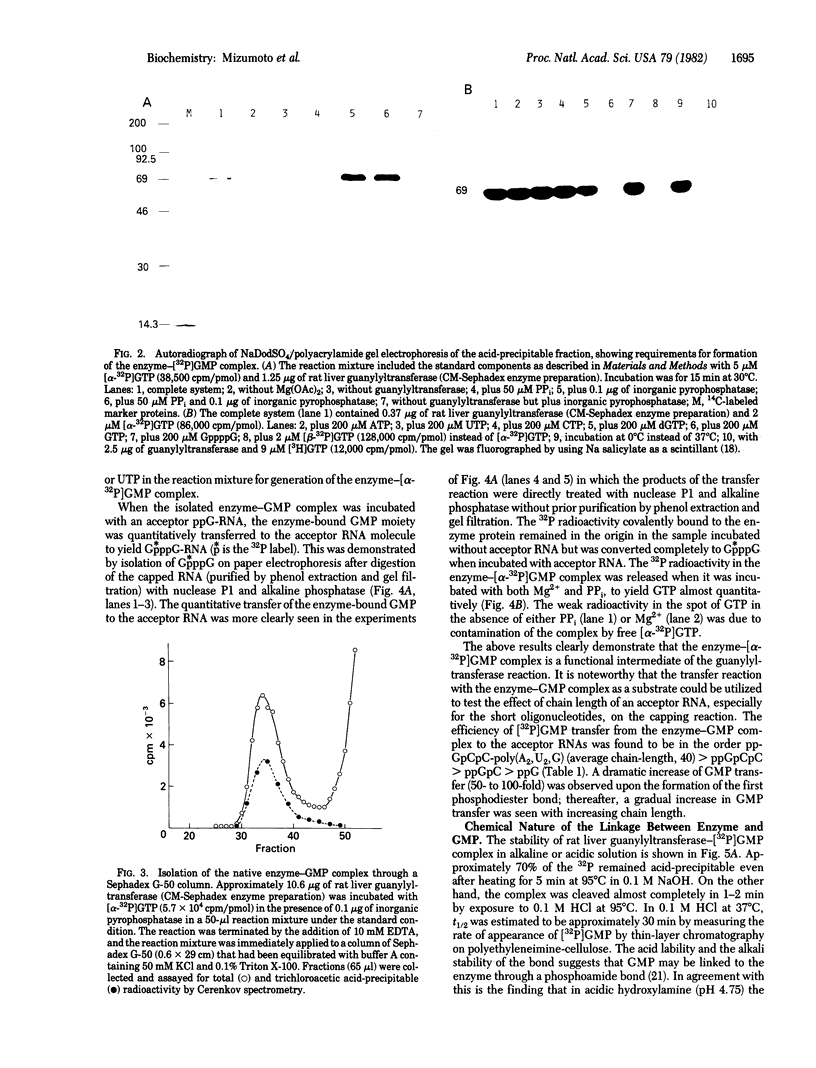
Rat liver RNA guanylyltransferase catalyzes a GTP-PPi exchange reaction in the absence of acceptor RNA [Mizumoto, K. & Lipmann, F. (1979) Proc. Natl. Acad. Sci. USA 76, 4961-4965] suggesting that the reaction proceeds through the formation of a covalent guanylylated intermediate. We now present more direct evidence for the existence of the enzyme-GMP intermediate: (i) the enzyme-[32P]GMP intermediate was formed on incubation of rat liver guanylyltransferase with [alpha-32P]GTP and migrated as a single radioactive band with Mr 69,000 on NaDodSO4/polyacrylamide gel electrophoresis, and (ii) the intermediate isolated on gel filtration can transfer its GMP moiety to ppGpCpC-poly(A2,U2,G) to form the capped RNA molecule or it can react with PPi to regenerate GTP. The formation of the intermediate was dependent on Mg2+ and was strongly inhibited by PPi. The addition of pyrophosphatase markedly increased the amount of the intermediate complex. On blue dextran-Sepharose affinity column chromatography, the activity of guanylyltransferase to form an enzyme-[32P]GMP intermediate comigrated with activities of cap formation and GTP-PPi exchange. A phosphoamide type linkage between GMP and enzyme is suggested by its acidlabile and alkali-stable nature and also by the susceptibility to acidic hydroxylamine. These results indicate that the reaction catalyzed by rat liver guanylyltransferase occurs through the following two partial steps: (i) E + GTP in equilibrium E-pG + PPi; and (ii) E-pG + ppN .....leads to GpppN .....+ E.
Full text
PDF
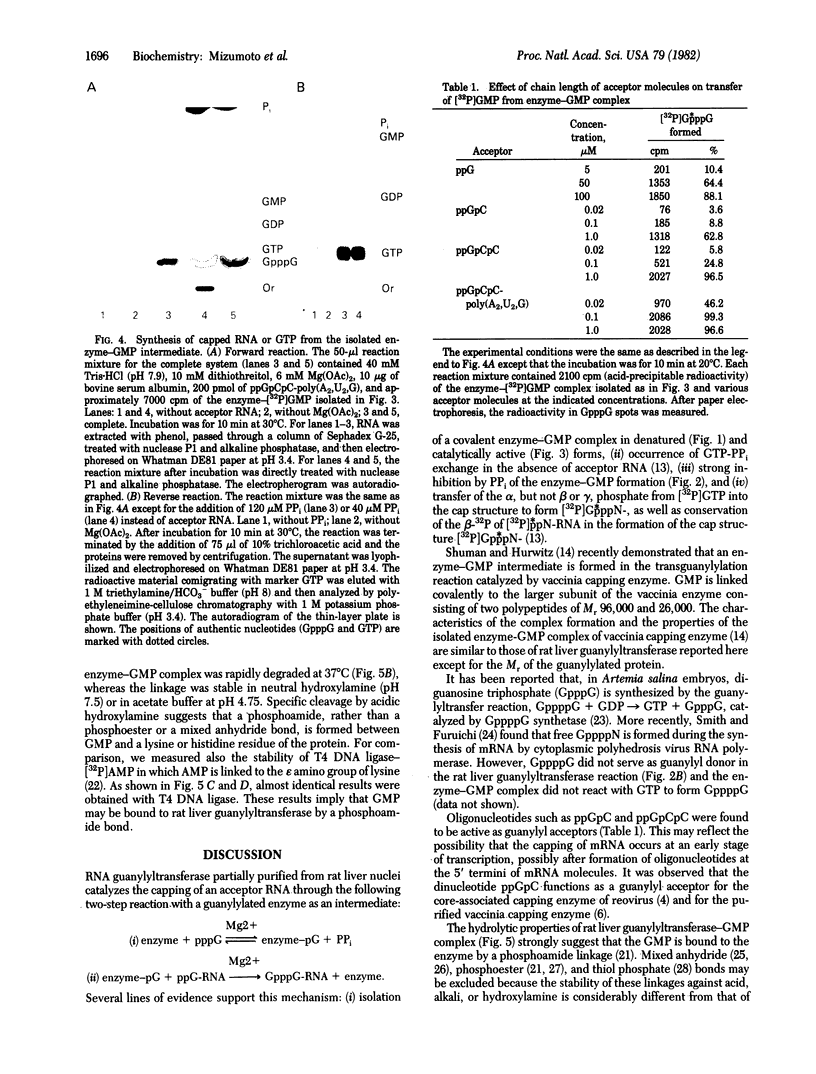
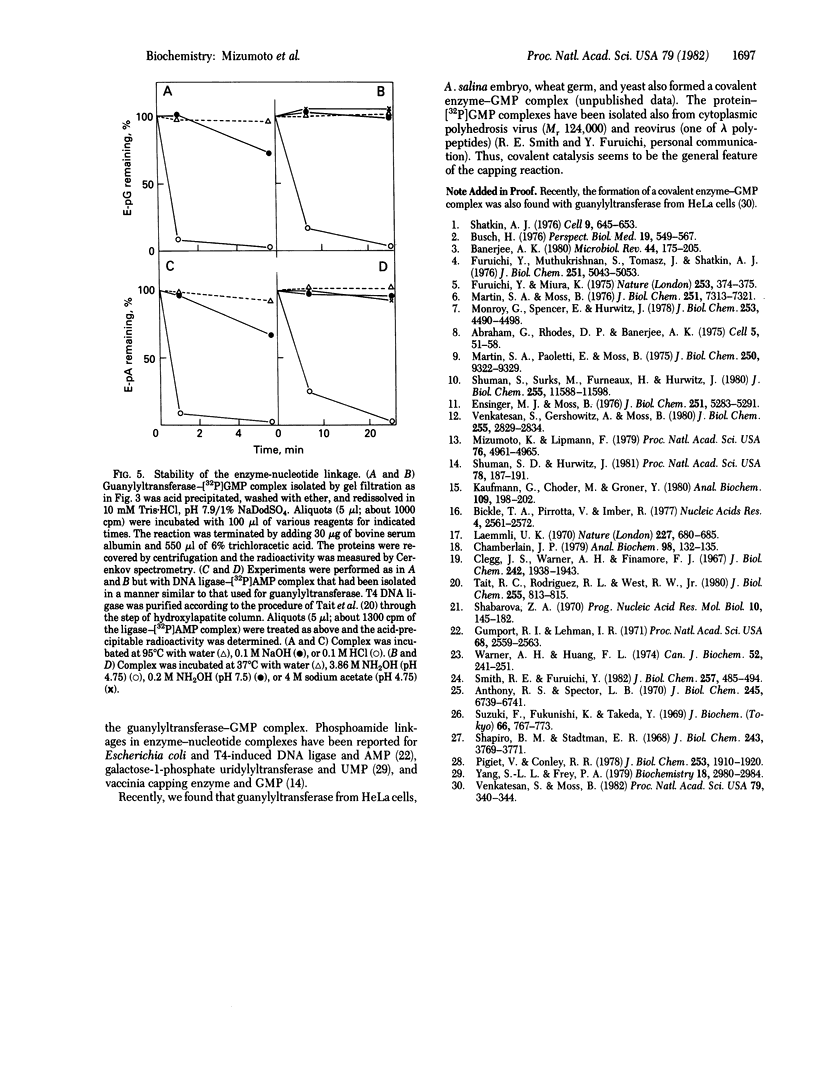
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Anthony R. S., Spector L. B. A phosphoenzyme intermediary in acetate kinase action. J Biol Chem. 1970 Dec 25;245(24):6739–6741. [PubMed] [Google Scholar]
- Banerjee A. K. 5'-terminal cap structure in eucaryotic messenger ribonucleic acids. Microbiol Rev. 1980 Jun;44(2):175–205. doi: 10.1128/mr.44.2.175-205.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch H. The function of the 5' cap of mRNA and nuclear RNA species. Perspect Biol Med. 1976 Summer;19(4):549–567. doi: 10.1353/pbm.1976.0064. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clegg J. S., Warner A. H., Finamore F. J. Evidence for the function of P-1, P-4-diguanosine 5'-tetraphosphate in the development of Artemia salina. J Biol Chem. 1967 Apr 25;242(8):1938–1943. [PubMed] [Google Scholar]
- Ensinger M. J., Moss B. Modification of the 5' terminus of mRNA by an RNA (guanine-7-)-methyltransferase from HeLa cells. J Biol Chem. 1976 Sep 10;251(17):5283–5291. [PubMed] [Google Scholar]
- Furuichi Y., Miura K. A blocked structure at the 5' terminus of mRNA from cytoplasmic polyhedrosis virus. Nature. 1975 Jan 31;253(5490):374–375. doi: 10.1038/253374a0. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Muthukrishnan S., Tomasz J., Shatkin A. J. Mechanism of formation of reovirus mRNA 5'-terminal blocked and methylated sequence, m7GpppGmpC. J Biol Chem. 1976 Aug 25;251(16):5043–5053. [PubMed] [Google Scholar]
- Gumport R. I., Lehman I. R. Structure of the DNA ligase-adenylate intermediate: lysine (epsilon-amino)-linked adenosine monophosphoramidate. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2559–2563. doi: 10.1073/pnas.68.10.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G., Choder M., Groner Y. Synthesis of carrier-free beta-32P-nucleosides-triphosphate in almost quantitative yields. Anal Biochem. 1980 Nov 15;109(1):198–202. doi: 10.1016/0003-2697(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976 Dec 10;251(23):7313–7321. [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Mizumoto K., Lipmann F. Transmethylation and transguanylylation in 5'-RNA capping system isolated from rat liver nuclei. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4961–4965. doi: 10.1073/pnas.76.10.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Spencer E., Hurwitz J. Characteristics of reactions catalyzed by purified guanylyltransferase from vaccinia virus. J Biol Chem. 1978 Jun 25;253(12):4490–4498. [PubMed] [Google Scholar]
- Pigiet V., Conley R. R. Isolation and characterization of phosphothioredoxin from Excherichia coli. J Biol Chem. 1978 Mar 25;253(6):1910–1920. [PubMed] [Google Scholar]
- Shabarova Z. A. Synthetic nucleotide-peptides. Prog Nucleic Acid Res Mol Biol. 1970;10:145–182. doi: 10.1016/s0079-6603(08)60564-4. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Shuman S., Hurwitz J. Mechanism of mRNA capping by vaccinia virus guanylyltransferase: characterization of an enzyme--guanylate intermediate. Proc Natl Acad Sci U S A. 1981 Jan;78(1):187–191. doi: 10.1073/pnas.78.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S., Surks M., Furneaux H., Hurwitz J. Purification and characterization of a GTP-pyrophosphate exchange activity from vaccinia virions. Association of the GTP-pyrophosphate exchange activity with vaccinia mRNA guanylyltransferase . RNA (guanine-7-)methyltransferase complex (capping enzyme). J Biol Chem. 1980 Dec 10;255(23):11588–11598. [PubMed] [Google Scholar]
- Smith R. E., Furuichi Y. A unique class of compound, guanosine-nucleoside tetraphosphate G(5')pppp(5')N, synthesized during the in vitro transcription of cytoplasmic polyhedrosis virus of Bombyx mori. Structural determination and mechanism of formation. J Biol Chem. 1982 Jan 10;257(1):485–494. [PubMed] [Google Scholar]
- Suzuki F., Fukunishi K., Takeda Y. Studies on ATP citrate lyase of rat liver. V. The binding site of phosphate. J Biochem. 1969 Dec;66(6):767–774. doi: 10.1093/oxfordjournals.jbchem.a129206. [DOI] [PubMed] [Google Scholar]
- Tait R. C., Rodriguez R. L., West R. W., Jr The rapid purification of T4 DNA ligase from a lambda T4 lig lysogen. J Biol Chem. 1980 Feb 10;255(3):813–815. [PubMed] [Google Scholar]
- Venkatesan S., Gershowitz A., Moss B. Purification and characterization of mRNA guanylyltransferase from HeLa cell nuclei. J Biol Chem. 1980 Apr 10;255(7):2829–2834. [PubMed] [Google Scholar]
- Venkatesan S., Moss B. Eukaryotic mRNA capping enzyme-guanylate covalent intermediate. Proc Natl Acad Sci U S A. 1982 Jan;79(2):340–344. doi: 10.1073/pnas.79.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. H., Huang F. L. Biosynthesis of the diguanosine nucleotides. II. Mechanism of action of GTP:GTP guanylyltransferase on nucleotide metabolism in brine shrimp embryos. Can J Biochem. 1974 Mar;52(3):241–251. doi: 10.1139/o74-037. [DOI] [PubMed] [Google Scholar]
- Yang S. L., Frey P. A. Nucleophile in the active site of Escherichia coli galactose-1-phosphate uridylyltransferase: degradation of the uridylyl-enzyme intermediate to N3-phosphohistidine. Biochemistry. 1979 Jul 10;18(14):2980–2984. doi: 10.1021/bi00581a011. [DOI] [PubMed] [Google Scholar]