Background: Mycobacterium tuberculosis survives within the host by modulating host immune responses.
Results: RD-1/ESAT-6 from M. tuberculosis induces IL-1β in dendritic cells to direct Th2 differentiation, which facilitates disease progression by inhibiting host protective Th1 responses.
Conclusion: Cytokines produced by M. tuberculosis-infected cells play a role in promoting Th2 responses to subvert host immunity.
Significance: These findings contribute to understanding the immune evasion mechanisms of M. tuberculosis.
Keywords: Cytokine, Dendritic Cells, Interleukin, Mycobacterium tuberculosis, T Cell
Abstract
Mycobacterium tuberculosis, the causative agent of tuberculosis (TB), resides and replicates within phagocytes and persists in susceptible hosts by modulating protective innate immune responses. Furthermore, M. tuberculosis promotes T helper 2 (Th2) immune responses by altering the balance of T cell polarizing cytokines in infected cells. However, cytokines that regulate Th2 cell differentiation during TB infection remain unknown. Here we show that IL-1β, produced by phagocytes infected by virulent M. tuberculosis strain H37Rv, directs Th2 cell differentiation. In sharp contrast, the vaccine strain bacille Calmette-Guérin as well as RD-1 and ESAT-6 mutants of H37Rv failed to induce IL-1β and promote Th2 cell differentiation. Furthermore, ESAT-6 induced IL-1β production in dendritic cells (DCs), and CD4+ T cells co-cultured with infected DCs differentiated into Th2 cells. Taken together, our findings indicate that IL-1β induced by RD-1/ESAT-6 plays an important role in the differentiation of Th2 cells, which in turn facilitates progression of TB by inhibiting host protective Th1 responses.
Introduction
Tuberculosis (TB)3 is a global health problem that causes >nine million new cases and claims two million lives each year (1). According to the World Health Organization (WHO), nearly 2 billion people or nearly one third of the global population is latently infected with Mycobacterium tuberculosis, the etiological agent of TB (2). Current therapy for TB is lengthy and consists of multiple antibiotics, which have the risk of generating drug-resistant variants of M. tuberculosis (3–5). In fact, almost all countries, irrespective of their socioeconomic status, are now under threat from multiple drug-resistant and extensively drug-resistant stains of M. tuberculosis (3, 5, 6). Unfortunately, the rate by which new drug-resistant variants of this deadly organism are generated is much faster than the rate by which new generation antibiotics are being discovered, which has already resulted in the appearance of totally drug-resistant TB strains (7, 8). Therefore, alternative treatment methods that avoid the generation of multiple drug-resistant strains are urgently needed. Immunotherapy is one possible option that might avoid such risks.
It has been well established that T helper 1 (Th1) cells producing interferon (IFN)-γ play a central role in host resistance to M. tuberculosis infection (9, 10). Consequently, interleukin (IL)-12-, IL-12 receptor-, signal transducer of activation and transcription (STAT)-4-, and T-bet-deficient animals, which are defective in the generation of Th1 responses, are highly susceptible to M. tuberculosis infection (9, 11). Similarly, individuals with mutations in IL-12 or its signaling components are highly susceptible to M. tuberculosis infection (12, 13). It has been well documented that M. tuberculosis inhibits Th1 cells by inhibiting IL-12 production in infected cells (14). On the other hand, transforming growth factor (TGF)-β produced by the infected cells directs the differentiation of induced regulatory T cells (Tregs) (15–17). In fact, it has been shown that Tregs are induced during the progression of TB, and this is associated with inhibition of protective T cell responses in the host (18, 19). M. tuberculosis-infected cells also produce IL-6, which, in combination with TGF-β, provides favorable conditions for the differentiation of Th17 cells (20, 21). All Th cell subsets, including Th1 cells, Th2 cells, Th17 cells, and Tregs are in a dynamic balance, and it has been therefore assumed that impaired Th1 responses during the progression of TB are associated with increased Th2 responses. However, the cytokines produced by the infected cells that play a role in the development of Th2 responses during progression of TB have not been identified.
It has previously been shown that M. tuberculosis-infected macrophages produce copious amounts of IL-1β (22, 23). IL-1β plays an important role in the differentiation of Th2 cells (24). Thus, we examined whether IL-1β produced by infected phagocytes is responsible for Th2 cell differentiation during progression of TB. We observed that dendritic cells (DCs) infected with the virulent M. tuberculosis strain H37Rv induced copious amounts of IL-1β, whereas the vaccine strain bacille Calmette-Guérin (BCG) and M. tuberculosis strains carrying mutations in the region of difference (RD)-1 (H37RvΔRD-1) or early secretory antigenic target (ESAT)-6 (H37RvΔESAT-6) failed to induce IL-1β. Importantly, complementation of H37RvΔESAT-6 with ESAT-6 restored IL-1β production in DCs. On the other hand, H37Rv, BCG, and all H37Rv mutants induced comparable amounts of IL-12. H37Rv-infected DCs induced both IFN-γ- and IL-4-producing CD4+ T cells, whereas BCG, H37RvΔRD-1, and H37RvΔESAT-6 selectively induced IFN-γ-producing cells. We confirmed these findings by adoptive transfer experiments with naïve CD4+ T cells isolated from IL-1βR knock-out mice. Taken together, our findings indicate that IL-1β produced by infected phagocytes plays an important role in Th2 cell differentiation during TB.
EXPERIMENTAL PROCEDURES
Ethics Statement
Animal experiments were performed according to the guidelines approved by the Institutional Animal Ethics Committee meeting held on November 22, 2007 at ICGEB (approval ID; ICGEB/IAEC/IMM-13/2007), New Delhi, India, and the Department of Biotechnology (DBT) guidelines, Government of India. All mice used for experiments were ethically killed by asphyxiation in carbon dioxide according to institutional and DBT regulations.
Mice
C57BL/6 and OT-II T cell receptor (TCR) transgenic mice (6–8 weeks of age) were initially purchased from the Jackson Laboratory. IL-1βR−/− mice were purchased from the Jackson Laboratory. All animals were subsequently bred and maintained in the animal facility of the International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi, India.
Bacteria
M. tuberculosis strain H37Rv was a kind gift from the Colorado State University repository. BCG, H37RvΔRD-1, H37RvΔESAT-6, and ESAT-6-complemented H37RvΔESAT-6 were a kind gift from Prof. David Sherman (SBRI, Seattle, WA). All mycobacterial strains were grown in 7H9 (Middlebrook, Difco) medium supplemented with 10% ADC (albumin, dextrose, and catalase; Difco) and with 0.05% Tween 80 and 0.2% glycerol, and cultures were grown to mid-log phase. Aliquots of the cultures in 20% glycerol were preserved at −80 °C, and these cryopreserved stocks were used for infections.
Reagents
Luminex kits were purchased from Bio-Rad. Granulocyte-monocyte colony-stimulating factor (GM-CSF) and IL-4 were obtained from R&D Biosystems. Purified anti-mouse IL-1β antibody (clone B122) was purchased from e-Biosciences.
Generation of DCs
Mice were euthanized, and the femurs were isolated. Bone marrow was flushed out with RPMI 1640 medium using a 2.0-ml syringe (26.5 gauge). The cells were washed twice with PBS and then cultured in complete RPMI 1640 medium (Invitrogen) supplemented with GM-CSF (40 ng/ml) and IL-4 (10 ng/ml) on 24-well plates (1 million cells/ml). On the 3rd day, 75% of the medium was replaced with fresh DC culture medium. On day 5, the suspended cells were removed, and the loosely adherent cells were collected as immature DCs (CD11c-positive cells were >90%). For mature DCs, immature DCs were stimulated with lipopolysaccharide (LPS, 1 μg/ml) for 24 h. FACS analysis using anti-CD11c, -CD80, -CD86, and -MHC class II antibodies suggested that >95% of the cells were conventional DCs.
Bacterial Infections and Co-culture of DCs with CD4+ T Cells
In Vitro
Bone marrow cells were isolated from mice (C57BL/6), differentiated into immature DCs as described above, and cultured in 24-well plates (1 × 106 cells/well). Cells were infected with H37Rv, H37RvΔRD-1, BCG, H37RvΔESAT-6, or ESAT-6-complemented H37RvΔESAT-6 (multiplicity of infection of 1:10). Supernatants from cells were collected at 24, 48, and 72 h for cytokine profiling. For T cell differentiation, CD4+ T cells (1 × 106) were purified by magnetic activated cell sorting method (CD4+ T cell isolation beads kit; Miltenyi Biotech) from OT-II TCR transgenic mice and co-cultured with immature DCs (1 × 106) infected with H37Rv, H37RvΔRD-1, BCG, H37RvΔESAT-6, or ESAT-6-complemented H37RvΔESAT-6 in the presence of ovalbumin (10 μg/ml) peptide (Thermo Scientific) for 72 h. Then, CD4+ T cells were harvested and subjected to intracellular staining for IL-4 and IFN-γ expression.
In Vivo
Mice (C57BL/6) used for these experiments were infected with H37Rv, H37RvΔRD-1, H37RvΔESAT-6, BCG, or ESAT-6-complemented H37RvΔESAT-6 through the aerosol route.
Detection of Cytokines
Cytokines in the culture supernatant of DCs were assayed by a Luminex microbead-based multiplexed assay using commercially available kits according to the manufacturer's protocol (BioPlex, Bio-Rad).
Antibody Treatment
For depletion of IL-1β, we cultured cells in the presence of anti-IL-1β mAb (10 μg/ml) and collected the supernatant for cytokine profiling after different time points.
T Cell Adoptive Transfer
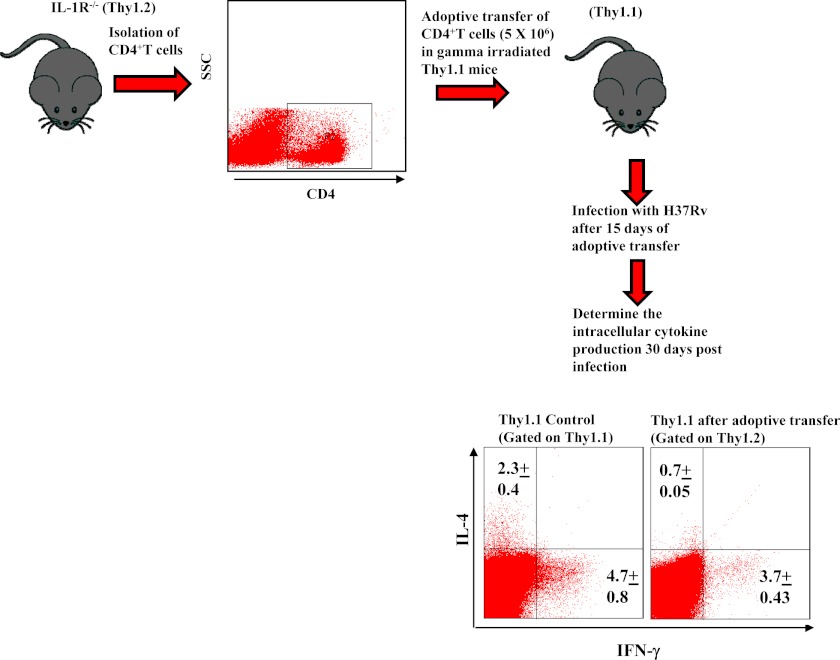
For adoptive transfer experiments, Thy1.1+ mice were γ-irradiated (8 rads/s for 100 s) and rested for a day. CD4+ T cells, isolated from lymph nodes of IL-1R−/− mice (Thy1.2+ background) were then adoptively transferred into the irradiated recipient mice (2–4 × 106 cells/mouse). After 15 days recipient mice were challenged with H37Rv through the aerosol route.
Intracellular Cytokine Staining
For intracellular cytokine staining, cells were treated with 50 ng/ml phorbol 12-myristate 13-acetate and 500 ng/ml ionomycin in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich) added during the last 6 h of culture. Cells were washed twice with PBS and resuspended in a permeabilization buffer (Cytofix/Cytoperm kit; BD Biosciences) and stained with the following fluorescently conjugated monoclonal antibodies: anti-CD4 (clone GK1.5)-allophycocyanin, anti-IFN-γ (clone XMG1.2)-fluorescein isothiocyanate (FITC), anti-IFN-γ (clone XMG1.2)- allophycocyanin, anti-IL-4 (clone GK1.5)-phycoerythrin (eBiosciences). Fluorescence intensity was measured by flow cytometry (FACS Calibur or FACS CantoII; BD Biosciences), and data were analyzed with FlowJo (Tree star).
Western Blot Analysis
DCs derived from C57BL/6 mice were infected with H37Rv or H37RvΔESAT-6. For T cell differentiation, CD4+ T cells (1 × 106) were purified by magnetic activated cell sorting from OT-II TCR transgenic mice and co-cultured with DCs (1 × 106) infected with H37Rv or H37RvΔESAT-6 in the presence of ovalbumin peptide (5 μg/ml). Whole cell lysate was prepared by using lysis buffer (50 mm Tris-HCl, pH 7.4, 5 mm EDTA, 120 mm NaCl, 0.5% Nonidet P-40, 0.5 mm NaF, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride) along with HALTTM phosphatase inhibitor mixture (78420; Thermo Scientific) and protease inhibitor mixture (78410; Thermo Scientific) for 1 h. Samples were electrophoresed on a 10% SDS-polyacrylamide gel and electroblotted onto polyvinylidene difluoride (PVDF) membranes. Blots were blocked for 1 h in 5% BSA in PBST (PBS with 0.1% Tween 20). STAT6, pSTAT6, STAT4, and pSTAT4 proteins were detected with anti-STAT6 (Sc-621), -pSTAT6 (Sc-11762-R), -STAT4 (Sc-486) and -pSTAT4 (Sc-16317-R) polyclonal antibodies, respectively, at a dilution of 1:250 and as recommended by the manufacturer (Santa Cruz Biotechnology). Goat anti-rabbit immunoglobulin G-conjugated horseradish peroxidase (Sc-2004) (diluted 1:2500) was used as a secondary antibody (Santa Cruz Biotechnology). Immunoblotting for GAPDH was carried out to confirm equal loading.
Statistical Analysis
All data were derived from at least three independent experiments. Statistical analyses were conducted using SPSS10 software, and values were presented as mean ± S.D. Significant differences between the groups were determined by ANOVA followed by Tukey's multiple comparison test (SPSS software). A value of p < 0.05 was accepted as an indication of statistical significance.
RESULTS
Virulent M. tuberculosis H37Rv but Not BCG Induces IL-1β and Th2 Cells
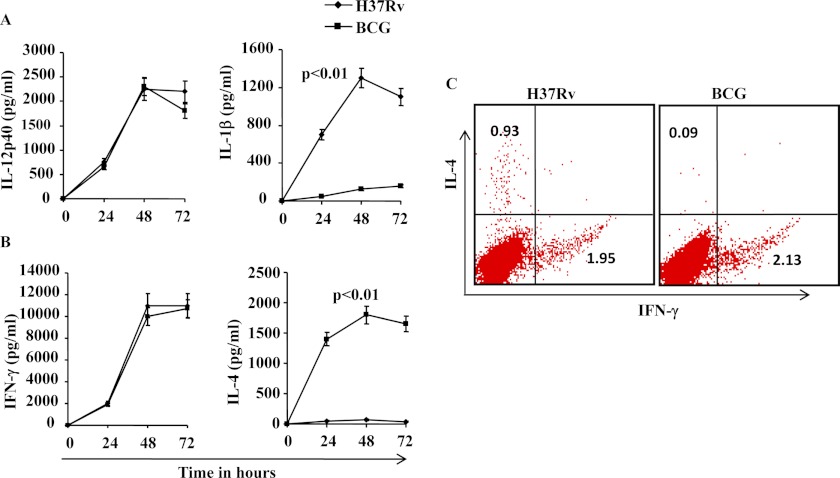
It is now well established that Th1 cell responses play a protective role (10) whereas Th2 and induced Tregs facilitate progression of TB (11, 18). Previous studies have provided evidence that BCG induces Th1 cell responses (25), whereas virulent H37Rv induces both Th1 and Th2 cell responses (26). Despite adequate Th1 cell responses, M. tuberculosis continues to progress toward disease in susceptible hosts, suggesting that Th1 immunity is not sufficient for optimal host protection. Differentiation of Th1 and Th2 subsets requires IL-12 and IL-4, respectively (14, 27). Whereas IL-12 produced by professional antigen-presenting cells (APCs) drives Th1 cell differentiation (13), professional APCs do not produce IL-4. However, it has been shown that under some circumstances, IL-1β produced by APCs plays an important role in Th2 cell differentiation (28, 29). Therefore, we determined the cytokines produced by M. tuberculosis- or BCG-infected DCs. We generated bone marrow-derived DCs from C57BL/6 mice and infected these cells with H37Rv or BCG. We determined the T cell-polarizing cytokines IL-12 and IL-1β. We found that both H37Rv and BCG induced IL-12 whereas only H37Rv induced IL-1β (Fig. 1A). We next determined whether these infected DCs differentially facilitate the differentiation of Th cells. To test this hypothesis, we co-cultured CD4+ T cells isolated from OT-II TCR transgenic mice in the presence of ovalbumin peptide. We observed that H37Rv-infected DCs induced large amounts of both IFN-γ and IL-4, whereas BCG-infected DCs induced only IFN-γ in T cells (Fig 1B). This observation suggests that M. tuberculosis induces either Th0 cells, which produce both IFN-γ and IL-4, or induce distinct populations of Th1 (IFN-γ producers) and Th2 (IL-4 producers) cells. Therefore, to distinguish between these possibilities, we performed intracellular staining of CD4+ T cells for IFN-γ and IL-4 production. We found that H37Rv induced few if any CD4+ T cells producing both IFN-γ and IL-4 simultaneously. In sharp contrast, BCG induced IFN-γ-producing cells only (Fig. 1C).
FIGURE 1.
Capacity of M. tuberculosis strains to induce IL-1β secretion in DCs co-cultured with OT-II CD4+ T cells and to promote Th2 cell differentiation. A, IL-12p40 and IL-1β levels after infection of DCs with H37Rv or BCG. IL-1β levels were significantly increased (p < 0.01) in H37Rv-infected DCs compared with DCs infected with BCG. B, IFN-γ and IL-4 levels after infection of DCs with H37Rv or BCG. IL-4 levels were significantly higher (p < 0.01) in H37Rv-infected DCs than BCG-infected DCs. C, intracellular IFN-γ and IL-4 levels produced by CD4+ T cells from OT-II TCR transgenic mice co-cultured with DCs infected with H37Rv or BCG. The results shown are representative of at least three or four independent experiments. Error bars, ±S.D.
RD-1/ESAT-6 Induces IL-1β Production and Th2 Cell Differentiation
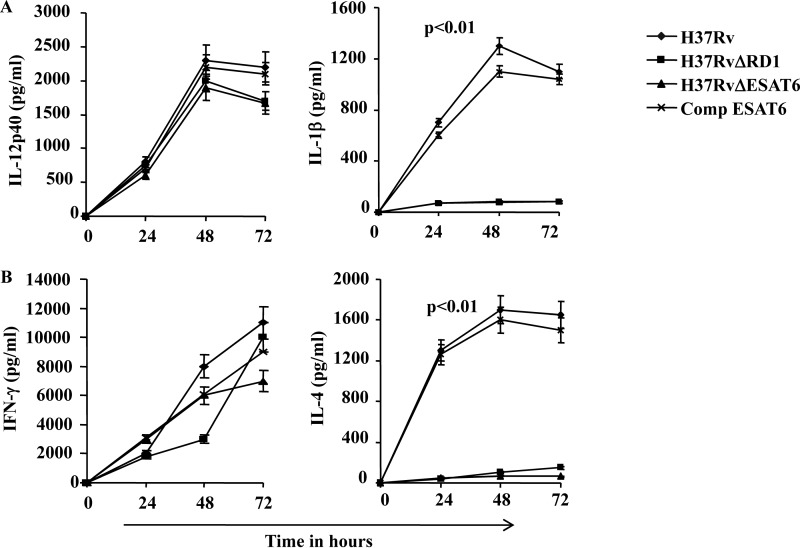
From the preceding section it was clear that H37Rv induces both Th1 and Th2 responses, whereas BCG selectively induces Th1 responses. Compared with H37Rv, BGC lacks several genomic segments that are called regions of difference (RD), and a total of 22 such RD regions have been characterized. Among them, RD-1 has been shown to play an important role in the pathogenicity of M. tuberculosis and its capacity to subvert the immune system (30, 31). Therefore, we tested whether RD-1 has a role in inducing IL-1β in DCs and to promote Th2 cell responses. ESAT-6 and CFP-10 are two important antigenic proteins encoded within the RD-1 region that are secreted as a complex. Thus, mutation in either protein impairs formation and secretion of the complex (30, 32, 33). Therefore, to understand the role of RD-1 we performed experiments with deletion mutants of H37Rv in RD-1 (H37RvΔRD-1) or ESAT-6 (H37RvΔESAT-6). Interestingly, neither the H37RvΔRD-1 mutant nor the H37RvΔESAT-6 mutant was able to induce IL-1β, whereas both mutants induced levels of IL-12 comparable with H37Rv and BCG (Fig. 2A). Taken together, these observations indicated that the RD-1 of H37Rv plays an important role in the production of IL-1β by infected DCs, but has little if any role in the production of IL-12 (Fig. 2A). Next we tested whether cytokines (and possibly IL-1β) produced by the H37Rv-infected DCs assist in the differentiation of Th2 cells. For this purpose, we co-cultured naïve CD4+ T cells from OT-II TCR transgenic mice with infected DCs pulsed with ovalbumin peptide. We determined cytokines produced in the culture supernatant as well as cytokines produced by the cells intracellularly. We found that DCs infected with H37Rv induced both IL-4- and IFN-γ-producing CD4+ T cells whereas BCG-infected DCs selectively induced IFN-γ-producing CD4+ T cells (Fig. 2B). These observations clearly indicate that the RD-1 region of virulent strain H37Rv plays a dominant role in inducing Th2 responses.
FIGURE 2.
Cytokine profiling from DCs infected with H37Rv or mutant strains. A, measurements of levels of IL-12p40 and IL-1β from supernatants of DCs infected with H37Rv or mutant strains (H37RvΔRD1, H37RvΔESAT-6, or ESAT-6-complemented H37RvΔESAT-6) of H37Rv at different points. B, levels of IFN-γ and IL-4 from the culture supernatants of DCs infected with H37Rv or mutants (H37RvΔRD1, H37RvΔESAT-6, or ESAT-6-complemented H37RvΔESAT-6). H37Rv and ESAT-6-complemented H37RvΔESAT-6 (Comp ESAT-6) direct differentiation of both Th1 (IL-12p40 and IFN-γ) and Th2 (IL-4) cytokine responses as well as IL-1β production, whereas H37RvΔRD1 and H37RvΔESAT-6 induce only Th1 cytokines in infected DCs co-cultured with naïve OT-II CD4+ T cells. IL-4 and IL-1β levels were significantly increased (p < 0.01) in H37Rv and Comp-ESAT-6 infected DCs compared with DCs infected with the other strains. The results shown are representative of at least three independent experiments. Error bars, ±S.D.
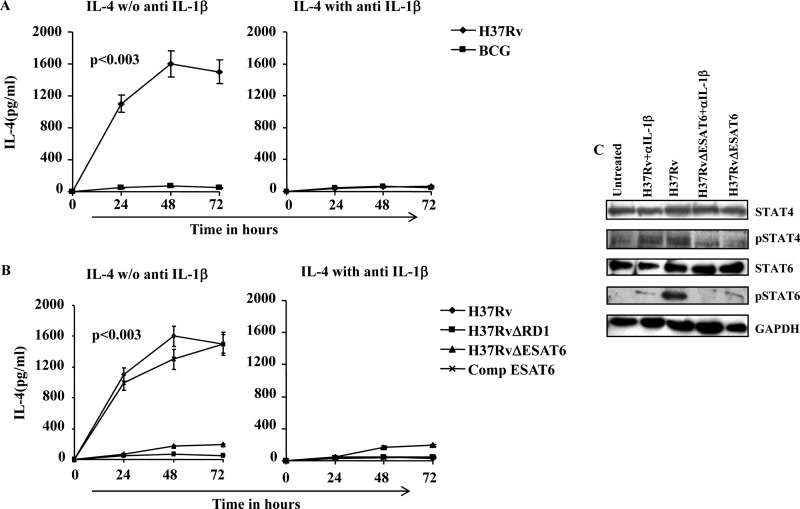
Neutralization of IL-1β Abrogates Th2 Cell Differentiation in T Cell Co-cultures with Infected DCs
To investigate further the role of IL-1β in Th2 cell differentiation induced by H37Rv, we performed T cell co-culture experiments with infected DCs in the presence of anti-IL-1β antibodies. Neutralization of IL-1β with antibody dramatically abrogated IL-4 production by CD4+ T cells (Fig. 3A). Interestingly, DCs infected with BCG, H37RvΔRD-1, or H37RvΔESAT-6 mutants selectively induced IFN-γ production, and this was not affected by anti-IL-1β antibodies, which thus served as a negative control (Fig. 3B). We also neutralized IL-1β in co-cultures of T cells with DCs infected with RD-1-complemented BCG. Similar to the results obtained for H37Rv, we found that neutralization of IL-1β dramatically abrogated Th2 cell differentiation induced by RD-1-complemented BCG. To confirm these findings, we investigated the molecular mechanism of Th cell differentiation in infected DCs co-cultured with T cells. For this purpose, we performed Western blot analysis of STAT6, pSTAT6, STAT4, and pSTAT4 proteins in OT-II CD4+ T cells from cultures with infected DCs. We observed phosphorylation of STAT-6 in CD4+ T cells co-cultured with H37Rv-infected DCs, and this was dramatically reduced when IL-1β was neutralized by antibodies (Fig. 3C). In contrast, CD4+ T cells co-cultured with H37RvΔESAT-6 were unable to induce STAT-6 phosphorylation. We also examined STAT-4 phosphorylation and found an opposite pattern (Fig. 3C). These data suggest that IL-1β plays an important role in Th2 cell polarization during M. tuberculosis infection and that ESAT-6 or factors controlled by ESAT-6 are responsible for induction of IL-1β.
FIGURE 3.
ESAT-6 induces Th2 cell differentiation via IL-1β production. A, IL-4 production in DCs co-cultured with naïve OT-II CD4+ T cells after infection with H37Rv or BCG in the presence or absence of anti-IL-1β. B, IL-4 production in DCs co-cultured with naïve OT-II CD4+ T cells after infection with H37Rv or mutant strains (H37RvΔRD1, H37RvΔESAT-6, and Comp ESAT-6) in the presence or absence of anti-IL-1β. IL-4 levels were higher in cultures with H37Rv or Comp ESAT-6 compared with cultures with BCG or other mutant strains (p < 0.003). C, Western blot analysis of STAT6, pSTAT6, STAT4, and pSTAT4 proteins from DCs infected with H37Rv or H37RvΔESAT-6 and co-cultured with OT-II CD4+ T cells, to show modulated Th1 and Th2 responses in the presence or absence of anti-IL-1β. The results shown are representative of at least four independent experiments. Error bars, ±S.D.
Cytokine Profiles in Lungs of Infected Animals
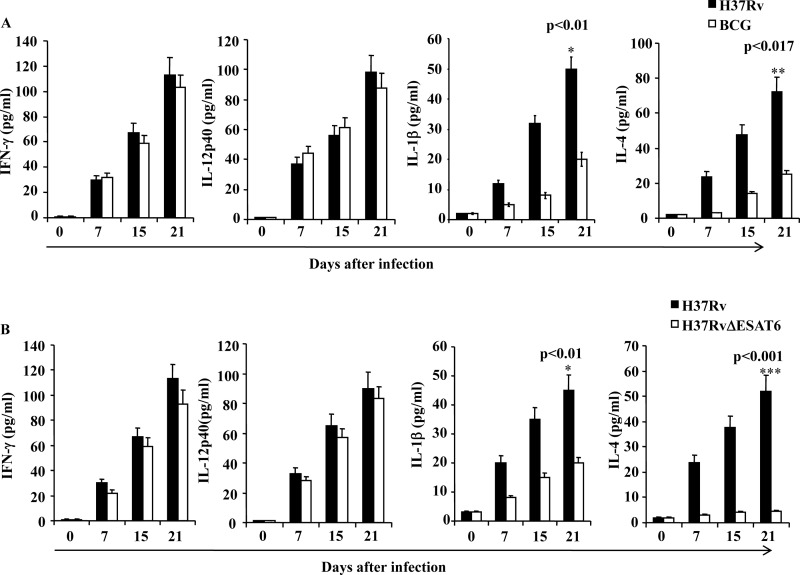
Next, we complemented our in vitro finding showing that the RD-1 region of virulent strain H37Rv induces IL-1β to promote Th2 cell differentiation, with in vivo experiments. We also performed cytokine profiling studies of lung homogenates obtained from C57BL/6 mice infected with H37Rv, BCG, or H37RvΔESAT-6. Consistent with our in vitro data, we observed that H37Rv-infected animals produced both IL-4 and IFN-γ, whereas BCG-infected animals induced only IFN-γ at all time points analyzed (Fig. 4A). To obtain further information about the relevant T cell-differentiating cytokines, we determined the levels of IL-12 and IL-1β, as in our in vitro experiments. We detected both IL-1β and IL-12 in lung homogenates from H37Rv-infected mice, whereas BCG-infected animals selectively produced IL-12, at levels similar to H37Rv-infected animals (Fig. 4A). We also determined cytokines in the lung homogenates of animals infected with H37RvΔRD-1 or H37RvΔESAT-6. As expected, both groups of infected animals produced IL-12 and IFN-γ but not IL-1β in their lungs (Fig 4B). In contrast, RD-1 complemented BCG-induced IL-12 as well as IL-1β, and both IFN-γ and IL-4 were present (Fig. 4B). Collectively these data indicate that the RD-1 region plays an important role in the induction of IL-1β by H37Rv-infected DCs, which in turn drives Th2 cell differentiation.
FIGURE 4.
H37Rv induces significantly higher Th2 responses and the proinflammatory cytokine IL-1β compared with BCG or H37RvΔESAT-6. A, cytokine levels in lung homogenates of mice at different time points after infection with H37Rv or BCG. B, cytokine levels in lung homogenates of mice at different time points after infection with H37Rv or mutant strain H37RvΔESAT-6. Statistical significance between different strains was determined by one-way ANOVA: *, p < 0.01; **, p < 0.017; ***, p < 0.001. The results shown are representative of at least three independent experiments with six mice within each group. Error bars, ±S.D.
IL-1β Acts Directly on CD4+ T Cells to Promote Th2 Cell Differentiation during M. tuberculosis Infection
To provide further evidence that IL-1β plays an important role in the differentiation of Th2 cells in M. tuberculosis-infected animals, we isolated CD4+ T cells from congenic wild-type Thy1.2 or IL-1βR−/− Thy1.2 mice and adoptively transferred these cells into irradiated Thy1.1 congenic animals followed by infection with H37Rv. Thirty days after infection, spleen cells from these animals were isolated and challenged with H37Rv complete soluble antigen ex vivo, and we stained T cells for the intracellular cytokines IL-4 and IFN-γ. Cytokines produced by adoptively transferred CD4+ T cells were determined by gating on Thy1.2. We observed that CD4+ T cells from wild-type animals had adopted a phenotype characterized by IFN-γ or IL-4 production. In contrast, CD4+ T cells from IL-1βR−/− mice differentiated predominantly into IFN-γ-producing cells (Fig. 5). Therefore, we conclude that IL-1β produced during the progression of TB disease is responsible for the generation of Th2 cell responses.
FIGURE 5.
IL-1β plays an important role in Th2 cell differentiation after infection with H37Rv without affecting Th1 cell differentiation. CD4+ T cells were isolated from congenic wild-type Thy1.2 or IL-1βR−/− Thy1.2 mice and adoptively transferred into irradiated Thy1.1 congenic animals followed by infection with H37Rv. Thirty days after infection, spleen cells from these animals were isolated and challenged with H37Rv complete soluble antigen ex vivo. T cells were then stained for the intracellular cytokines IL-4 and IFN-γ. The results shown are representative of at least three independent experiments with three mice within each group.
DISCUSSION
M. tuberculosis survives within susceptible hosts by altering host protective Th cell responses. IFN-γ-induced macrophage activation that results in the production of nitric oxide radicals is the key mechanism for elimination of the harbored pathogens. Thus, animals that are defective in the production of IL-12, inducible nitric-oxide synthase, IFN-γ, or proteins involved in their signaling pathways exhibit increased susceptibility to M. tuberculosis infection (34). However, M. tuberculosis successfully inhibits IL-12 production in susceptible hosts and thus inhibits the development of Th1 responses (35). In addition, it has clearly been shown that M. tuberculosis not only prevents Th1 responses, but also facilitates Th2 responses (36), which counterregulate host protective Th1 responses. Phagocytes, the natural home for M. tuberculosis, are the predominant source of IL-12 that drives Th1 cell differentiation. In contrast, IL-4, the key cytokine required for the differentiation of Th2 cells, is not produced by APCs. Thus, it is likely that another cytokine is involved in promoting Th2 cell differentiation during the progression of TB. Interestingly, previous studies have shown that IL-1β can act as a co-stimulatory factor for Th2 cells (29). Indeed, we found that IL-1β is induced by M. tuberculosis-infected DCs. We further showed that IL-1β is directly involved in Th2 cell differentiation during M. tuberculosis infection. Therefore, it appears that M. tuberculosis ensures survival not only by directly inhibiting Th1 responses, but also indirectly by mounting Th2 responses. In fact, it has been reported that inhibition of Th2 responses or genetic deficiency in the generation of Th2 responses confers partial resistance to M. tuberculosis infection (37).
Previously, it has been shown that IL-1β-mediated signaling plays an important role in host resistance to M. tuberculosis infection (22, 38). In fact, IL-1β- and IL-1βR-deficient animals have increased susceptibility to M. tuberculosis infection (39). Similarly, mice deficient in the IL-1β signaling adaptor molecule MyD88 are highly susceptible to M. tuberculosis infection (40). However, the precise nature of the host protective immune responses induced by IL-1β during M. tuberculosis infection is unclear. Our results demonstrate that IL-1β induces Th2 cell differentiation, which has been shown to promote progression of TB by inhibiting host protective Th1 responses (11). Therefore, IL-1β might originate two divergent immune responses: (i) IL-1β might induce the MyD88 signaling pathway to promote protective innate host immune responses; and (ii) IL-1β might have a direct role in the differentiation of Th2 cells, which counterregulates protective host adaptive immune responses. Therefore, it will be of future interest to delineate the conditions and mechanisms by which IL-1β elicits these divergent responses.
Previously, we have shown that BCG, M. tuberculosis H37Rv, and mutant strains H37RvΔRD-1 and H37RvΔESAT-6 induce IL-12 in DCs in a Toll-like receptor-2-independent but MyD88-dependent manner (41), suggesting that production of IL-12 is independent of RD-1. In this report we have shown that IL-1β production is dependent on RD-1/ESAT-6. Thus, M. tuberculosis has at least two different sets of factors that control IL-12 and IL-1β production to modulate Th cell differentiation during the progression of TB. Furthermore, it is known that Tregs also assist in the progression of TB (19), whereas the role of Th17 cells remains unclear and might differ during primary and secondary infection (41). Taken together, these data indicate that Th1 cells are protective whereas Th2 cells and Tregs promote disease. These Th cell subsets are in a dynamic balance during M. tuberculosis infection. In this context, it has been recently shown that mycobacterial strains that can mount Th1 responses but are defective in the induction of Th2 cells or Tregs exhibit dramatically increased vaccine efficacy (36, 42). Consistent with these observations, our recent data indicate that animals that are unable to mount Th2 cells and Tregs (STAT-6−/−TGF-βRIIDN mice) are completely resistant to M. tuberculosis infection.4 Therefore, in future studies it will be interesting to identify the components that are responsible for the induction of Th2 cells and Tregs, which will enable us to design improved vaccines and therapies.
In conclusion, we have demonstrated that the RD-1 region of M. tuberculosis is responsible for the capacity of this organism to promote Th2 cell differentiation. One of our future goals will be to identify the bacterial component(s) that induce(s) Tregs during TB disease. Our findings suggest that M. tuberculosis mutants that are defective in the induction of both Th2 cells and Tregs may serve as superior vaccines.
D. Bhattacharya and G. Das, unpublished observations.
- TB
- tuberculosis
- APC
- antigen-presenting cell
- BCG
- bacille Calmette-Guérin
- DC
- dendritic cell
- ESAT
- early secretory antigenic target
- RD-1
- region of difference 1
- TCR
- T cell receptor
- Th cell
- T helper cell
- Treg
- regulatory T cell.
REFERENCES
- 1. WHO (2007) Global Tuberculosis Control Surveillance, Planning, Financing, World Health Organization, Geneva, Switzerland [Google Scholar]
- 2. WHO (2004) BCG vaccine. Weekly Epidemiol. Record 79, 27–38 [Google Scholar]
- 3. Almeida Da Silva P. E., Palomino J. C. (2011) Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J. Antimicrob. Chemother. 66, 1417–1430 [DOI] [PubMed] [Google Scholar]
- 4. Davies J. (1996) Origins and evolution of antibiotic resistance. Microbiologia 12, 9–16 [PubMed] [Google Scholar]
- 5. Gillespie S. H. (2002) Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob. Agents Chemother. 46, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah N. S., Wright A., Bai G. H., Barrera L., Boulahbal F., Martín-Casabona N., Drobniewski F., Gilpin C., Havelková M., Lepe R., Lumb R., Metchock B., Portaels F., Rodrigues M. F., Rüsch-Gerdes S., Van Deun A., Vincent V., Laserson K., Wells C., Cegielski J. P. (2007) Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 13, 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Velayati A. A., Farnia P., Masjedi M. R., Ibrahim T. A., Tabarsi P., Haroun R. Z., Kuan H. O., Ghanavi J., Farnia P., Varahram M. (2009) Totally drug-resistant tuberculosis strains: evidence of adaptation at the cellular level. Eur. Respir. J. 34, 1202–1203 [DOI] [PubMed] [Google Scholar]
- 8. Velayati A. A., Masjedi M. R., Farnia P., Tabarsi P., Ghanavi J., Ziazarifi A. H., Hoffner S. E. (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136, 420–425 [DOI] [PubMed] [Google Scholar]
- 9. Flynn J. L., Chan J. (2001) Immunology of tuberculosis. Annu. Rev. Immunol. 19, 93–129 [DOI] [PubMed] [Google Scholar]
- 10. Sweeney K. A., Dao D. N., Goldberg M. F., Hsu T., Venkataswamy M. M., Henao-Tamayo M., Ordway D., Sellers R. S., Jain P., Chen B., Chen M., Kim J., Lukose R., Chan J., Orme I. M., Porcelli S. A., Jacobs W. R., Jr. (2011) A recombinant Mycobacterium smegmatis induces potent bactericidal immunity against Mycobacterium tuberculosis. Nat. Med. 17, 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lienhardt C., Azzurri A., Amedei A., Fielding K., Sillah J., Sow O. Y., Bah B., Benagiano M., Diallo A., Manetti R., Manneh K., Gustafson P., Bennett S., D'Elios M. M., McAdam K., Del Prete G. (2002) Active tuberculosis in Africa is associated with reduced Th1 and increased Th2 activity in vivo. Eur. J. Immunol. 32, 1605–1613 [DOI] [PubMed] [Google Scholar]
- 12. Cooper A. M., Solache A., Khader S. A. (2007) Interleukin-12 and tuberculosis: an old story revisited. Curr. Opin. Immunol. 19, 441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trinchieri G. (2003) Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3, 133–146 [DOI] [PubMed] [Google Scholar]
- 14. Hickman S. P., Chan J., Salgame P. (2002) Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naïve T cell polarization. J. Immunol. 168, 4636–4642 [DOI] [PubMed] [Google Scholar]
- 15. Chen W., Konkel J. E. (2010) TGF-β and “adaptive” Foxp3+ regulatory T cells. J. Mol. Cell. Biol. 2, 30–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scott-Browne J. P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J. D., Rudensky A. Y., Bevan M. J., Urdahl K. B. (2007) Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204, 2159–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimura A., Wakabayashi Y., Mori T. (2010) Cellular and molecular basis for the regulation of inflammation by TGF-β. J. Biochem. 147, 781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kursar M., Koch M., Mittrücker H. W., Nouailles G., Bonhagen K., Kamradt T., Kaufmann S. H. (2007) Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J. Immunol. 178, 2661–2665 [DOI] [PubMed] [Google Scholar]
- 19. Shafiani S., Tucker-Heard G., Kariyone A., Takatsu K., Urdahl K. B. (2010) Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J. Exp. Med. 207, 1409–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 21. Mangan P. R., Harrington L. E., O'Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. (2006) Transforming growth factor-βinduces development of the Th17 lineage. Nature 441, 231–234 [DOI] [PubMed] [Google Scholar]
- 22. Juffermans N. P., Florquin S., Camoglio L., Verbon A., Kolk A. H., Speelman P., van Deventer S. J., van Der Poll T. (2000) Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182, 902–908 [DOI] [PubMed] [Google Scholar]
- 23. Mayer-Barber K. D., Barber D. L., Shenderov K., White S. D., Wilson M. S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., Schlueter D., Flavell R. A., Sutterwala F. S., Sher A. (2010) Caspase-1 independent IL-1β production is critical for host resistance to Mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ben-Sasson S. Z., Hu-Li J., Quiel J., Cauchetaux S., Ratner M., Shapira I., Dinarello C. A., Paul W. E. (2009) IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. U.S.A. 106, 7119–7124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fine P. E. (1995) Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346, 1339–1345 [DOI] [PubMed] [Google Scholar]
- 26. Bhattacharyya S., Singla R., Dey A. B., Prasad H. K. (1999) Dichotomy of cytokine profiles in patients and high-risk healthy subjects exposed to tuberculosis. Infect. Immun. 67, 5597–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noben-Trauth N., Hu-Li J., Paul W. E. (2002) IL-4 secreted from individual naïve CD4+ T cells acts in an autocrine manner to induce Th2 differentiation. Eur. J. Immunol. 32, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 28. Hebel K., Rudolph M., Kosak B., Chang H. D., Butzmann J., Brunner-Weinzierl M. C. (2011) IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J. Immunol. 187, 5627–5635 [DOI] [PubMed] [Google Scholar]
- 29. Mejri N., Brossard M. (2007) Splenic dendritic cells pulsed with Ixodes ricinus tick saliva prime naïve CD4+ T to induce Th2 cell differentiation in vitro and in vivo. Int. Immunol. 19, 535–543 [DOI] [PubMed] [Google Scholar]
- 30. Guinn K. M., Hickey M. J., Mathur S. K., Zakel K. L., Grotzke J. E., Lewinsohn D. M., Smith S., Sherman D. R. (2004) Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51, 359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherman D. R., Guinn K. M., Hickey M. J., Mathur S. K., Zakel K. L., Smith S. (2004) Mycobacterium tuberculosis H37Rv: delta RD1 is more virulent than M. bovis bacille Calmette-Guerin in long-term murine infection. J. Infect. Dis. 190, 123–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McLaughlin B., Chon J. S., MacGurn J. A., Carlsson F., Cheng T. L., Cox J. S., Brown E. J. (2007) A Mycobacterium ESX-1-secreted virulence factor with unique requirements for export. PLoS Pathog. 3, e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Majlessi L., Brodin P., Brosch R., Rojas M. J., Khun H., Huerre M., Cole S. T., Leclerc C. (2005) Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J. Immunol. 174, 3570–3579 [DOI] [PubMed] [Google Scholar]
- 34. Flynn J. L., Chan J., Triebold K. J., Dalton D. K., Stewart T. A., Bloom B. R. (1993) An essential role for interferon γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178, 2249–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pathak S. K., Basu S., Bhattacharyya A., Pathak S., Kundu M., Basu J. (2005) Mycobacterium tuberculosis lipoarabinomannan-mediated IRAK-M induction negatively regulates Toll-like receptor-dependent interleukin-12 p40 production in macrophages. J. Biol. Chem. 280, 42794–42800 [DOI] [PubMed] [Google Scholar]
- 36. Rook G. A. (2007) Th2 cytokines in susceptibility to tuberculosis. Curr. Mol. Med. 7, 327–337 [DOI] [PubMed] [Google Scholar]
- 37. Potian J. A., Rafi W., Bhatt K., McBride A., Gause W. C., Salgame P. (2011) Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J. Exp. Med. 208, 1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilkinson R. J., Patel P., Llewelyn M., Hirsch C. S., Pasvol G., Snounou G., Davidson R. N., Toossi Z. (1999) Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1β on tuberculosis. J. Exp. Med. 189, 1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamada H., Mizumo S., Horai R., Iwakura Y., Sugawara I. (2000) Protective role of interleukin-1 in mycobacterial infection in IL-1 α/β double-knockout mice. Lab. Invest. 80, 759–767 [DOI] [PubMed] [Google Scholar]
- 40. Fremond C. M., Togbe D., Doz E., Rose S., Vasseur V., Maillet I., Jacobs M., Ryffel B., Quesniaux V. F. (2007) IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179, 1178–1189 [DOI] [PubMed] [Google Scholar]
- 41. Chatterjee S., Dwivedi V. P., Singh Y., Siddiqui I., Sharma P., Van Kaer L., Chattopadhyay D., Das G. (2011). Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog. 7, e1002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koch M. A., Tucker-Heard G., Perdue N. R., Killebrew J. R., Urdahl K. B., Campbell D. J. (2009) The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]