Background: The molecular mechanisms associated with insulin-induced chondrocyte differentiation are unknown.
Results: Insulin-induced chondrocyte differentiation occurred with an early accumulation of O-linked N-acetylglucosamine-modified proteins. O-GlcNAcylation accumulation per se led to chondrocyte differentiation in vitro and in vivo.
Conclusion: O-GlcNAcylation induces chondrocyte differentiation.
Significance: This is the first evidence of O-GlcNAcylation regulation of chondrogenic differentiation.
Keywords: Cell Differentiation, Chondrocytes, Chondrogenesis, Insulin, O-GlcNAcylation, Endochondral Ossification
Abstract
Insulin is an inducer of chondrocyte hypertrophy and growth plate chondrogenesis, although the specific molecular mechanisms behind these effects are mostly unknown. Our aim was to investigate whether insulin-induced chondrocyte hypertrophy occurs through a modification in the amount of O-linked N-acetylglucosamine (O-GlcNAc)-modified proteins and in the expression of the key enzymes of this pathway, O-GlcNAc transferase and O-GlcNAcase (OGA). We also studied if O-GlcNAc accumulation per se, induced by an OGA inhibitor, was able to induce pre-hypertrophic chondrocyte differentiation both in vitro and in vivo. Insulin-induced differentiation of ATDC5 pre-chondrocytes occurred alongside a gradual increase in the accumulation of O-GlcNac-modified proteins (O-GlcNAcylated proteins), as well as an increase in the expression of O-GlcNAc transferase and OGA. In the absence of insulin, O-GlcNAc accumulation induced by thiamet-G, a specific OGA inhibitor, was able to increase the gene expression of differentiation markers, as well as the activity of MMP-2 and -9. Thiamet-G also activated pERK, p-JNK, and p-p38 and the O-GlcNAcylation of Akt. Thiamet-G administration to C57/bl mice induced a significant expansion in the growth plate height and in the hypertrophic zone height. Therefore, our results show that O-GlcNAc glycosylation has chondromodulating activity.
Introduction
Skeletal growth is achieved by endochondral ossification (EO)2 in the growth plate cartilage during the firsts stages of life (1). EO is initiated by the condensation and differentiation of mesenchymal progenitor cells into chondrocytes. The cascade of chondrogenic differentiation events sequentially includes cell proliferation, extracellular matrix synthesis, cellular hypertrophy, matrix mineralization, vascular invasion, and eventually apoptosis, which allow cartilage remodeling into bone (1). Chondrocyte hypertrophy plays a pivotal role in coordinating both chondrogenesis and osteogenesis, as they provide a scaffold for bone formation and secrete different mediators that control the activity of other cells involved in EO. Numerous skeletal diseases are caused by cellular proliferation dysfunction and hypertrophic chondrocyte differentiation, such as a large number of skeletal dysplasias (2, 3).
The regulation of the processes of EO in the growth plate occurs through tight interactions between circulating systemic hormones and locally produced growth factors. Among them, insulin and its structural and functional analog insulin growth factor-I (IGF-I) are strong stimulators of chondrogenesis and EO (4, 5). IGF-I null mice have a reduced growth plate height (6). Insulin treatment has been shown to ameliorate impaired bone growth and impaired bone healing both in vitro and in vivo (5, 7–9). However, little is known about the specific molecular mechanisms responsible for the insulin-mediated induction of the chondrogenic differentiation.
The incorporation of O-linked N-acetylglycosamine (O-GlcNAc) moieties is the major glycosylation type found within the cytosolic and nuclear compartments of eukaryotic cells, and it constitutes a dynamic and reversible mechanism of post-translational modification of proteins (10–15). The monosaccharide N-acetylglucosamine is linked to serine or threonine residues by the O-GlcNAc transferase (OGT) (11), which uses UDP-GlcNAc as a donor. O-GlcNAc can be removed by the O-GlcNAc-glucosaminidase (OGA) (16). The concentration of UDP-GlcNAc is regulated by glucose levels and is synthesized by the hexosamine biosynthesis pathway (HBP) (17, 18). OGT and OGA could be compared with the kinase/phosphatase system. In fact, phosphorylation and O-GlcNAc residue modification (O-GlcNAcylation) are often reciprocal, constituting a complex and dynamic interplay of protein activation (19). To date, many O-GlcNAcylated proteins that belong to different classes of mediators have been identified as follows: transcription factors, enzymes, chaperones, cytoskeletal proteins, signaling molecules, etc. (reviewed in Refs. 12, 15), suggesting that O-GlcNAcylation a key regulator of many different cellular processes, including signal transduction, transcription, and proteosomal degradation. Alterations in protein O-GlcNAcylation have been implicated in various human diseases, including diabetes mellitus, immunological disorders, cardiovascular disease, neurodegeneration, and cancer (10, 15).
The ATDC5 cell line exhibits a process of chondrogenic differentiation, which is analogous to that observed during endochondral bone formation. Although it is widely known that insulin signaling enhances chondrocyte differentiation through the stimulation of chondrocyte proliferation and glucose uptake, the mechanisms by which this occurs are poorly understood. So, the aim of this work was to investigate whether insulin-induced chondrocyte differentiation occurs through a modification of the amount of O-GlcNAcylated proteins. Furthermore, we sought to ascertain whether O-GlcNAc accumulation was able to induce hypertrophic chondrocyte differentiation both in vitro and in vivo.
EXPERIMENTAL PROCEDURES
Cell Culture
Pre-chondrogenic ATDC5 cells were purchased from the RIKEN Cell Bank (Ibaraki, Japan). ATDC5 cells were cultured in a 1:1 mixture of Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 medium (DMEM/F12) (Lonza, Walkersville, MD) containing 5% fetal bovine serum (FBS, Lonza, Walkersville, MD), penicillin (100 units/ml), and streptomycin (100 units/ml) (Lonza, Walkersville, MD). To induce cell differentiation, the medium was then replaced with 5% FBS/DMEM/F-12 containing 10 μg/ml transferrin (Sigma), 10−8 m sodium selenite (Sigma), and 10 μg/ml insulin (Sigma). The cells were then cultured at 37 °C for different periods of time up to 21 days at 5% CO2. When indicated, the highly selective OGA inhibitor thiamet-G (20, 21) (a kind gift from Dr. Natasha E. Zachara, The Johns Hopkins University School of Medicine, Baltimore, MD) delivered at a dose of 1 μm or ascorbic acid at a dose of 50 μg/ml or sterile water (vehicle) was added instead of insulin. To suppress the presence of UDP-GlcNAc (the donor of O-GlcNAc), we inhibited the activity of the enzyme glutamine:fructose-6-phosphate-amidotransferase-1 (GFAT-1) employing 6-diazo-5-oxo-l-norleucine (DON; Sigma) at a dose of 10−5 m, in the presence or absence of insulin. The culture medium was changed every 2 to 3 days.
Gene Expression Studies
For gene expression studies, cells were harvested at the time indicated, and total RNA was obtained using TriPure Isolation Reagent (Roche Diagnostics). The RNA (1 μg) was reverse-transcribed with the high capacity cDNA kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions, and RNA expression was quantified by single-reporter real time PCR using the Step One Plus detection system (Applied Biosystems) as described previously (22). The specific oligonucleotide primer pairs labeled with a fluorescent dye (FAMTM) at the 5′ end, as well as the specific FAM TaqMan probe for collagen type IIa1 (Col II) (assay ID Mm01309565_m1), collagen type X (Col X) (Mm00487041_m1), aggrecan-1 (Agg) (Mm00545794_m1), parathyroid hormone receptor-1 (Mm01250244_m1), Indian hedgehog (IHH) (Mm00439613_m1), Runx2 (Mm00501584_m1), and alkaline phosphatase (ALP Mm00475834_m1) were purchased from Applied Biosystems. A pre-designed GAPDH FAM/MGB probe (rRNA) assay (4352932E, Applied Biosystems) was also used as an endogenous control, and mRNA expression was normalized to that of GAPD RNA in each well. The experimental values for each gene were then normalized to the calibrator value (cells at day 0 of differentiation were chosen as the calibrator = 1 for each gene).
Alcian Blue Staining
ATDC5 cells were plated in p65 plates and cultured in the different medium tested. At each time point, cells were rinsed with PBS and fixed with methanol for 3 min at −20 °C. They were then stained with 0.1% Alcian blue 8GS (Sigma) in 0.1 m HCl for 2 h. The stained culture plates were rinsed with phosphate buffer saline (PBS) three times and extracted with 2 ml of 6 m guanidine-HCl for 2 h at room temperature. The absorbance of aliquots of the extracted dye was measured at 620 nm in a plate reader.
Zymography
Activity of the gelatinases matrix metalloprotease (MMP)-2 and MMP-9 was determined in ATDC5 cell culture supernatants from 6-well plates seeded with 6 × 103 cells. Equal amounts of proteins were loaded on a 10% polyacrylamide gel containing 0.1% of gelatin. Proteins were separated by SDS-PAGE under nonreducing conditions at 4 °C. Afterward, the gels were washed with 2.5% Triton X-100 and incubated with proteolysis buffer (50 mm Tris-HCl, pH 7.6, 0.2 mm NaCl, and 5 mm CaCl2, 0.2% Nonidet P-40 and 0.01% Tween 20) for 16 h at 37 °C. Next, the gels were incubated with staining buffer (0.05% Coomassie Blue, 50% methanol, 10% acetic acid), followed by destaining buffer (4% methanol, 8% acetic acid). The gels were scanned using the Gel Doc EZ scanner (Bio-Rad).
Western Blot Studies
Twenty micrograms of total protein from ATDC5 cultures were loaded and then resolved on SDS-acrylamide gels to determine the amount of O-GlcNAc-modified proteins, as well as the presence of OGT, OGA, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38, Akt, glycogen synthase kinase 3 β (GSK3β), and β-catenin. The gels were transferred to nitrocellulose membranes (Bio-Rad) that were blocked with 3% skim milk in Tris-buffered saline (TBS), 0.5% Tween 20 at room temperature for 1 h, and then incubated overnight at 4 °C with RL2 (Thermo Fisher Scientific, Waltham, MA), anti-OGT ab59135 (Abcam, Cambridge, UK), anti-OGA (ab345, a kind gift from Dr. Gerald W. Hart, of the Johns Hopkins University School of Medicine, Baltimore, MD), anti-ERK, anti-phospho-ERK, anti-JNK, anti-phospho-JNK, anti-p38, anti-phospho-p38, anti-GSK3β, anti-phospho- GSK3β or anti-β-catenin (all from Cell Signaling, Danvers, MA), or anti-Akt1/2/3 or phospho-Akt (Ser-474) (both from Santa Cruz Biotechnology), diluted in blocking buffer. The antibody binding was visualized by enhanced chemiluminescence employing the corresponding peroxidase-conjugated secondary antibodies and an ECL kit (Amersham Biosciences). The bands were quantified by densitometry, and the results were expressed in arbitrary units employing the Quantity One software (Version 4.6.3). For total O-GlcNAc quantification, all the immunoreactive bands were merged.
Detection of O-GlcNAcylated Akt
To detect O-GlcNAcylation of Akt, 80 μg of total protein from ATDC5 culture was incubated with 50 μl of magnetic beads (Dynabeads Protein G; Invitrogen) coupled according to the manufacturer's directions with 4 μl of anti-Akt1/2/3 rabbit polyclonal antibody (Santa Cruz Biotechnology) overnight at 4 °C. Then the magnetic beads were collected, and the supernatant was saved to measure the amount of unbound Akt. The magnetic beads were washed three times with PBS and then mixed with loading buffer and placed at 70 °C for 10 min. Eluted proteins were then separated from magnetic beads and loaded onto SDS-acrylamide gels and then immunoblotted with RL2 antibody as described above.
Experimental Animals
For the thiamet-G dose dependence study, six 23-day-old male C57BL/6 mice (Harlan Interfauna Ibérica, S.A., Barcelona, Spain) received single intraperitoneal injections of either 0, 10, 20, 100, 200, or 500 mg/kg of thiamet-G dissolved in phosphate-buffered saline (PBS) and then were euthanized 8 h later to evaluate the O-GlcNAc levels in different tissues (brain, liver, muscle, and knee). The time of sacrifice was chosen on the basis of previously published data on thiamet-G in rodents, which demonstrated that the peak level of O-GlcNAc proteins following administration of the drug was achieved after 8–10 h (20, 21, 23). Tissues were collected immediately after sacrifice, flash-frozen in liquid nitrogen, and stored at −80 °C until required for use.
For in vivo chondrocyte hypertrophy studies, eight 23-day-old male C57BL/6 mice received daily intraperitoneal injections of 20 mg/kg thiamet-G over 15 days, whereas the control group (n = 8) was intraperitoneally injected with PBS. The mice were weighed at base line and at the end of the study. All of the procedures involving animals were performed according to the International and Institutional Animal Research Committee guidelines.
Histological Examination
Immediately after each animal was sacrificed, both tibias were dissected free from soft tissue and measured under a dissecting microscope. Then tissues were fixed in 4% formaldehyde for 24 h and decalcified with Osteosoft® (VWR International Eurolab, Barcelona, Spain) for 2 days. Then, the tibias were dehydrated through ethanol series and embedded in paraffin, and paraffin sections (5 μm) were stained with hematoxylin and eosin. Two stained sections per growth plate were viewed at ×20 magnification, and images were captured with the use of a Leica DMD108 microscope (Leica Microsystems, Barcelona, Spain). The images were aligned so that the direction of growth was vertical on the computer screen. Then each growth plate was measured for plate height and hypertrophic zone height by delineating the top of the growth plate, the junction between the proliferative zone and the hypertrophic zone, and the chondro-osseous junction. These zones were established based on the morphological characteristics of the chondrocytes, and on the changes in matrix staining (24, 25). The vertical height of each zone was measured in the central part of the image at similar intervals across the sections (n = 9 or 10 per section). Values for each individual mouse were obtained by averaging all 40 measurements (2 sections × 10 locations × 2 growth plates (left and right)) per animal. For cell count in the hypertrophic zone, we counted the number of hypertrophic chondrocytes in areas of 1000 μm2 (200 × 50 μm). We counted three different areas in each growth plate, and the mean of these measures was taken as the number of cells/1000 μm2 in each growth plate.
Immunohistochemistry
Paraffin sections were also employed for Col X immunohistochemical staining, employing a rabbit anti-Col X (a kind gift from Dr. Danny Chan, University of Hong Kong, China). An antigen-retrieval protocol consisting of hyaluronidase treatment (0.8 mg/ml for 30 min at 37 °C) was used. Biotinylated secondary antibody in conjunction with the ABC elite kit (Vector Labs, Burlingame, CA) were applied following the manufacturer's specifications.
Statistical Analysis
Results are expressed as means ± S.E. and were analyzed using the Mann-Whitney U test. Where multiple comparisons were performed, the Kruskal-Wallis test was used. The null hypothesis was rejected in each statistical test when the p value was <0.05. All statistical analyses were performed using Windows SPSS version 11.0 software (SPSS, Chicago, IL).
RESULTS
ATDC5 Differentiation Induces O-GlcNAc Accumulation
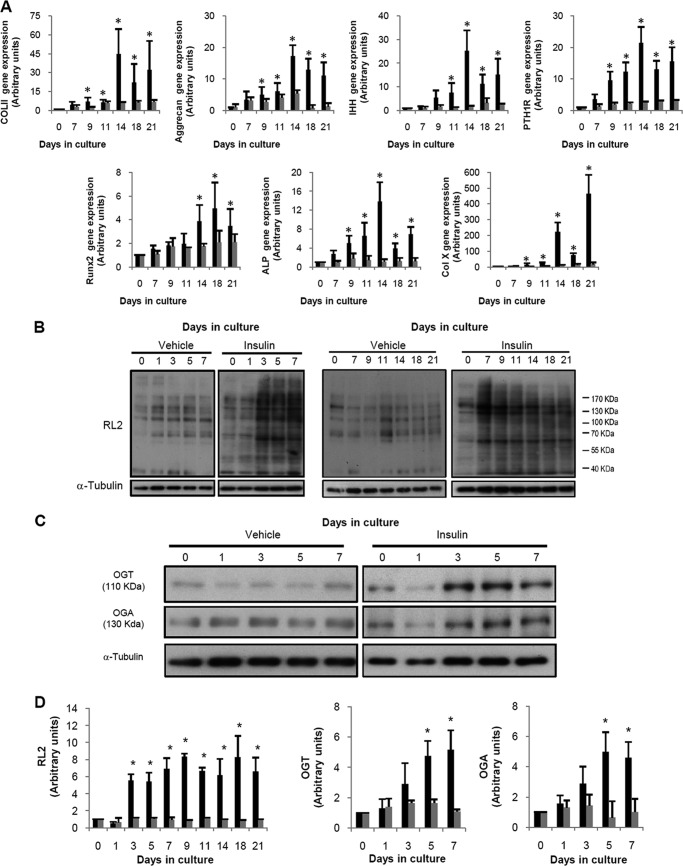
The cell line ATDC5 was chosen for these studies because it has been shown to be a useful in vitro model for examining chondrogenic differentiation (5, 26, 27). We first analyzed the time course differentiation of ATDC5 cells induced by insulin by assessing the increase observed in the gene expression of both early and late differentiation markers. To do this, cells were incubated in the absence or presence of 10 μg/ml insulin and harvested after different periods of culture, up to a maximum of 21 days. As can be observed in Fig. 1A, insulin induced a gradual increase in the gene expression of all the differentiation markers measured as follows: Col II, Agg, parathyroid hormone receptor type 1, IHH, Runx2, ALP, and Col X. The increase in the gene expression of the differentiation markers was statistically significant since day 9 in comparison with the expression of each gene at day 0, which was always normalized to 1 (Fig. 1A). In the presence of insulin, the expression of each gene was also significantly different from that observed in the absence of insulin at the same time since day 9. We then measured whether ATDC5 differentiation was associated with an alteration in the amount of O-GlcNAcylated proteins employing the RL2 antibody that specifically recognizes O-GlcNAc residues. Our experiments demonstrated that insulin-induced differentiation gradually increased the amount of O-GlcNAcylated proteins, an increase that was significant since day 3 in comparison with the amount observed at day 0 (Fig. 1, B and D), whereas the absence of insulin did not evoke any change in RL2 staining. The most extensive increase in the O-GlcNAcylation induced by the presence of insulin was found in proteins between 110 and 170 kDa and in proteins around 55–70 kDa (Fig. 1B). The increased in the amount of O-GlcNAcylated proteins remained high from day 3 until the end of the study, without significant differences between the different days of study. Furthermore, ATDC5 differentiation was also characterized by an increase in the presence of the key enzymes that regulate the addition and removal of this moiety. The presence of both OGT and OGA was significantly increased since day 3 of differentiation (Fig. 1, C and D) in comparison with day 0, whereas no effect was noted in the absence of insulin. The expression of these enzymes remained increased until the end of the study (data not shown). Therefore, we observed that the activation of the O-GlcNAc modification system preceded the increase in the gene expression of the differentiation markers that we measured.
FIGURE 1.
ATDC5 differentiation induces the accumulation of O-GlcNAc-modified proteins. ATDC5 cells were differentiated with 10 mg/ml insulin (black bars) or treated with the vehicle (gray bars) for different periods of time, up to 21 days. A, gene expression of differentiation markers Col II, Agg, IHH, PTH1R, Runx2, ALP, Col X, and GAPDH was examined by real time PCR. Results are expressed as fold induction expressed as mean ± S.E. of six independent experiments in comparison with day 0. B, Western blot studies of O-GlcNAcylated proteins. C, representative Western blot studies of OGT and OGA synthesis. D, densitometric analysis of four independent experiments of RL2, OGT, and OGA Western blot experiments. Bars show the mean ± S.E. *, p < 0.05 versus day 0 and versus vehicle at the corresponding time.
Ascorbic acid (AA) has been described previously as a differentiation agent in ATDC5 cells (28). Therefore, we analyzed whether a chondrogenic stimuli, nondirectly related to glucose metabolism, such as AA, would also be able to modify the amount of O-GlcNAcylated proteins in ATDC5 cells in the absence of insulin. AA evoked a clear increase in the accumulation of O-GlcNAcylated proteins since day 3 of incubation (supplemental Fig. S1). Both OGT and OGA synthesis were also significantly induced by the presence of AA (supplemental Fig. S1).
O-GlcNAc Accumulation Is Able to Induce ATDC5 Differentiation
Because both insulin and AA induced an early accumulation of O-GlcNAcylated proteins, we subsequently examined whether O-GlcNAc accumulation per se was able to induce ATDC5 differentiation. To do this, ATDC5 cells were incubated with the selective OGA inhibitor thiamet-G at a concentration of 1 μm (21) added in lieu of insulin. Thiamet-G was generated as a potent and selective inhibitor of human OGA which, unlike other OGA inhibitors, does not inhibit hexosaminidase-β activity (21, 29). We first studied the effect of thiamet-G in the accumulation of O-GlcNAcylated proteins and in the regulation of the enzymes responsible for this modification. As expected, thiamet-G induced a clear increase in the accumulation of O-GlcNAcylated proteins since day 1 of incubation, in comparison with day 0 (Fig. 2A). Regarding the enzyme regulation, we showed that thiamet-G induced a significant increase in the synthesis of OGA since day 1 postincubation, probably due to a feedback regulation in response to the enzymatic activity inhibition of this enzyme (Fig. 2A). In contrast, OGT synthesis was significantly inhibited by thiamet-G (Fig. 2A).
FIGURE 2.
O-GlcNAc accumulation in ATDC5 leads to an increase in differentiation markers. A, Western blot studies of ATDC5 cells treated with 1 mm thiamet-G for different periods of time. Upper panels show representative Western blots, and lower panels show the densitometric analyses of four independent experiments as fold change versus day 0 ± S.E. B, gene expression of differentiation markers Col II, aggrecan, IHH, PTH1R, Runx2, ALP, Col X, and GAPDH was examined by real time PCR in ATDC5 stimulated with thiamet-G. Results are expressed as fold induction expressed as mean ± S.E. in comparison with day 0. Bars show the mean ± S.E. of seven independent experiments. *, p < 0.05 versus day 0.
In addition, the presence of this drug in the absence of insulin led to a significant increase in the gene expression of the differentiation markers measured as follows: Col II, Agg, parathyroid hormone receptor type 1, IHH, Runx2, ALP, and Col X in comparison with the expression at day 0 (Fig. 2B). These increases were also significantly different from those observed without stimuli at the corresponding time of incubation. When compared with the gene expression induced by insulin, no significant differences were observed, probably due to the standard deviation of the experiments, although the fold inductions for thiamet-G treatment seemed lower than those induced with insulin (Figs. 1A and 2B).
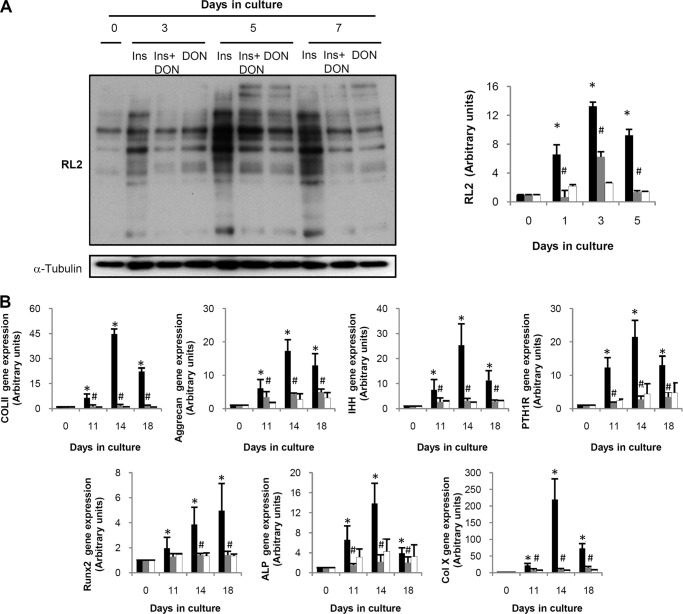
To ensure the role of O-GlcNAcylation in chondrocyte differentiation, we tested the effects of an HBP inhibitor on ATDC5 differentiation induced by insulin. To do so, the inhibitor of the HBP rate-limiting enzyme GFAT-1, DON, was added to the medium in the presence of insulin (Fig. 3A). We observed that the elevation in O-GlcNAcylated proteins induced by insulin was significantly blocked by treating with DON. These results indicate that the increase in protein O-GlcNAcylation during ATDC5 differentiation may be caused by HBP activation. We then tested whether the inhibition of O-GlcNAcylation could have any effect on the increase of the differentiation markers induced by insulin. As shown in Fig. 3B, the presence of DON almost completely blocked the increase in the differentiation markers induced by insulin.
FIGURE 3.
DON treatment in ATDC5 leads to a decrease in O-GlcNAcylation and in the differentiation markers. A, Western blot study of O-GlcNAcylated proteins of cells treated with insulin in the absence (black bars) or presence of DON (gray bars). White bars indicate the effect of DON in the absence of insulin. Left panel shows a representative Western blot, and right panel shows the densitometric analysis of three independent experiments. Results are shown as fold induction versus day 0 ± S.E. *, p < 0.05 versus day 0; #, p < 0.05 versus insulin at the corresponding time. B, gene expression of differentiation markers Col II, aggrecan, IHH, PTH1R, Runx2, ALP, Col X, and GAPDH was examined by real time PCR in ATDC5 stimulated with insulin in the absence (black bars) or presence of DON (gray bars). White bars indicate the effect of DON in the absence of insulin. Results are expressed as fold induction expressed as mean ± S.E. in comparison with day 0. Bars show the mean ± S.E. of three independent experiments. *, p < 0.05 versus day 0; #, p < 0.05 versus insulin at the corresponding time.
O-GlcNAc Accumulation Regulation of Proteoglycan Synthesis
We then tested whether thiamet-G treatment was also able to increase proteoglycan synthesis measured by the Alcian blue staining. As can be observed in Fig. 4A, the presence of insulin progressively increased the proteoglycan content in ATDC5 cells during the time of study. In contrast, only a slight increase was observed in thiamet-G-stimulated cells, which was significantly lower than that observed for insulin (Fig. 4A).
FIGURE 4.
A, Alcian blue staining of ATDC5 cells after 21 days in culture with insulin (black bars) or thiamet-G (gray bars). Lower panel shows the quantification of the absorbance at 620 nm. Bars show the mean ± S.E. of four independent experiments. *, p < 0.05 versus day 0; #, p < 0.05 versus insulin at the corresponding time. B, gelatin zymography showing MMP-9 and MMP-2 activities in ATDC5 cell media ATDC5. Cells were treated with 10 μg/ml insulin or 1 μm thiamet-G over 14 or 21 days.
O-GlcNAc Accumulation Up-regulates MMP-9 and MMP-2 Activities
Another hallmark of chondrocyte differentiation is enzymatic remodeling of the extracellular matrix. The MMP family of proteases, which play a crucial role in remodeling and degradation of the extracellular matrix, are involved in the preservation of extracellular matrix integrity and vascularization of the growth plate (30, 31). An increase in the activity of MMP-2 and MMP-9 has been previously related to an increase in ATDC5 differentiation (32). We studied the effect of O-GlcNAc accumulation on MMP-2 and MMP-9 activity in the supernatants of ATDC5 chondrocytes cultured for 14 and 21 days. Gel zymography detected 92- and 72-kDa bands corresponding to MMP-9 and MMP-2, respectively (Fig. 4B). The activities of both MMPs were higher on days 14 and 21 in cells differentiated in the presence of insulin than those observed at day 0. O-GlcNAc accumulation induced by thiamet-G also evoked a clear increase in the activity of these MMPs in comparison with the activity on day 0 on both days tested. The induction of these MMPs was similar to that induced by insulin (Fig. 4B).
O-GlcNAc Accumulation Activates the Expression and Activation of Different Signaling Molecules in ATDC5 Cells
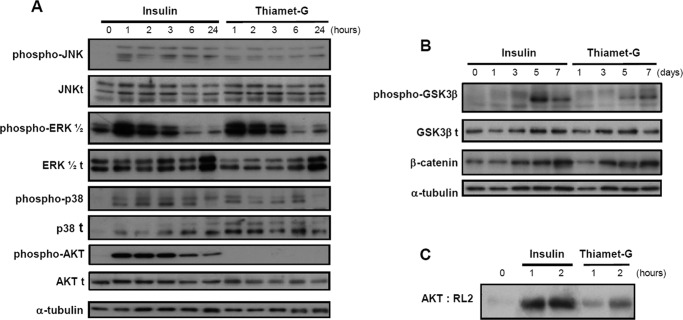
To establish whether the molecular mediators that are induced during insulin-induced ATDC5 differentiation were similar or not to those induced by thiamet-G, we examined the activation of different mitogen-activated protein kinases (MAPKs), and the Akt activation, which are involved in signal transduction networks and have been implicated in a variety of cellular events, such as differentiation, proliferation, migration, and apoptosis (33–35). To do this, we compared the effects of thiamet-G with those of insulin at different times of incubation during the first 24 h of differentiation. Immunoblot signals show that thiamet-G induced the phosphorylation of JNK, ERK, and p38, in comparison with the activation observed at 0 h, with peaking occurring around 1–2 h for all three mediators (Fig. 5). Insulin also activated the phosphorylation of these three mediators (Fig. 5A). However, thiamet-G did not induce the phosphorylation of Akt, whereas a clear induction was observed with insulin (Fig. 5A).
FIGURE 5.
A and B, representative Western blot analysis of MAPKs and Akt1/2/3 activation in ATDC5 pre-chondrocyte cells incubated with 10 μg/ml insulin or 1 μm thiamet-G for the indicated periods of times. Cell extracts were analyzed using specific antibodies against phosphorylated and unphosphorylated forms of the proteins. C, proteins were immunoprecipitated with total Akt antibody, and then the eluted proteins were immunoblotted with RL2 antibody in ATDC5 cells incubated with 10 μg/ml insulin or 1 μm thiamet-G for the indicated periods of times.
We also investigated whether a downstream pathway of MAPKs and Akt, such as glycogen synthase kinase-3β (GSK3β) that has been largely associated with enhanced chondrocyte differentiation (36), could be induced by insulin and thiamet-G treatment. Both insulin- and thiamet-G-induced differentiation strongly increased the phosphorylation of GSK3β (Fig. 5B), a kinase that phosphorylates β-catenin and that is inactive upon phosphorylation. In addition, GSK3β inactivation induced by insulin or thiamet-G, was associated with increased levels of β-catenin (Fig. 5B).
Because the activation of the Akt pathway has also been associated with its dynamic O-GlcNAcylation and phosphorylation, we studied whether O-GlcNAcylation accumulation induced by insulin and thiamet-G could also regulate this modification of Akt. Protein extracts from insulin or thiamet-G-treated cells were immunoprecipitated with an Akt antibody, and the magnetic bead-bound proteins were eluted and immunoblotted with RL2 antibody. As can be observed in Fig. 5C, both insulin- and thiamet-G-treated cells showed an increase in O-GlcNAcylated Akt in comparison with the O-GlcNAcylated Akt in undifferentiated cells. Thus, our results showed that insulin differentiation simultaneously induced both the phosphorylation and the O-GlcNAcylation of Akt, whereas thiamet-G induced its O-GlcNACylation.
O-GlcNAc Accumulation Increases Growth Plate Chondrocyte Differentiation in Vivo
We next wanted to test whether O-GlcNAcylation accumulation in vivo was able to induce chondrocyte differentiation. Therefore, we studied the effect of thiamet-G administration on the endochondral plate height in newborn mice. Preliminary experiments were carried out to assess the effectiveness of thiamet-G in C57BL/6 mice. We were particularly interested in the dose-dependent effect of thiamet-G on O-GlcNAc levels, since this long term study requires a dosing regimen that maintains increased O-GlcNAc levels through the entire course of study. To investigate this issue, mice were given different doses of thiamet-G by a single intraperitoneal injection and sacrificed after 8 h. We then evaluate the amount of O-GlcNAcylated proteins in the target organs where this modification was assessed (21, 23). Evaluation of homogenized brain, liver, muscle, and knee by Western blot analysis using the RL2 antibody revealed that O-GlcNAc levels in brain, liver, and knee were increased in a dose-dependent manner in response to thiamet-G administration, although no effect was observed in muscle with a single dose of thiamet-G within the given range of doses administered (Fig. 6A). Furthermore, we did not observe further increases in O-GlcNAcylation for thiamet-G doses higher than 20 mg/kg. Therefore, this dose was chosen for further experiments. Thiamet-G treatment during 15 days increased the amount of O-GlcNAcylated proteins in all the tissues tested (Fig. 6B).
FIGURE 6.
A, dose-dependent analysis of the effect of thiamet-G on the amount of O-GlcNAcylated proteins in different mice tissues 8 h after the injection of the drug. B, representative Western blots showing O-GlcNAc levels in different tissues of mice treated during 15 days with 20 mg/kg thiamet-G or vehicle. Right panel, bars show the densitometric analysis of O-GlcNAcylated proteins in the different tissues. n = 8 for each group. Results are expressed as mean ± S.E. *, p < 0.05 versus vehicle.
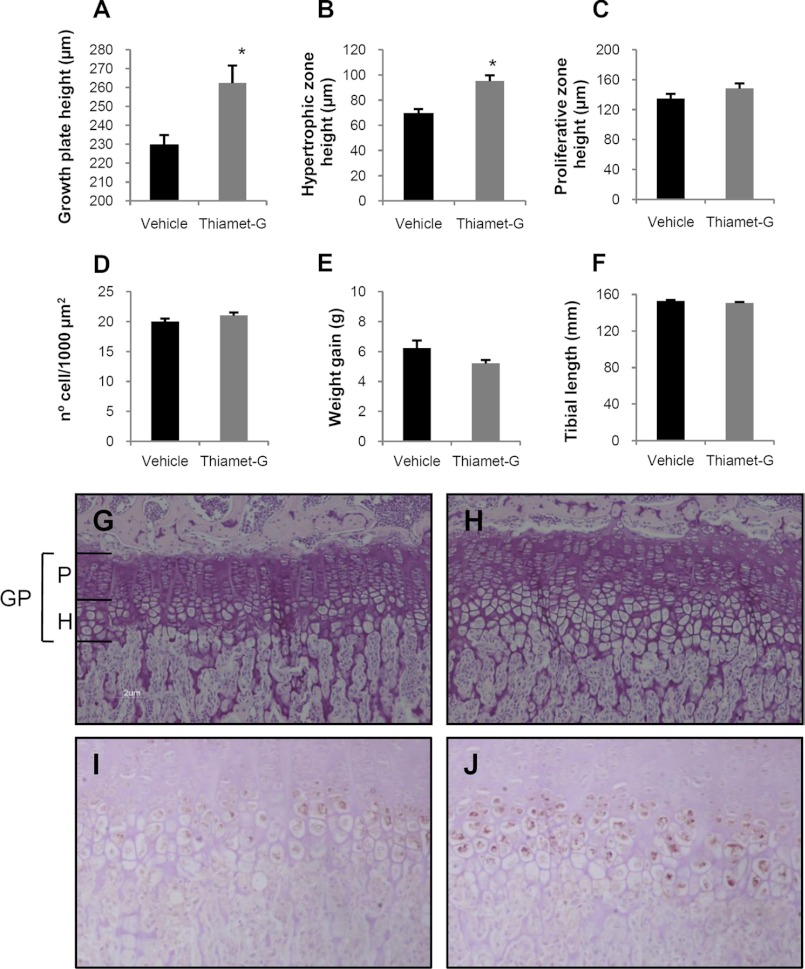
We next turned our attention to elucidating the effect O-GlcNAc accumulation induced by thiamet-G might have on growth plate height and hypertrophic zone height, as these are the zones of the growth plate where chondrocyte differentiation takes place. For this purpose, we examined the endochondral plate of the tibias of mice treated with thiamet-G during 15 days and compared them with the tibias of saline-treated mice. The endochondral plate is clearly identifiable in hematoxylin and eosin staining mainly due to its structure and the change in the stain between the calcified bone and the chondrocytes. Treatment with thiamet-G increased both tibial growth plate and hypertrophic zone height, in comparison with the heights observed in vehicle-treated mice (Fig. 7, A, B, G, and H), reflecting a stimulatory effect of O-GlcNAc accumulation on growth plate chondrocyte differentiation. The effect of thiamet-G on the hypertrophic zone seemed specific, since the proliferating zone height was not significantly modified by thiamet-G (Fig. 7C). To check whether the increase in the hypertrophic zone was due to an increase in the cell size, we count the number of hypertrophic chondrocytes in 1000 μm2 in the growth plates in our mice. As shown in Fig. 7D, thiamet-G did not evoke any change in the number of cells counted in the same area, thus indicating that the hypertrophic chondrocyte size was similar in the two groups studied. Additionally, we did not observe any significant effect of thiamet-G administration on tibial length or weight gain of the mice (Fig. 7, E and F).
FIGURE 7.
A–D, measurements in the growth plate of mice treated with thiamet-G or vehicle. Bars show the effect of thiamet-G treatment on growth plate height (A), hypertrophic zone height (B), proliferative zone height (C), and number of hypertrophic chondrocytes (D) in 1000 μm2. E indicates weight gain, and F indicates tibial length. G and H, photomicrographs showing hematoxylin-eosin staining of the growth plate from representative vehicle-treated mice (G) and thiamet-G treated mice (H). I and J, photomicrographs showing collagen X staining in the hypertrophic zone of vehicle-treated mice (I) and thiamet-G-treated mice (J). Magnifications are as follows: G and H, ×200; I and J, ×240. *, p < 0.05 versus vehicle. GP, growth plate; P, proliferating zone; H, hypertrophic zone.
Subsequently, we analyzed the presence of the hypertrophic marker Col X in the growth plate of these animals. As can be observed in Fig. 7, I and J, col X staining was more intense in the thiamet-G-treated animals than in saline-treated mice.
DISCUSSION
In this work, we have shown for the first time that insulin-induced ATDC5 differentiation occurred following an increase in the accumulation of O-GlcNAc-modified proteins. The accumulation of this type of modified residues was paralleled by an increased presence of the enzymes responsible for O-GlcNAc cycling, OGT and OGA. We have shown that, in the absence of insulin, OGA inhibition induced an increase in O-GlcNAcylation together with an increase in various differentiation markers.
The increase in protein O-GlcNAcylation associated with differentiation that we have demonstrated encompasses a large number of proteins. Proteomic analysis has identified nearly 1000 proteins susceptible to undergoing O-GlcNAcylation (37) and belonging to almost all functional classes of proteins (11, 38). We have not identified which specific protein targets of O-GlcNAc modification are functionally linked to the mechanisms of endochondral differentiation and cell hypertrophy. However, different major determinants that function in these processes are known to be heavily modified by O-GlcNAc. Different proteins involved in the Wnt pathway have been shown to be modified by O-GlcNAc, such as plakoglobin (39), β-catenin (40), or cAMP-responsive element-binding protein, in addition to calcium/calmodulin-dependent kinases (41) and phosphatidylinositol 3-kinase/Akt (42). We showed a special increase in the O-GlcNAcylation of proteins around 110–130 kDa. Similar results were described for the O-GlcNAcylation in the rat diabetic heart (43). We also observed a clear increase in O-GlcNAcylated proteins around 55–70 kDa, in line with previous results in diabetic mouse hearts (44). In future work, our group will go deeper into the identification of these proteins, because these comparisons are only superficial and are probably tissue- and antibody-dependent.
Differentiation of the ATDC5 chondrogenic cell line is typically induced by exposing the cells to insulin through the stimulation of insulin and insulin growth factor receptors (5), which boost glucose uptake and utilization (45). Proliferative and early hypertrophic chondrocytes accumulate glycogen that is employed during the maturation phase (45, 46). Therefore, it seems conceivable that O-GlcNAcylation, which has been described as a nutrient sensor that may link cell responses to the cell nutrient status (18, 47), can be modified during ATDC5 differentiation.
The enhancement of global O-GlcNAcylation induced by insulin and the link between insulin resistance and aberrant GlcNAcylation has been extensively studied, especially in processes associated with diabetes and cardiovascular disorders (18, 47). Some studies have shown that increased O-GlcNAc could dampen the insulin response-inhibiting Akt pathway in insulin target tissues, such as adipocytes and muscle cells (48–51). However, in other systems it has been demonstrated that an increase in O-GlcNAcylation enhanced insulin signaling (52, 53). In line with our data, it has also been shown that preadipocyte differentiation is induced through an increase in O-GlcNAc protein modification (54), whereas adipocyte differentiation can be blocked inhibiting the HBP (55). To our knowledge, no studies have been published regarding the regulation of O-GlcNAc incorporation in hypertrophic or articular chondrocytes. Recent preliminary data from our laboratory revealed that there is also an accumulation of O-GlcNAcylated proteins in the cartilage of human osteoarthritic patients (56). Remarkably, some characteristics of osteoarthritis, i.e. articular chondrocyte proliferation, the gene expression of differentiation markers, MMP synthesis, and matrix remodeling, resemble the chondrocyte differentiation process during EO (57, 58).
We have shown that the accumulation of O-GlcNAcylated proteins induced by thiamet-G evoked an increase in the gene differentiation markers and in the activity of MMP-2 and -9 in ATDC5 chondroprogenitors, thus resembling the effect of insulin. We found little difference between insulin and thiamet-G in the intensity of the induction of the differentiation markers and a clear difference in the proteoglycan synthesis activation, at least as measured by Alcian blue staining. Furthermore, the profile of MAPK phosphorylation induced by both treatments was nearly the same; insulin and thiamet-G induced an increase in p-p38, p-ERK1/2, and p-JNK, in line with previous data in other cell types (59, 60). However, thiamet-G failed in the induction of Akt phosphorylation, as has been described for other OGA inhibitors (42). Interestingly, previous data showed that insulin-induced proteoglycan synthesis in chondrocytes would specifically require Akt phosphorylation (61). Regarding GSK3β and β-catenin pathway activation, we have also shown that O-GlcNAcylation accumulation exactly replicated the effect of insulin in ATDC5 pre-chondrocytes.
We also studied whether a specific differentiation mediator was a target for O-GlcNAcylation during chondrogenic differentiation. As has been described, there is an extensive cross-talk between O-GlcNAcylation and phosphorylation of Akt that regulates its signaling (42, 62). We were able to detect changes in the Akt O-GlcNAcylation that were induced both with insulin and with thiamet-G. According to our data, Akt O-GlcNAcylation and Akt phosphorylation can be simultaneously induced, in line with previously published data (42). Furthermore, Akt O-GlcNAcylation was even more intense when Akt phosphorylation was activated, as was the case for insulin-induced differentiation. The effect of Akt O-GlcNAcylation regarding its enzymatic activity or its cellular localization is still under discussion. A decrease in Akt phosphorylation and/or Akt activity associated with an increase in Akt O-GlcNAcylation has been described by some investigators (49, 50, 62). However, it has also been demonstrated that Akt O-GlcNAcylation did not inhibits its phosphorylation, having no effect or a stimulating effect on its enzymatic activity (42, 52, 53). Irrespective of the modulation of Akt activity, O-GlcNAcylation can also modulate the cellular distribution of the enzyme, probably inducing further changes in the enzyme targets (42). Therefore, further experiments are needed to describe the specific effect of Akt O-GlcNAcylation on chondrocyte differentiation.
Overall, and keeping in mind that slight differences were observed between a growth hormone, such as insulin, and a specific OGA inhibitor, as thiamet-G, our data suggest that the accumulation of O-GlcNAcylated proteins may be an implicit part of the cell mechanism for chondrocyte differentiation.
Among the different factors that may be responsible for the increase in O-GlcNAcylation observed during chondrocyte differentiation induced by insulin, we have shown that the increase in the synthesis of OGT, the enzyme responsible for the addition of this kind of modification, is potentially responsible for this effect. The increase in the presence of this enzyme has been associated with an increase in this post-translational modification both in response to acute stress (63, 64) and in response to chronic damage (65). In addition, we have also observed an increased presence of the enzyme responsible for O-GlcNAc removal, OGA, during ATDC5 hypertrophy. Our results are in line with recently published data, suggesting that under certain conditions OGT and OGA may act in tandem to regulate O-GlcNAc signaling within the cell (65, 66). Interestingly, the increase in global O-GlcNAcylation in response to acute stress did seem to depend on OGT induction, as well as a decrease in the presence of OGA (63). In contrast, in response to chronic stimuli, both enzymes seem to be regulated in the same direction. The increase in O-GlcNAcylated proteins in human cardiac hypertrophy was associated with an increased synthesis of both OGT and OGA (65), and we obtained the same results for the increase in O-GlcNAcylated proteins in human osteoarthritic cartilage (56). Interestingly, the regulation in the same direction for OGT and OGA has been also described in chronic studies when the result is a global decrease in O-GlcNAcylation of proteins, where the author showed a decrease in the synthesis of both enzymes (66). In fact, it has also been described that OGT null cells also knocked down OGA expression in response to this manipulation (67). In this context, our results of OGT and OGA regulation during thiamet-G treatment seem relevant. We have shown that the accumulation of O-GlcNAcylated proteins due to the pharmacological inhibition of OGA activity led to an induction on its synthesis and to an inhibition of OGT. Therefore, one is tempted to speculate that it is the activity of both enzymes, more than the protein synthesis, that may be regulated in tandem. However, further studies are needed to go deeper into the regulation of OGT and OGA expression, synthesis, and activity.
To further confirm that O-GlcNAc accumulation per se was able to increase chondrocyte differentiation, we administered the OGA inhibitor thiamet-G to young mice. Thiamet-G and other thiazolines have been previously employed to study the effect of the increase in O-GlcNAcylation avoiding the toxic effects of other OGA inhibitors and with better results than studies overexpressing OGT (20). Furthermore, it has been demonstrated that the administration of improved OGA inhibitors such as thiazolines or 6-acetamido-6-deoxy-castanospermine in vivo does not seem to cause insulin resistance or glucohomeostasis perturbations (20, 23).3 Our data show that thiamet-G administration induces a significant increase in endochondral plate height, probably due to an increased length of the hypertrophic zone. However, we did not find this effect to be paralleled by an increase in tibial length. Based on our experiments, we were unable to determine whether a longer treatment with thiamet-G would induce an increase in tibial length. Furthermore, we cannot rule out the possibility that the effect of thiamet-G on chondrocyte differentiation was not followed by an induction of other events that participate in bone enlargement, such as the induction of vascularization or the calcification process.
In summary, this study shows that insulin-induced chondrocyte differentiation is associated with an increase in O-GlcNAcylated proteins due at least in part to an increase in the presence of OGT. Furthermore, the accumulation of O-GlcNAcylated proteins induced by OGA inhibition, in the absence of insulin, induced both chondrocyte differentiation and matrix remodeling through the increased presence of different MMPs. Most importantly, the in vivo administration of thiamet-G led to an increase in endochondral plate height in mice. Together, these findings indicate that O-GlcNAcylation is likely to play a vital role in chondrogenesis and that a greater understanding of OGT and OGA activities will potentially provide new avenues to investigate the control of cartilage formation, growth, metabolism, and repair.
Supplementary Material
Acknowledgments
We are indebted to Dr. Natasha E. Zachara and to Dr. Gerald W. Hart (The Johns Hopkins University School of Medicine, Baltimore, MD) for their donation of thiamet-G and anti-OGA345 antibody and to Dr. Danny Chan (University of Hong Kong) for collagen X antibody. We also thank Dr. D. J. Vocadlo for help in the discussion.
This work was supported by Instituto de Salud Carlos III Grants PS09/0034 (to R. L.) and PS09/01625 (to G. H.-B.).

This article contains supplemental Fig. S1.
D. J. Vocadlo, unpublished data.
- EO
- endochondral ossification
- O-GlcNAc
- O-linked N-acetylglucosamine
- OGT
- O-GlcNAc transferase
- OGA
- O-GlcNac glycanase
- HBP
- hexosamine biosynthesis pathway
- Col II
- collagen type II
- Agg
- aggrecan-1
- Col X
- collagen type X
- IHH
- Indian hedgehog
- Runx2
- runt-related transcription factor 2
- ALP
- alkaline phosphatase
- AA
- ascorbic acid
- DON
- 6-diazo-5-oxo-l-norleucine
- ALP
- alkaline phosphatase
- MMP
- matrix metalloprotease.
REFERENCES
- 1. Kronenberg H. M. (2003) Developmental regulation of the growth plate. Nature 423, 332–336 [DOI] [PubMed] [Google Scholar]
- 2. De Luca F. (2006) Impaired growth plate chondrogenesis in children with chronic illnesses. Pediatr. Res. 59, 625–629 [DOI] [PubMed] [Google Scholar]
- 3. LuValle P., Beier F. (2000) Cell cycle control in growth plate chondrocytes. Front. Biosci. 5, D493–D503 [DOI] [PubMed] [Google Scholar]
- 4. Hutchison M. R., Bassett M. H., White P. C. (2007) Insulin-like growth factor-I and fibroblast growth factor, but not growth hormone, affect growth plate chondrocyte proliferation. Endocrinology 148, 3122–3130 [DOI] [PubMed] [Google Scholar]
- 5. Phornphutkul C., Wu K. Y., Gruppuso P. A. (2006) The role of insulin in chondrogenesis. Mol. Cell. Endocrinol. 249, 107–115 [DOI] [PubMed] [Google Scholar]
- 6. Liu J. P., Baker J., Perkins A. S., Robertson E. J., Efstratiadis A. (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75, 59–72 [PubMed] [Google Scholar]
- 7. Beam H. A., Parsons J. R., Lin S. S. (2002) The effects of blood glucose control upon fracture healing in the BB Wistar rat with diabetes mellitus. J. Orthop. Res. 20, 1210–1216 [DOI] [PubMed] [Google Scholar]
- 8. Gandhi A., Beam H. A., O'Connor J. P., Parsons J. R., Lin S. S. (2005) The effects of local insulin delivery on diabetic fracture healing. Bone 37, 482–490 [DOI] [PubMed] [Google Scholar]
- 9. Kayal R. A., Alblowi J., McKenzie E., Krothapalli N., Silkman L., Gerstenfeld L., Einhorn T. A., Graves D. T. (2009) Diabetes causes the accelerated loss of cartilage during fracture repair which is reversed by insulin treatment. Bone 44, 357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanover J. A., Krause M. W., Love D. C. (2010) The hexosamine signaling pathway. O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 1800, 80–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart G. W., Housley M. P., Slawson C. (2007) Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 [DOI] [PubMed] [Google Scholar]
- 12. Love D. C., Hanover J. A. (2005) The hexosamine signaling pathway. Deciphering the “O-GlcNAc code.” Sci. STKE 2005, re13. [DOI] [PubMed] [Google Scholar]
- 13. Vosseller K., Sakabe K., Wells L., Hart G. W. (2002) Diverse regulation of protein function by O-GlcNAc. A nuclear and cytoplasmic carbohydrate post-translational modification. Curr. Opin. Chem. Biol. 6, 851–857 [DOI] [PubMed] [Google Scholar]
- 14. Zachara N. E., Hart G. W. (2004) O-GlcNAc a sensor of cellular state. The role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim. Biophys. Acta 1673, 13–28 [DOI] [PubMed] [Google Scholar]
- 15. Zachara N. E., Hart G. W. (2006) Cell signaling, the essential role of O-GlcNAc! Biochim. Biophys. Acta 1761, 599–617 [DOI] [PubMed] [Google Scholar]
- 16. Wells L., Gao Y., Mahoney J. A., Vosseller K., Chen C., Rosen A., Hart G. W. (2002) Dynamic O-glycosylation of nuclear and cytosolic proteins. Further characterization of the nucleocytoplasmic β-N-acetylglucosaminidase, O-GlcNAcase. J. Biol. Chem. 277, 1755–1761 [DOI] [PubMed] [Google Scholar]
- 17. Slawson C., Copeland R. J., Hart G. W. (2010) O-GlcNAc signaling. A metabolic link between diabetes and cancer? Trends Biochem. Sci. 35, 547–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells L., Vosseller K., Hart G. W. (2003) A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell. Mol. Life Sci. 60, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kamemura K., Hart G. W. (2003) Dynamic interplay between O-glycosylation and O-phosphorylation of nucleocytoplasmic proteins. A new paradigm for metabolic control of signal transduction and transcription. Prog. Nucleic Acids Res. Mol. Biol. 73, 107–136 [DOI] [PubMed] [Google Scholar]
- 20. Macauley M. S., Vocadlo D. J. (2010) Increasing O-GlcNAc levels. An overview of small molecule inhibitors of O-GlcNAcase. Biochim. Biophys. Acta 1800, 107–121 [DOI] [PubMed] [Google Scholar]
- 21. Yuzwa S. A., Macauley M. S., Heinonen J. E., Shan X., Dennis R. J., He Y., Whitworth G. E., Stubbs K. A., McEachern E. J., Davies G. J., Vocadlo D. J. (2008) A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat. Chem. Biol. 4, 483–490 [DOI] [PubMed] [Google Scholar]
- 22. Alvarez-Soria M. A., Herrero-Beaumont G., Moreno-Rubio J., Calvo E., Santillana J., Egido J., Largo R. (2008) Long term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthritis Cartilage 16, 1484–1493 [DOI] [PubMed] [Google Scholar]
- 23. Macauley M. S., Shan X., Yuzwa S. A., Gloster T. M., Vocadlo D. J. (2010) Elevation of global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem. Biol. 17, 949–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bailón-Plaza A., Lee A. O., Veson E. C., Farnum C. E., van der Meulen M. C. (1999) BMP-5 deficiency alters chondrocytic activity in the mouse proximal tibial growth plate. Bone 24, 211–216 [DOI] [PubMed] [Google Scholar]
- 25. Mikic B., Clark R. T., Battaglia T. C., Gaschen V., Hunziker E. B. (2004) Altered hypertrophic chondrocyte kinetics in GDF-5 deficient murine tibial growth plates. J. Orthop. Res. 22, 552–556 [DOI] [PubMed] [Google Scholar]
- 26. Fukai A., Kawamura N., Saito T., Oshima Y., Ikeda T., Kugimiya F., Higashikawa A., Yano F., Ogata N., Nakamura K., Chung U. I., Kawaguchi H. (2010) Akt1 in murine chondrocytes controls cartilage calcification during endochondral ossification under physiologic and pathologic conditions. Arthritis Rheum. 62, 826–836 [DOI] [PubMed] [Google Scholar]
- 27. Ijiri K., Zerbini L. F., Peng H., Correa R. G., Lu B., Walsh N., Zhao Y., Taniguchi N., Huang X. L., Otu H., Wang H., Wang J. F., Komiya S., Ducy P., Rahman M. U., Flavell R. A., Gravallese E. M., Oettgen P., Libermann T. A., Goldring M. B. (2005) A novel role for GADD45β as a mediator of MMP-13 gene expression during chondrocyte terminal differentiation. J. Biol. Chem. 280, 38544–38555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Temu T. M., Wu K. Y., Gruppuso P. A., Phornphutkul C. (2010) The mechanism of ascorbic acid-induced differentiation of ATDC5 chondrogenic cells. Am. J. Physiol. Endocrinol. Metab. 299, E325–E334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Macauley M. S., Bubb A. K., Martinez-Fleites C., Davies G. J., Vocadlo D. J. (2008) Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J. Biol. Chem. 283, 34687–34695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brochhausen C., Lehmann M., Halstenberg S., Meurer A., Klaus G., Kirkpatrick C. J. (2009) Signaling molecules and growth factors for tissue engineering of cartilage. What can we learn from the growth plate? J. Tissue Eng. Regen. Med. 3, 416–429 [DOI] [PubMed] [Google Scholar]
- 31. Vu T. H., Shipley J. M., Bergers G., Berger J. E., Helms J. A., Hanahan D., Shapiro S. D., Senior R. M., Werb Z. (1998) MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93, 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Challa T. D., Rais Y., Ornan E. M. (2010) Effect of adiponectin on ATDC5 proliferation, differentiation, and signaling pathways. Mol. Cell. Endocrinol. 323, 282–291 [DOI] [PubMed] [Google Scholar]
- 33. Bobick B. E., Kulyk W. M. (2008) Regulation of cartilage formation and maturation by mitogen-activated protein kinase signaling. Birth Defects Res. C Embryo Today 84, 131–154 [DOI] [PubMed] [Google Scholar]
- 34. Watanabe H., de Caestecker M. P., Yamada Y. (2001) Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-β-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem. 276, 14466–14473 [DOI] [PubMed] [Google Scholar]
- 35. Ulici V., Hoenselaar K. D., Gillespie J. R., Beier F. (2008) The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev. Biol. 8, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawasaki Y., Kugimiya F., Chikuda H., Kamekura S., Ikeda T., Kawamura N., Saito T., Shinoda Y., Higashikawa A., Yano F., Ogasawara T., Ogata N., Hoshi K., Hofmann F., Woodgett J. R., Nakamura K., Chung U. I., Kawaguchi H. (2008) Phosphorylation of GSK-3β by cGMP-dependent protein kinase II promotes hypertrophic differentiation of murine chondrocytes. J. Clin. Invest. 118, 2506–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J., Torii M., Liu H., Hart G. W., Hu Z. Z. (2011) dbOGAP. An integrated bioinformatics resource for protein O-GlcNAcylation. BMC Bioinformatics 12, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chatham J. C., Nöt L. G., Fülöp N., Marchase R. B. (2008) Hexosamine biosynthesis and protein O-glycosylation. The first line of defense against stress, ischemia, and trauma. Shock 29, 431–440 [DOI] [PubMed] [Google Scholar]
- 39. Hatsell S., Medina L., Merola J., Haltiwanger R., Cowin P. (2003) Plakoglobin is O-glycosylated close to the N-terminal destruction box. J. Biol. Chem. 278, 37745–37752 [DOI] [PubMed] [Google Scholar]
- 40. Sayat R., Leber B., Grubac V., Wiltshire L., Persad S. (2008) O-GlcNAc-glycosylation of β-catenin regulates its nuclear localization and transcriptional activity. Exp. Cell Res. 314, 2774–2787 [DOI] [PubMed] [Google Scholar]
- 41. Dias W. B., Cheung W. D., Wang Z., Hart G. W. (2009) Regulation of calcium/calmodulin-dependent kinase IV by O-GlcNAc modification. J. Biol. Chem. 284, 21327–21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gandy J. C., Rountree A. E., Bijur G. N. (2006) Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1. FEBS Lett. 580, 3051–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fülöp N., Mason M. M., Dutta K., Wang P., Davidoff A. J., Marchase R. B., Chatham J. C. (2007) Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am. J. Physiol. Cell Physiol. 292, C1370–C1378 [DOI] [PubMed] [Google Scholar]
- 44. Hu Y., Belke D., Suarez J., Swanson E., Clark R., Hoshijima M., Dillmann W., H. (2005) Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res. 13, 1006–1013 [DOI] [PubMed] [Google Scholar]
- 45. Wang J., Zhou J., Bondy C. A. (1999) Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 13, 1985–1990 [DOI] [PubMed] [Google Scholar]
- 46. Pritchard J. J. (1952) A cytological and histochemical study of bone and cartilage formation in the rat. J. Anat. 86, 259–277 [PMC free article] [PubMed] [Google Scholar]
- 47. Lefebvre T., Dehennaut V., Guinez C., Olivier S., Drougat L., Mir A. M., Mortuaire M., Vercoutter-Edouart A. S., Michalski J. C. (2010) Dysregulation of the nutrient/stress sensor O-GlcNAcylation is involved in the etiology of cardiovascular disorders, type-2 diabetes, and Alzheimer disease. Biochim. Biophys. Acta 1800, 67–79 [DOI] [PubMed] [Google Scholar]
- 48. Patti M. E., Virkamäki A., Landaker E. J., Kahn C. R., Yki-Järvinen H. (1999) Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance of early postreceptor insulin signaling events in skeletal muscle. Diabetes 48, 1562–1571 [DOI] [PubMed] [Google Scholar]
- 49. Vosseller K., Wells L., Lane M. D., Hart G. W. (2002) Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U.S.A. 99, 5313–5318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang X., Ongusaha P. P., Miles P. D., Havstad J. C., Zhang F., So W. V., Kudlow J. E., Michell R. H., Olefsky J. M., Field S. J., Evans R. M. (2008) Phosphoinositide signaling links O-GlcNAc transferase to insulin resistance. Nature 451, 964–969 [DOI] [PubMed] [Google Scholar]
- 51. Whelan S. A., Dias W. B., Thiruneelakantapillai L., Lane M. D., Hart G. W. (2010) Regulation of insulin receptor substrate 1 (IRS-1)/AKT kinase-mediated insulin signaling by O-linked β-N-acetylglucosamine in 3T3-L1 adipocytes. J. Biol. Chem. 285, 5204–5211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park S., Park S. H., Baek J. Y., Jy Y. J., Kim K. S., Roth J., Cho J. W., Choe K. M. (2011) Protein O-GlcNAcylation regulates Drosophila growth through the insulin signaling pathway. Cell. Mol. Life Sci. 68, 3377–3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Krześlak A., Jóźwiak P., Lipińska A. (2011) Down-regulation of β-N-acetyl-d-glucosaminidase increases Akt1 activity in thyroid anaplastic cancer cells. Oncol. Rep. 26, 743–749 [DOI] [PubMed] [Google Scholar]
- 54. Ishihara K., Takahashi I., Tsuchiya Y., Hasegawa M., Kamemura K. (2010) Characteristic increase in nucleocytoplasmic protein glycosylation by O-GlcNAc in 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 398, 489–494 [DOI] [PubMed] [Google Scholar]
- 55. Hsieh T. J., Lin T., Hsieh P. C., Liao M. C., Shin S. J. (2012) Suppression of glutamine:fructose-6-phosphate amidotransferase-1 inhibits adipogenesis in 3T3-L1 adipocytes. J. Cell. Physiol. 227, 108–115 [DOI] [PubMed] [Google Scholar]
- 56. Tardio L., Herrero-Beaumont G., Gomez-Barrena E., Largo R. (2010) Osteoarthritis leads to increased levels of protein O-linked N-Acetylglucosamine in the cartilage of patients. Osteoarthritis Cartilage 18, 111 (abstr.) [DOI] [PubMed] [Google Scholar]
- 57. Dreier R. (2010) Hypertrophic differentiation of chondrocytes in osteoarthritis. The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 12, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tchetina E. V. (2011) Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis Res. Ther. 2011, 683970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldberg H., Whiteside C., Fantus I. G. (2011) O-Linked N-acetylglucosamine supports p38 MAPK activation by high glucose in glomerular mesangial cells. Am. J. Physiol. Endocrinol. Metab. 301, E713–E726 [DOI] [PubMed] [Google Scholar]
- 60. Tallent M. K., Varghis N., Skorobogatko Y., Hernandez-Cuebas L., Whelan K., Vocadlo D. J., Vosseller K. (2009) In vivo modulation of O-GlcNAc levels regulates hippocampal synaptic plasticity through interplay with phosphorylation. J. Biol. Chem. 284, 174–181 [DOI] [PubMed] [Google Scholar]
- 61. Starkman B. G., Cravero J. D., Delcarlo M., Loeser R. F. (2005) IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem. J. 389, 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang S., Huang X., Sun D., Xin X., Pan Q., Peng S., Liang Z., Luo C., Yang Y., Jiang H., Huang M., Chai W., Ding J., Geng M. (2012) Extensive cross-talk between O-GlcNAcylation and phosphorylation regulates Akt signaling. PLoS One 7, e37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taylor R. P., Parker G. J., Hazel M. W., Soesanto Y., Fuller W., Yazzie M. J., McClain D. A. (2008) Glucose deprivation stimulates O-GlcNAc modification of proteins through up-regulation of O-linked N-acetylglucosaminyltransferase. J. Biol. Chem. 283, 6050–6057 [DOI] [PubMed] [Google Scholar]
- 64. Zachara N. E., O'Donnell N., Cheung W. D., Mercer J. J., Marth J. D., Hart G. W. (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J. Biol. Chem. 279, 30133–30142 [DOI] [PubMed] [Google Scholar]
- 65. Lunde I. G., Aronsen J. M., Kvaløy H., Qvigstad E., Sjaastad I., Tønnessen T., Christensen G., Grønning-Wang L. M., Carlson C. R. (2012) Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiol. Genomics. 44, 162–172 [DOI] [PubMed] [Google Scholar]
- 66. Belke D. D. (2011) Swim-exercised mice show a decreased level of protein O-GlcNAcylation and expression of O-GlcNAc transferase in heart. J. Appl. Physiol. 111, 157–162 [DOI] [PubMed] [Google Scholar]
- 67. Kazemi Z., Chang H., Haserodt S., McKen C., Zachara N. E. (2010) O-Linked β-N-acetylglucosamine (O-GlcNAc) regulates stress-induced heat shock protein expression in a GSK-3β-dependent manner. J. Biol. Chem. 285, 39096–39107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.