Background: Nothing is known regarding the anti-apoptotic effect of HO-1 on hCSCs.
Results: HO-1 expression induced by CoPP enhances hCSC survival through activation of the ERK/NRF2 signaling pathway and cytokine release.
Conclusion: CoPP is a cytoprotective agent that could improve the efficacy of CSC-based therapies for heart disease.
Significance: Strategies to enhance donor cell survival would have enormous therapeutic implications for patients with ischemic heart disease.
Keywords: Apoptosis, Cytokine, ERK, NRF2, Oxidative Stress, CoPP, HO-1, Human Cardiac Stem Cell
Abstract
Intracoronary delivery of c-kit-positive human cardiac stem cells (hCSCs) is a promising approach to repair the infarcted heart, but it is severely limited by the poor survival of donor cells. Cobalt protoporphyrin (CoPP), a well known heme oxygenase 1 inducer, has been used to promote endogenous CO generation and protect against ischemia/reperfusion injury. Therefore, we determined whether preconditioning hCSCs with CoPP promotes CSC survival. c-kit-positive, lineage-negative hCSCs were isolated from human heart biopsies. Lactate dehydrogenase release assays demonstrated that preconditioning CSCs with CoPP markedly enhanced cell survival after oxidative stress induced by H2O2, concomitant with up-regulation of heme oxygenase 1, COX-2, and anti-apoptotic proteins (BCL2, BCL2-A1, and MCL-1) and increased phosphorylation of NRF2. Apoptotic cytometric assays showed that pretreatment of CSCs with CoPP enhanced the cells' resistance to apoptosis induced by oxidative stress. Conversely, knocking down HO-1, COX-2, or NRF2 by shRNA gene silencing abrogated the cytoprotective effects of CoPP. Further, preconditioning CSCs with CoPP led to a global increase in release of cytokines, such as EGF, FGFs, colony-stimulating factors, and chemokine ligand. Conditioned medium from cells pretreated with CoPP conferred naive CSCs remarkable resistance to apoptosis, demonstrating that cytokines released by preconditioned cells play a key role in the anti-apoptotic effects of CoPP. Preconditioning CSCs with CoPP also induced an increase in the phosphorylation of Erk1/2, which are known to modulate multiple pro-survival genes. These results potentially provide a simple and effective strategy to enhance survival of CSCs after transplantation and, therefore, their efficacy in repairing infarcted myocardium.
Introduction
Stem cell-based therapies have shown great potential for repairing cardiac damage due to ischemia/reperfusion injury in patients (1–4). Among the many types of cells being investigated, c-kit+ cardiac stem cells (CSCs)3 appear to be particularly promising because they normally reside in the adult myocardium and have repeatedly been shown to be capable of differentiating into all three major cardiac cell types (cardiomyocytes, smooth muscle cells, and endothelial cells) as well as improving heart function after myocardial infarction in various animal models (5–7). However, the bulk of clinical data to date suggests that we are still in the early days of cell-based therapy for heart disease, with a number of negative, marginal, and transient effects recorded in larger scale double-blind placebo-controlled trials (8). One of the main challenges associated with cell transplantation is the fact that the majority of infused cells that manage to stay in the heart will not survive. It has been estimated that more than 90% of transplanted cells will eventually die (9, 10). Thus, strategies that enhance cardiac stem cell survival after adoptive transfer would have enormous therapeutic implications for patients with ischemic heart disease and post-myocardial infarction heart failure.
Several methods to increase cell survival have been described, including heat shock of the cells prior to transplantation, forced expression of survival factors in the donor cells, and exposure of cells to soluble pro-survival factors (11, 12). Numerous studies have demonstrated that stem cells contribute to tissue repair and regeneration by releasing important growth factors, including cytokines and chemokines, in a dynamic spatial-temporal manner that can promote donor cell survival, angiogenesis, tissue repair, and remodeling (12–18). Thus, genetically modulating the cells by overexpressing growth factors has also been used as an alternative strategy to precondition stem cells (19–22). Although these interventions do seem to increase cell survival, there is clearly room for additional improvement in cardiac stem cell survival.
Heme oxygenase (HO) is an important enzyme that maintains intracellular heme levels. HO degrades heme, producing equimolar quantities of biliverdin (which is converted to bilirubin by biliverdin reductase), iron, and carbon monoxide (23). HO exists in three isoforms: HO-1, HO-2, and HO-3. HO-1 is a stress-inducible protein that induces cellular protection in the event of injury, inflammation, oxidative stress, etc. Considerable evidence supports the protective functions of HO-1 in the cardiovascular system, which are ascribed to its end products, biliverdin and carbon monoxide (CO) (24, 25). HO-2 and HO-3 are constitutive forms. HO-2 is mainly expressed in the brain and testes, and HO-3 is found in rat brain and has little enzymatic activity (26, 27). Expression of HO-1 is modulated by transcriptional regulation, including ERK-dependent signaling and NRF2 (nuclear factor (erythroid-derived 2)-like 2), a transcription factor that plays a key role in the expression of antioxidant genes, HO-1 (28), and cyclooxygenase-2 (COX-2) (29). Cobalt protoporphyrin (CoPP), an HO-1 inducer, has been shown to enhance the survival of cardiomyocytes and restore contractility to unvascularized three-dimensional adult cardiomyocyte grafts implanted in vivo (30) as well as to protect the heart from damage during ischemia in both normal and diabetic rats (31). Although the cytoprotective effect of HO-1 has been extensively studied in the setting of myocardial ischemia-reperfusion injury, nothing is known regarding the anti-apoptotic effect of HO-1 on hCSCs.
The first-in-human clinical study of c-kit+ hCSCs is ongoing in Louisville, with initial results that are very encouraging (32). Here we present evidence that preconditioning hCSCs with CoPP promotes cell survival and resistance to oxidative stress. The mechanism appears to involve activation of survival signaling pathways, such as the ERK/NRF2 pathway, as well as paracrine effects via the increased release of cytokines. These results provide a simple and effective strategy to enhance survival of hCSCs after transplantation and, therefore, their efficacy in repairing infarcted myocardium in patients with ischemic heart disease.
EXPERIMENTAL PROCEDURES
Isolation and Culture of hCSCs
Human c-kit+/Lin− CSCs were isolated from the right atria appendages in patients with ischemic cardiomyopathy as described previously (7, 32, 33). Briefly, myocardial tissues were minced into small pieces and enzymatically dissociated with collagenase II (30 units/ml) with gentle shaking at 37 °C for 1 h. After incubation on ice for 10 min, the undigested clumps were separated by gravity. Supernatant was transferred into a 15-ml tube and centrifuged at 1,400 rpm for 5 min. The collected cell pellet was suspended and cultured in CSC growth medium consisting of Ham's F-12 (Invitrogen), 10% FBS (Hyclone), 10 ng/ml human basic FGF (Sigma), 0.005 unit/ml human EPO (erythropoietin) (Sigma), 0.2 mm l-glutathione (Sigma), and penicillin (100 units/ml)/streptomycin (100 μg/ml). The day after, growth medium was refreshed to remove cell debris, and cells were maintained in a humidified environment at 37 °C and 5% CO2. 4–5 days later, cells were sorted using the c-kit MACS kit according to the manufacturer's instructions (Miltenyi Biotec), expanded, and characterized by FACS analysis to obtain lineage-negative hCSCs. Human CSCs at passage 8–15 were employed for in vitro studies.
Preconditioning of hCSCs with CoPP
The day before experiments, hCSCs were cultured on Petri dishes at a density of 3000 cells/cm2. To optimize the dose and time for preconditioning, cells were treated with several doses of CoPP (0–80 μm) for 24 h and challenged with 2 mm H2O2 for 3 h in serum-free F-12 medium thereafter. The protective effect of CoPP preconditioning on oxidative stress was examined by detecting the lactate dehydrogenase (LDH) release according to the manufacturer's instructions (Takara). The optimal dose of CoPP (10 μm) was then used to treat cells for a period of time (24 h) to determine an optimized time point for preconditioning by LDH assay. In addition, the efficacy of cytoprotection by recovery from CoPP preconditioning was also performed for up to 48 h.
Lactate Dehydrogenase Leakage
Detection of cell membrane integrity is a rapid and simple approach to determine cell viability via measuring cellular LDH leakage in damaging cells. Following the manufacturer's instructions from the lactate dehydrogenase assay kit (Takara), samples in different hCSC treatment groups were centrifuged at 250 × g for 10 min, and 100 μl of the supernatant was then mixed with an equal volume of premixed reagent (catalyst/dye solution = 1:45) for 30 min at room temperature in a 96-well plate. The absorbance of each sample at 490 nm was measured using a Bio-Rad iMarkTM microplate reader, and the percentage of LDH release for each sample was normalized according to the absorbance reading from samples treated with 0.5% Triton X-100.
Apoptosis Assay with FITC-labeled Annexin V and Propidium Iodide (PI) Staining
The percentage of apoptotic, necrotic (late apoptotic), and viable hCSCs in different treatment groups was determined using the annexin V/PI cell apoptosis kit (Invitrogen) according to the instructions from the manufacturer. Briefly, after 12-h preconditioning with 10 μm CoPP and recovery for 24 h, hCSCs at 80% confluence were challenged with 1 mm H2O2 in serum-free F-12 medium. 2 h later, cells were trypsinized, washed in PBS twice, and suspended in 100 μl of annexin-binding buffer with 1 μl of 100 μg/ml PI and 5 μl of Alexa Fluor 488 annexin V solution. After room temperature incubation for 15 min, 400 μl of 1× annexin-binding buffer was added with gentle mixing, and samples were immediately analyzed by LSRII flow cytometry (BD Biosciences). The early apoptotic cells were stained with annexin V only, and necrotic or late apoptotic cells displayed both PI and annexin V staining, whereas live cells remained unstained.
shRNA Lentiviral Transfection
shRNA lentiviral particles for knockdown of HO-1, NRF2, and COX-2 were used to infect hCSCs according to the manufacturer's instructions (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Scrambled shRNA and GFP lentiviral particles were also applied to infect hCSCs as negative and positive controls. hCSCs were placed in a 12-well plate 24 h prior to viral infection. At 50% confluence, cells were refreshed with 1 ml of growth medium containing 5 μg/ml Polybrene. Lentiviral particles (20 μl) were then directly added into the culture medium with gentle swirling afterward. The day after, culture medium was replaced with normal growth medium for recovery. 48 h after infection, cells were cultured in the growth medium with 5 μg/ml puromycin to diminish non-infected cells. The puromycin-containing growth medium was replaced every 2 days for up to 8–10 days, when almost 100% of cells with GFP fluorescence were detected in the positive control group. The efficiency of gene knockdown was examined by Western blot.
Western Blot
Western blotting was performed using standard techniques as described previously (34, 35). After treatment, hCSCs were harvested and lysed with ice-cold modified radioimmune precipitation assay buffer (150 mm NaCl, 5 mm EDTA, 1% Nonidet P-40, 20 mm Tris-HCl, pH 7.5) plus protease and phosphatase inhibitor mixtures (Sigma). 20 μg of each sample was separated on 4–12% SDS-polyacrylamide gradient gels (Invitrogen). After transfer to PVDF membrane, primary and secondary antibodies were applied, and the signals were detected with ECL-plus reagent. Primary antibodies against HO-1, NRF2, COX-2, phospho-ERK, and ERK were purchased from Santa Cruz Biotechnology, Inc., and phospho-NRF2 antibody was obtained from Epitomics.
Real-time PCR for Gene Library Screening Array
Real-time PCR was performed for quantification of the change of cytokine or anti-apoptotic gene after preconditioning, using primer libraries designed by RealTimePrimers. The total RNA was isolated and purified using an RNA isolation kit (Qiagen), and the concentration of purified RNA was determined by a Nano-Drop 2000C spectrophotometer (Thermo Scientific). After RNA was reverse-transcribed to cDNA, sample preparation for real-time PCR was performed according to the manufacturer's instructions of the iQ SYBR Green Supermix (Bio-Rad) kit, and PCR was run with a Bio-Rad iQ5 optical module. The cycling conditions were set at 95 °C for 3 min as initial denaturation, 40 cycles of denaturation at 95 °C for 30 s, annealing at 59.5 °C for 40 s, and extension at 72 °C for 50 s. The data were acquired during the extension step. Melting curves were obtained at the end of the reaction by gradually raising the temperature by 1 °C/min from 59.5 to 95 °C over a time period of 35 min.
Statistical Analysis
Experiments were performed in quadruplicate and repeated at least three times. Data are expressed as mean ± S.E. Statistical significance was assessed by analysis of variance followed by Bonferroni/Dunn testing, or unpaired t test. A p value less than or equal to 0.05 was considered statistically significant.
RESULTS
Protective Effects of Preconditioning hCSCs with CoPP
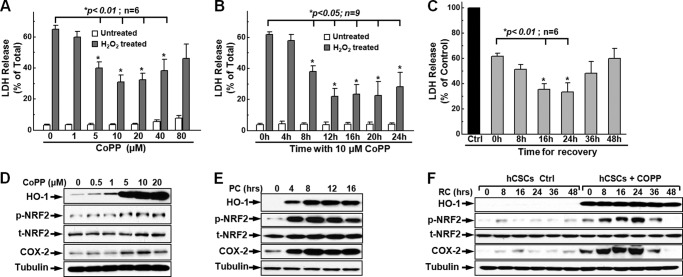
Previous studies have established that CoPP protects cardiomyocytes against ischemia-reperfusion injury (30, 36). However, little is known about whether it is protective for adult cardiac stem cells against apoptosis induced by extracellular or intracellular stimuli during stem cell transplantation. To test the hypothesis that CoPP is cytoprotective for hCSCs, we performed LDH release assays to determine the survival of CoPP-preconditioned hCSCs following oxidative stress induced by H2O2 (Fig. 1). We first optimized the concentration of CoPP for cell preconditioning using the LDH release assay and found that 10–40 μm CoPP was not toxic for hCSCs up to a 24-h treatment, as indicated by the minimal LDH release in the resting state without H2O2 treatment (Fig. 1A, white columns). Under oxidative stress conditions induced by 2 mm H2O2, cells pretreated with 5–40 μm CoPP showed significant decreased LDH release (Fig. 1A, gray columns), indicating greater survival after oxidative stress in cells pretreated with 5–40 μm CoPP. The data also showed that cells pretreated with 10 μm CoPP exhibited the minimum release of LDH, indicating maximal protection of cells against oxidative stress at this concentration; thus, we used 10 μm as the optimal concentration of CoPP to precondition hCSCs in the following work.
FIGURE 1.
Enhanced hCSC survival following preconditioning with CoPP. A, following preconditioning, 5–40 μm CoPP dramatically enhanced hCSC survival as indicated by the significant decrease of LDH release (gray columns); these concentrations of CoPP had no toxicity for hCSCs, as indicated by the minimum release of LDH (white columns). B, pretreatment of hCSCs with 10 μm CoPP for 8–24 h significantly increased cell survival, as indicated by the decrease of LDH release (gray columns). C, the cytoprotective effect of CoPP lasts up to 48 h after recovery, and a significant increase in hCSC survival was observed at 16- or 24-h recovery after preconditioning with 10 μm CoPP for 12 h as evidenced by the significant decrease of LDH release. D, Western blot showed a significant increase in expression of HO-1 and COX-2 and phosphorylation of NRF2 after preconditioning hCSCs with increasing concentrations of CoPP for 12 h. E, Western blotting showed the time dependence of preconditioning hCSCs with 10 μm CoPP, as indicated by the increased level of expression of HO-1 and COX-2, as well as the phosphorylation of NRF2 following preconditioning hCSCs at various time points (0, 4, 8, 12, and 16 h) (n = 3). F, Western blotting showed the persistence of significantly increased levels of expression of HO-1 and COX-2 and phosphorylation of NRF2 during the recovery time (0–48 h). p-NRF2, phosphorylated NRF2; t-NRF2, total NRF-2. Error bars, S.E.
We then assessed the optimal preconditioning time for hCSCs with 10 μm CoPP. As shown in Fig. 1B, we found that there was no toxicity when hCSCs were treated with 10 μm CoPP up to 24 h, as indicated by the minimal LDH release from 4–24-h treatment without oxidative stress induced by H2O2 (Fig. 1B, white columns). After an 8–20-h pretreatment with 10 μm CoPP, there was a significant increase of cell survival as indicated by the dramatic decrease of LDH release following oxidative stress induced by 2 mm H2O2 for 3 h (Fig. 1B, gray columns), indicating a significantly greater survival in this time scale of hCSCs preconditioned with 10 μm CoPP. Up to 3-fold less LDH (∼20% versus ∼60%) was released at 12 h post-treatment with 10 μm CoPP, suggesting significant enhancement of cell survival by preconditioning of hCSCs with CoPP. To determine how long the cytoprotection of CoPP may last after hCSC preconditioning, preconditioned hCSCs were subjected to recovery for 8–48 h, and the preconditioning (PC) medium was replaced with fresh culture medium for different periods immediately following a 12-h PC. We have observed that additional 16–24-h recovery (RC) showed better cytoprotective effects compared with no recovery (Fig. 1C). Because 10 μm CoPP induced a significant cytoprotective effect without inducing cell death at 12-h PC and 24-h RC, these concentrations and time points were selected for preconditioning hCSCs in the following experiments unless specified.
It has been well established that CoPP, as an HO-1 inducer, enhances the survival of cardiomyocytes and protects the heart from damage during ischemia in various animal models through the up-regulation of cytoprotective proteins and transcriptional factors (30, 31). To confirm the expression of HO-1, Western blotting against HO-1 was performed. As shown in Fig. 1D, preconditioning hCSCs induced a marked increase in HO-1 expression after a 12-h pretreatment, which was dependent on the concentration of CoPP from 0–20 μm CoPP; meanwhile, we found that the expression of COX-2 and the phosphorylation of NRF2 were also significantly up-regulated. Furthermore, we found that the up-regulation of the expression of HO-1 and COX-2 and the phosphorylation of NRF2 were time-dependent for preconditioning. As shown in Fig. 1E, the HO-1 expression level increased after 4-h pretreatment and stabilized following 8–24-h pretreatment. Meanwhile, it was also observed that the up-regulation of expression of COX-2 and phosphorylation of NRF2 were dependent on the preconditioning time. The expression levels of these proteins were also examined during recovery; Western blots showed that HO-1 maintained significant expression levels for up to 48 h of recovery. However, the expression of COX-2 and phosphorylation of NRF2 were further enhanced immediately after recovery, and their expression reached their peak level at 24 h of recovery, and then gradually decreased following recovery for longer times (Fig. 1F), which consistently elucidated the significant enhancement of hCSC survival after 24 h of recovery as shown in Fig. 1C. All of these data suggest that preconditioning hCSCs with CoPP significantly enhances the cell survival ability against oxidative stress induced by H2O2, which is dependent on the concentration of CoPP and time for preconditioning and recovery, and is correlated with the up-regulation of expression of HO-1 and COX-2 as well as the activation of phosphorylation of NRF2.
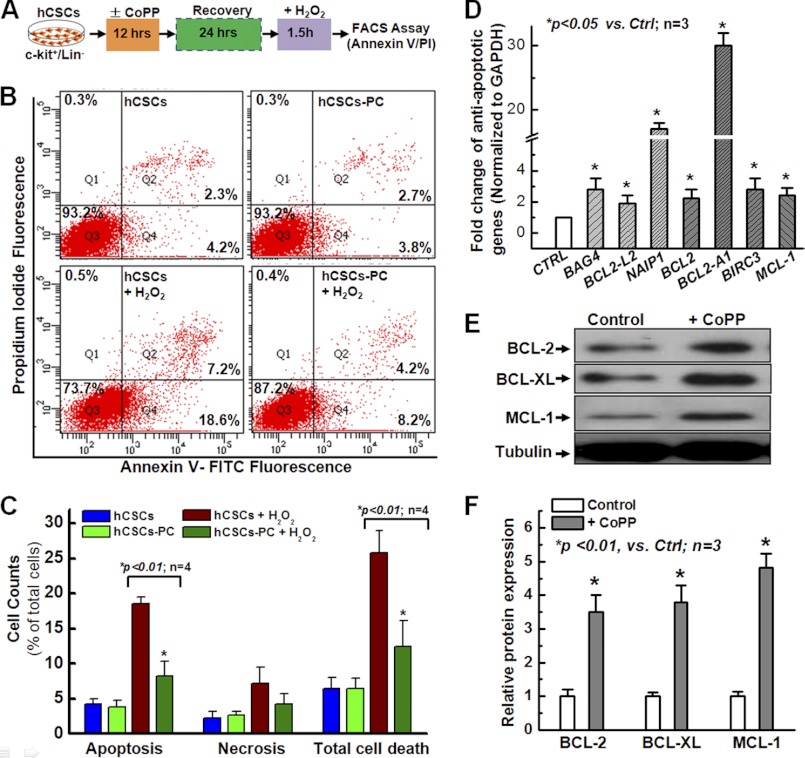
Resistance to Apoptosis following Preconditioning of hCSCs with CoPP
Based on the data presented in Fig. 1, we have established a protocol to examine cell apoptosis with FACS analysis. As shown in Fig. 2A, we first preconditioned hCSCs with 10 μm CoPP for 12 h, and then cells were allowed to recover for 24 h in fresh culture medium. Finally, cells were harvested and stained with FITC-labeled annexin V and PI for flow cytometric analysis. Cells were divided into the following four groups: 1) control cells pretreated with DMSO but not subjected to H2O2-induced oxidative stress; 2) cells subjected to CoPP preconditioning but no H2O2-induced oxidative stress; 3) cells pretreated with DMSO and subjected to oxidative stress induced by H2O2; 4) cells subjected to CoPP preconditioning and H2O2-induced oxidative stress. As shown in Fig. 2B, following flow cytometry analysis, the majority of cells are divided into three populations: live cells in the Q3 region, apoptotic cells in the Q4 region, and necrotic (or late apoptotic cells) in the Q2 region. Total cell death was defined by the difference between live cells and total cells (as a percentage). The quantitative data are shown in Fig. 2C. The results show that there was no significant change in cell apoptosis, cell necrosis, and total cell death under resting conditions without oxidative stress for both groups with or without CoPP preconditioning (blue column versus green column). After oxidative stress induced by H2O2, the apoptotic cell number was significantly decreased for CoPP-preconditioned cells compared with control cells without CoPP preconditioning, but there was no significant difference for necrotic cells between these two groups. However, total cell death was dramatically decreased after preconditioning with CoPP, which was mainly due to the decrease in cell apoptosis. This result is consistent with the data from the LDH release assay and further confirmed that preconditioning hCSCs with CoPP greatly enhances cell survival.
FIGURE 2.
CoPP promotes resistance of hCSCs to apoptosis. A, schematic protocol to examine apoptotic hCSCs by FACS following preconditioning with CoPP and annexin V/PI staining. B, FACS analysis with annexin V/PI staining shows a significant decrease in cell apoptosis and total cell death after preconditioning hCSCs with CoPP; cells pretreated with DMSO served as control. C, quantitative analysis for B, which further shows that preconditioning hCSCs with CoPP significantly enhances resistance to apoptosis, as indicated by the decrease in both apoptosis and total cell death. D, real-time quantitative PCR analysis with an anti-apoptotic primer library screening shows a significant increase of the indicated anti-apoptotic gene expression following pretreatment of hCSCs with 10 μm CoPP. E, Western blotting confirmed the increase in some of the anti-apoptotic protein expression, including BCL2, BCL-XL, and MCL-1, following pretreatment of hCSCs with 10 μm CoPP for 12 h and recovery for 24 h. F, quantitative data for the band density of the protein expression. Error bars, S.E.
To understand how preconditioning hCSCs with CoPP enhances the cell survival ability against apoptosis induced by oxidative stress (Fig. 2C), we examined anti-apoptotic gene expression by quantitative real-time RT-PCR with a PCR primer library screening and found a significant increase in the expression of a number of anti-apoptotic genes, including a ∼2.5-fold increase in the expression of BAG4, BCL2, BCL2-L2, BIRC3, and XIAP at the mRNA levels and a more than 20-fold increase for NAIP1 and BCL2-A1 (Fig. 2D). Increased protein expression of BCL-2, BCL-XL, and MCL-1 was further confirmed by Western blotting analysis following 12-h pretreatment of hCSCs with CoPP and 24-h recovery (Fig. 2E). The quantitative data with a band densitometric analysis are shown in Fig. 2F, indicating a marked increase in the expression of anti-apoptotic proteins, including BCL2, BCL-XL, and MCL-1. These data suggest that the enhanced hCSC survival after preconditioning with CoPP may be due to the up-regulation of anti-apoptotic genes and that preconditioning with HO-1 inducers has anti-apoptotic effects on hCSCs.
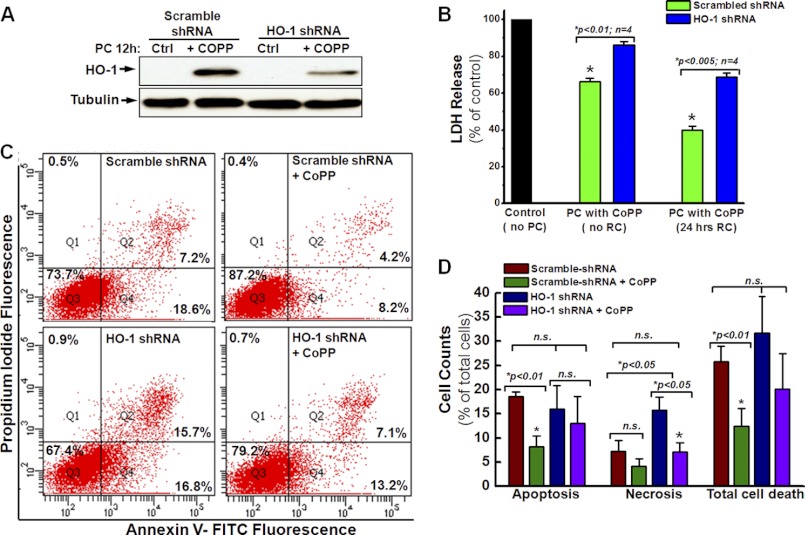
Effect of HO-1/NRF2/COX-2 Down-regulation on hCSC Survival after CoPP Preconditioning
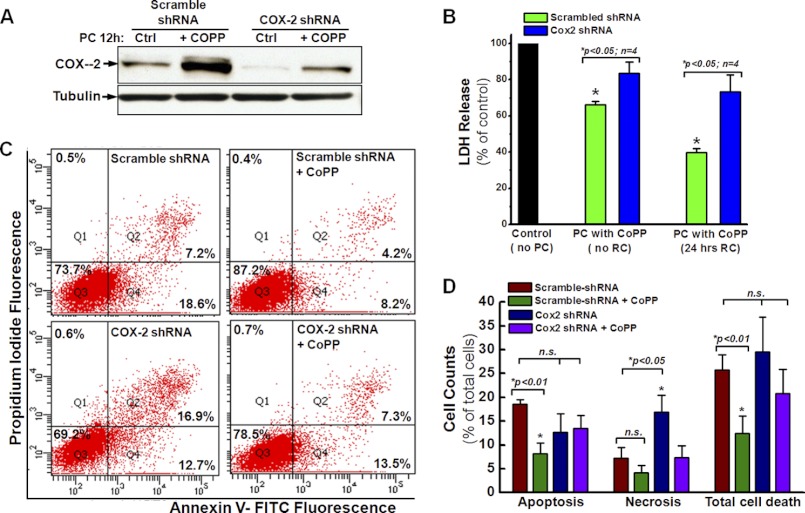
Based on the data shown in Fig. 1, D–F, it can be postulated that CoPP enhances hCSC survival through the up-regulation of HO-1 and COX-2 expression as well as the activation of NRF2 phosphorylation. To test whether the cell survival enhancement following preconditioning with CoPP is specifically dependent on the up-regulation of HO-1, we infected hCSCs with lentivirus encoding HO-1 shRNA as well as scrambled shRNA as control. The down-regulation of HO-1 was confirmed by Western blot with an HO-1 antibody (Fig. 3A), which showed a significantly decreased expression of HO-1 after 12-h CoPP PC. Concomitantly, the cytoprotective effect of CoPP was also diminished by HO-1 gene silencing, as indicated by the significant increase of LDH release following 12-h CoPP preconditioning (Fig. 3B, blue column versus green column). Furthermore, FACS analysis with annexin V and PI staining showed that knocking down HO-1 with shRNA gene silencing significantly increased the level of both cell apoptosis and total cell death in hCSCs preconditioned with CoPP compared with cells expressing scrambled shRNA (Fig. 3, C and D), indicating a reduction in the cytoprotective effect of CoPP preconditioning. These data provide a possible direction for further studies: the molecular mechanism for HO-1-induced hCSC therapeutic efficacy.
FIGURE 3.
Knocking down of HO-1 diminishes the cytoprotective effect of CoPP. A, Western blotting shows that HO-1 shRNA significantly decreases the expression of HO-1 after pretreatment with 10 μm CoPP for 12 h compared with the cells expressing scramble shRNA. The control (Ctrl) group consists of cells without CoPP pretreatment (n = 3). B, LDH release assays show that the down-regulation of HO-1 by shRNA gene silencing removes the cytoprotective effect of CoPP preconditioning. C, FACS analysis with annexin V/PI staining shows that HO-1 knockdown abrogates the cytoprotective effect of CoPP in cells infected with HO-1 shRNA, as indicated by apoptosis and total cell death similar to those of cells infected with scrambled shRNA as control. D, quantitative analysis of the FACS assay shown in C further confirms the essential role of HO-1 in CoPP-induced cytoprotection of hCSCs. n.s., not significant. Error bars, S.E.
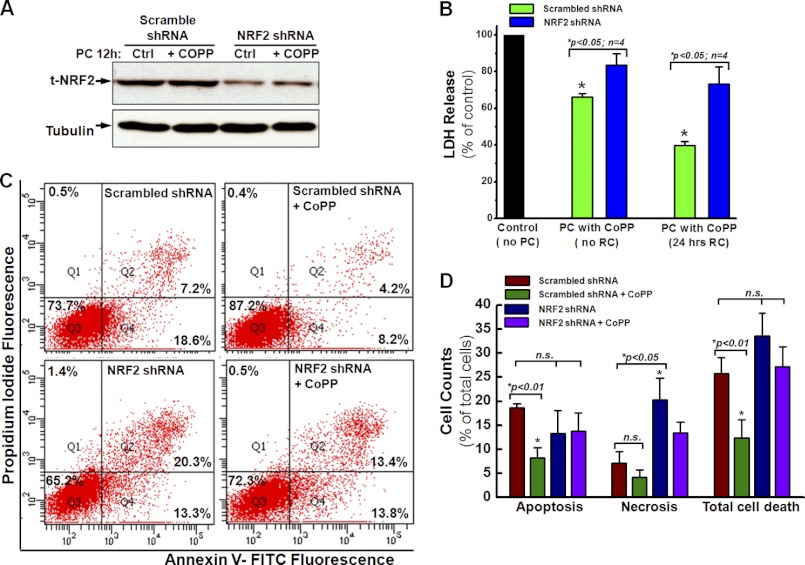
To understand whether the activation of NRF2 is specifically required for the enhancement of hCSC survival after preconditioning with CoPP, the expression of NRF2 was knocked down by shRNA gene silencing, which was confirmed by the Western blotting. As shown in Fig. 4A, the expression of total NRF2 was decreased by more than 80%. Contemporaneously, the cytoprotective effect of CoPP was also diminished by NFE2L2 gene silencing for NRF2, as indicated by a significant increase in LDH release following 12-h CoPP preconditioning (Fig. 4B, blue column versus green column) with or without 24-h recovery. Furthermore, FACS analysis with annexin V and PI staining showed that the knocking down of NRF2 by shRNA gene silencing significantly increased the level of both cell apoptosis and total cell death in hCSCs preconditioned with CoPP compared with cells expressing scrambled shRNA (Fig. 4, C and D), indicating a decreased cytoprotective effect of preconditioning hCSCs with CoPP. However, knocking down NRF2 also increased the level of late apoptosis to an extent greater than that observed in cells expressing HO-1 shRNA (Fig. 3D versus Fig. 4D), suggesting that NRF2 is upstream of HO-1 in this regulating pathway.
FIGURE 4.
Knockdown of NRF2 removes the cytoprotective effect of CoPP. A, Western blotting shows that NRF2 shRNA significantly decreases the expression of NRF2 after pretreatment with or without 10 μm CoPP for 12 h. The control (Ctrl) group consists of cells without CoPP pretreatment (n = 3). B, LDH release assays show that the down-regulation of NRF2 by shRNA gene silencing removes the cytoprotective effect of PC with CoPP, followed by 24-h RC or no RC, as indicated by the increased LDH release in hCSCs infected with NRF2 shRNA compared with hCSCs infected with scramble shRNA. C, FACS assay with annexin V/PI staining shows that NRF2 knockdown diminishes the cytoprotective effect of CoPP in cells infected with NRF2 shRNA, as indicated by apoptosis and total cell death similar to those of cells infected with scrambled shRNA as control. D, quantitative analysis of the FACS assay shown in C confirms that the down-regulation of NRF2 diminishes the cytoprotective effect of CoPP in hCSCs. t-NRF2, total NRF2. Error bars, S.E.
Because COX-2 was up-regulated after preconditioning of hCSCs with CoPP (Fig. 1, D–F), the expression of COX-2 was also knocked down by shRNA gene silencing. As shown in Fig. 5A, Western blotting confirmed the decrease of COX-2 expression after CoPP preconditioning in hCSCs expressing COX-2 shRNA compared with the control cells expressing scrambled shRNA. Interestingly, the cytoprotective effect of CoPP was also diminished after knocking down COX-2, as indicated by the significant increase of LDH release following 12-h CoPP preconditioning (Fig. 5B, blue column versus green column) with or without 24-h recovery. At the same time, knocking down COX-2 significantly increased the level of both cell apoptosis and total cell death for hCSCs preconditioned with CoPP compared with cells expressing scrambled shRNA (Fig. 5, C and D), indicating a decreased cytoprotective effect of CoPP preconditioning. These data indicate that COX-2 plays an essential role in enhancing hCSC survival following preconditioning with CoPP.
FIGURE 5.
Knocking down of COX-2 reduces the cytoprotective effect of CoPP. A, Western blotting shows that COX-2 shRNA significantly decreases the expression of COX-2 after pretreatment with or without 10 μm CoPP for 12 h. The control (Ctrl) group consists of cells without CoPP pretreatment (n = 3). B, LDH release assays show that the down-regulation of COX-2 by shRNA gene silencing removes the cytoprotective effect of preconditioning cells with CoPP, followed by 24-h recovery or no recovery, as indicated by increased LDH release of hCSCs infected with COX-2 shRNA compared with hCSCs infected with scramble shRNA. C, FACS assay with annexin V/PI staining shows that COX-2 knockdown diminishes the cytoprotective effect of CoPP in cells infected with COX-2 shRNA, as indicated by apoptosis and total cell death similar to those of cells infected with scrambled shRNA as control. D, quantitative analysis of the FACS assay shown in C confirms that knockdown of COX-2 diminishes CoPP-induced cytoprotection of hCSCs. Error bars, S.E.
Effect of CoPP on the Release of Growth Factors
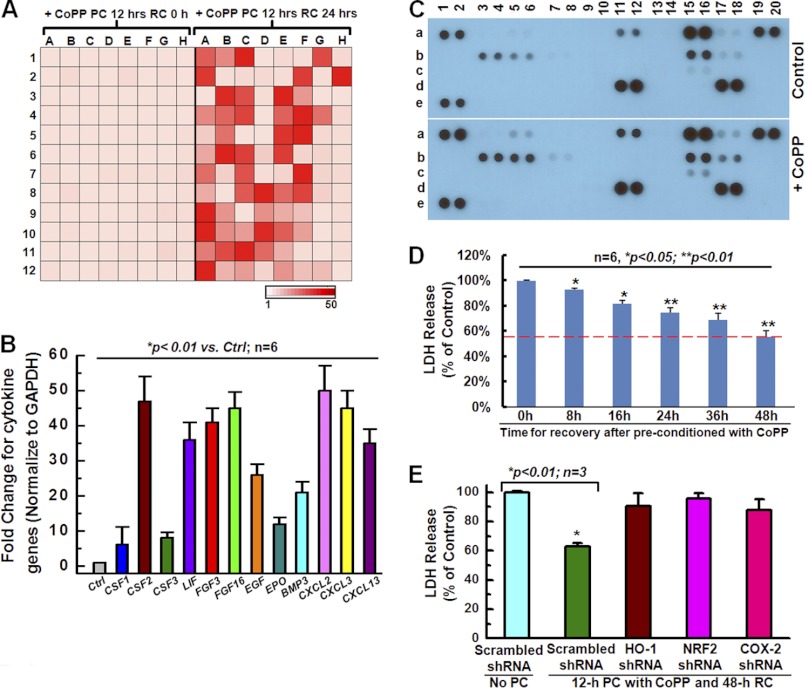
Increasing evidence suggests that stem cell-mediated tissue repair takes place via the release of paracrine factors into the surrounding tissue that subsequently direct a number of restorative processes, including myocardial protection, revascularization, and cardiac remodeling (13). To test the effect of CoPP on the expression of growth factors, we first performed a cytokine primer library array with 96-well format by real-time qRT-PCR. A global increase in gene expression of growth factors was observed in the cell group preconditioned with CoPP for 12- and 24-h recovery, compared with the group with 0-h recovery (Fig. 6A). Then the mRNA expression of several cytokines known to play beneficial roles in cell survival, proliferation, and migration was further confirmed by real-time qRT-PCR. Compared with non-preconditioned hCSCs (Ctrl), preconditioned hCSCs exhibited increased mRNA levels of CSF1/2/3, LIF (leukemia inhibitory factor), FGF2/3/18, EGF, EPO, BMP3, and CXCL2/3/13 (Fig. 6B).
FIGURE 6.
Effect of CoPP on cytokines and hCSC survival. A, the heat map shows a global increase in cytokine gene expression following 12 h of preconditioning and a 24-h recovery (right versus left), as a result of real-time PCR library screening with a 96-well format. B, real-time quantitative PCR confirmed a significant increase in cytokine gene expression, including IL-2, CSF1/2/3, FGFs, EGF, and CXCLs. C, a human cytokine antibody array showed up-regulation of the protein expression of several cytokines (a5/6, a11/12, b3/4, b5/6, b15/16, b17/18, and c15/16) in cells subjected to 12-h preconditioning with CoPP and 24-h recovery compared with the control cells. D, conditioned medium from hCSCs pretreated with 10 μm CoPP for 12 h and an 8–48-h recovery produces a cytoprotective effect for naive hCSCs, as indicated by the decrease in LDH release. E, LDH release assays show that conditioned medium from CoPP-preconditioned hCSCs, which were infected with HO-1, NRF2, or COX-2 shRNA, does not produce cytoprotective effects for naive hCSCs, as indicated by the increased LDH release following oxidative stress induced by H2O2, compared with the conditioned medium from CoPP-preconditioned hCSCs that were infected with scramble shRNA. Error bars, S.E.
Using a cytokine antibody array that assessed 36 soluble cytokines (R&D Systems), we observed a marked elevation in the protein expression of several cytokines secreted by CoPP-preconditioned cells, including CD40 ligand (a5/6), CXCL1 (a11/12), IL-1α (b3/4), IL-1β (b5/6), IL-6 (b15/16), CXCL8 (b17/18), and IL-23 (c15/16) (Fig. 6C, +CoPP versus Control), which is consistent with the gene expression analysis by real-time quantitative PCR. These data further confirmed the up-regulation of cytokines at the protein expression level following CoPP preconditioning. To test whether the cytokines produced by hCSCs are sufficient to induce cytoprotective effects, we harvested the conditioned medium from hCSCs pretreated with CoPP for 12 h followed by recovery for 0, 8, 16, 24, and 48 h and then cultured a new batch of naive hCSCs with this conditioned medium. LDH release assays showed that cells cultured with conditioned medium from hCSCs pretreated with 10 μm CoPP for 12 h, followed by a 24–48-h recovery, exhibited a significant decrease in LDH release, indicating that cells cultured with conditioned medium gained enhanced anti-apoptotic ability against oxidative stress (Fig. 6D). However, the conditioned medium harvested from hCSCs expressing HO-1/NRF2/COX-2 shRNA did not show such a protective effect for naive hCSCs compared with the conditioned medium harvested from hCSCs expressing scrambled shRNA following 12-h preconditioning and 48-h recovery, as indicated by the significant increase in LDH release in hCSCs expressing HO-1/NRF2/COX-2 shRNA after the oxidative stress induced by H2O2 (Fig. 6E). These results confirm that the up-regulation of HO-1/NRF2/COX-2 is involved in the enhancement of hCSC survival following CoPP preconditioning and that this up-regulation also plays a role in the release into the medium of cytokines that protect the cell from oxidative stress.
ERK/NRF2 Signaling Pathway in CoPP-mediated hCSC Survival
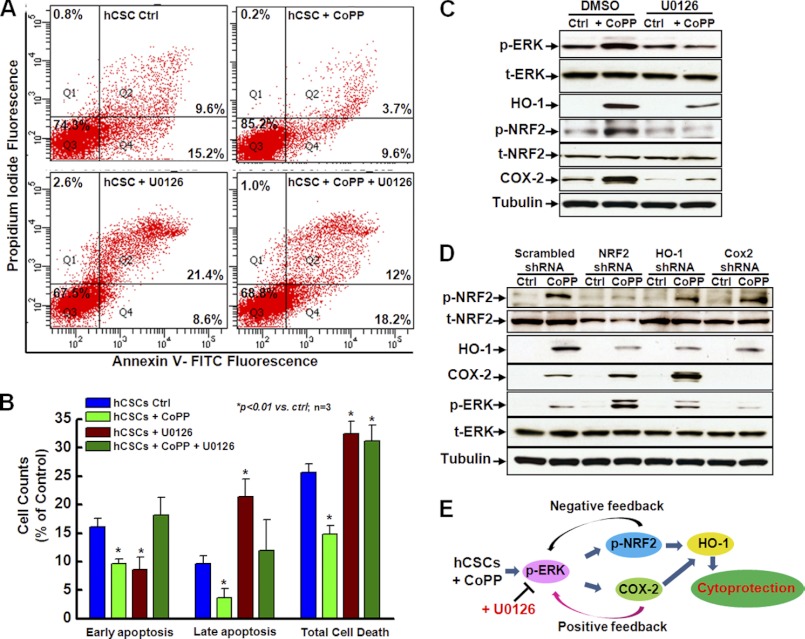
To further define the critical role of the ERK signaling pathway in the cytoprotection afforded by CoPP preconditioning, we examined the effect of ERK inhibition on oxidative stress-induced cell apoptosis with FACS analysis following annexin V/PI staining. As shown in Fig. 7, A and B, compared with the control cells (blue column), CoPP treatment significantly decreased both early and late apoptosis (green column), whereas the inhibition of ERK by U0126 significantly decreased early apoptosis but increased late apoptosis (red column), and this inhibition of ERK by U0126 significantly diminished the protective effect of CoPP against both early and late apoptosis (olive column). Thus, total cell death was decreased by preconditioning with CoPP but increased by inhibition of ERK activity with U0126, and the CoPP protection against oxidative stress-induced total cell death was abrogated by ERK inhibition (Fig. 7B), indicating that the ERK signaling pathway plays an important role in CoPP-mediated hCSC survival.
FIGURE 7.
The ERK/NRF2 signaling pathway is associated with enhanced hCSC survival following preconditioning with CoPP. A, FACS analysis with annexin V/PI double staining shows that specific inhibition of ERK by U0126 diminishes the cytoprotective effect of CoPP for hCSCs, as indicated by the increase of both cell apoptosis and total cell death compared with cells without U0126 treatment as control. B, quantitative analysis of the FACS assay shown in A confirms that the ERK signaling pathway is involved in CoPP-induced cytoprotection of hCSCs. C, Western blotting shows that U0126 specifically inhibits the phosphorylation of ERK, which also significantly inhibits the expression of HO-1 and COX-2 and the phosphorylation of NRF2, compared with cells treated with DMSO as control that were preconditioned with or without 10 μm CoPP for 12 h (n = 3). D, Western blotting shows that shRNA knockdown of NRF2 and HO-1 results in increased expression of COX-2 and phosphorylation of ERK, indicating a negative feedback regulation of NRF2 and HO-1 on COX-2 expression and phosphorylation of ERK. However, the shRNA knockdown of COX-2 leads to decreased expression of HO-1 and phosphorylation of ERK, indicating its positive feedback regulation of HO-1 expression and phosphorylation of ERK (n = 3). E, the schematic diagram shows the proposed molecular mechanism for CoPP-induced hCSC survival, which indicates that the ERK/NRF2 signaling pathway is essential in enhancing hCSC survival following preconditioning with CoPP. t-, total; p-, phosphorylated. Error bars, S.E.
Western blot confirmed that treatment of hCSCs with U0126 significantly inhibited the phosphorylation of ERK but did not alter total ERK expression. In addition, the phosphorylation of NRF2 and the expression of HO-1 and COX-2 were significantly decreased after inhibition of ERK by U0126 regardless of whether the cells were preconditioned with or without CoPP (Fig. 7C), suggesting that the ERK signaling pathway may be involved in CoPP-promoted hCSC survival through regulation of NRF2 activity and gene expression of HO-1 and COX-2.
As shown in Figs. 3–5, we found that knockdown of HO-1, NRF2, and COX-2 directly affected the cytoprotective effect of CoPP preconditioning. To understand the role of the ERK signaling pathway in this process, we examined the expression of HO-1 and COX-2, and the phosphorylation of NRF2 and ERK after knocking down the expression of HO-1, NRF2, and COX-2. As shown in Fig. 7D, following preconditioning of hCSCs with CoPP, cells expressing NRF2 shRNA showed a significant decrease in the expression of HO-1, whereas the expression of COX-2 and the phosphorylation of ERK were significantly increased in these cells compared with control cells expressing scramble shRNA. This suggested that HO-1 was up-regulated by NRF2, whereas NRF2 was downstream of ERK in this signal pathway, and the negative feedback regulation caused the increase of ERK phosphorylation when NRF2 was knocked down. Cells expressing HO-1 shRNA exhibited a slight decrease in the phosphorylation of NRF2 but showed a significant increase in the expression of COX-2 and phosphorylation of ERK, further indicating downstream feedback regulation of HO-1/NRF2 in the ERK signal pathway. However, cells expressing COX-2 shRNA showed a significant decrease in the phosphorylation of ERK and in the expression of HO-1 but not in NRF2 phosphorylation, implying a positive feedback regulating of COX-2 by the ERK/NRF2 signal pathway.
Taking the functional data together with the Western blotting assay, we propose that the following mechanism underlies CoPP-induced hCSC survival (Fig. 8E). In this scheme, we propose that preconditioning hCSCs with CoPP activates the phosphorylation of ERK, which then up-regulates HO-1 expression through either NRF2 or COX-2 signaling pathways and increases the expression of pro-survival genes, cytokines, and antioxidant genes, finally leading to cytoprotective effects and promotion of cell survival. Inhibition of ERK activity might disrupt this signal pathway and result in a decrease in the cytoprotective effect of CoPP preconditioning. However, inhibition of either NRF2 or COX-2 expression by shRNA knockdown is accompanied by a compensatory enhancement of other pathways.
The goal of this study was to examine the cytoprotective effect of CoPP on hCSCs with the goal of enhancing their efficacy after transplantation into an infarct heart. It is not known whether treatment with CoPP leads to adverse effects on the cellular ability for proliferation and differentiation. In order to answer this question, we first examined cell proliferation after preconditioning hCSCs with CoPP. As shown in supplemental Fig. S1, after incorporating BrdU into hCSC during cell culture, we performed both immunocytochemistry (supplemental Fig. S1A) and flow cytometry analysis (supplemental Fig. S1B) on hCSCs subjected to either CoPP preconditioning or no preconditioning. The quantitative data showed no significant difference in the PI+BrdU+ cell population (supplemental Fig. S1C), indicating that CoPP did not affect the cells' ability to proliferate, which was also confirmed by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assay (supplemental Fig. S1D). Second, both control cells and CoPP-preconditioned cells were subjected to differentiation using standard methods (7). Real-time PCR analysis showed that both control cells and CoPP-treated cells exhibited a significant increase in the expression of troponin I, a gene marker for adult cardiomyocytes, with no difference between these two groups (supplemental Fig. S2A). This was also confirmed by Western blotting with an antibody against MG53, a muscle-specific protein (34, 35). Both groups showed significant expression of MG53 compared with undifferentiated control cells, but there was no significant difference between CoPP-treated and untreated groups (supplemental Fig. S2A), indicating that preconditioning hCSCs with CoPP did not alter the cells' ability to proliferate and differentiate.
DISCUSSION
Many strategies have been tested to enhance the survival of donor cells, including exposure to hypoxia or anoxia (37), delivery of growth factor genes (18), anti-inflammatory or immune treatment, heat shock treatment, optimization of injection procedures, etc. (38). In this study, we have shown for the first time that exposure (preconditioning) of hCSCs to CoPP, a small molecule that serves as an HO-1 inducer, increases the resistance of these cells to oxidative stress-induced apoptosis. Our results provide evidence that CoPP preconditioning protects hCSCs through the activation of the ERK/NRF2 or ERK/COX-2 signal pathways at the transcriptional level and the consequent up-regulation of the HO-1 and COX-2 genes. Several lines of evidence support these conclusions.
The cytoprotective effects of HO-1 are mediated by its product CO (which is generated exclusively by the HO system), the production of the antioxidants biliverdin and bilirubin, and the degradation of excess amounts of the pro-oxidant heme (39, 40). Previous studies have shown cardioprotective actions of HO-1 in models of acute stress (e.g. ischemia/reperfusion injury) (41–45). HO-1 induction by CoPP treatment has been reported to inhibit obesity (46, 47), recruit endothelial progenitor cells in diabetes (48), protect neurons against neurotoxicity (49), and improve insulin sensitivity (50). However, the role of HO-1 in protecting CSCs against apoptosis is virtually unknown. It is also unknown whether CoPP-dependent HO-1 induction exerts cytoprotective effects following cell transplantation. Here, we demonstrate that HO-1 induction has the ability to enhance hCSC resistance to oxidative stress. Our data show that CoPP preconditioning significantly enhanced hCSC survival after oxidative stress-induced cell death, indicated by the decrease in LDH release, and that this salubrious effect was accompanied by the activation of the ERK/NRF2 signal pathway and the up-regulation of HO-1 and COX-2 expression. However, this treatment did not alter the cells' ability for self-renewal and cardiac differentiation. Our study suggests that augmentation of HO-1 expression may be a novel therapeutic strategy to enhance the efficacy of CSC therapy in patients with ischemic heart disease.
One important, although not exclusive, mechanism of the beneficial effects of CoPP appears to be the limitation of apoptosis. Although HO-1 is known to inhibit apoptosis (39, 40, 51), the precise mechanisms by which this occurs remain unclear. Apoptosis in the failing heart involves multiple mechanisms that produce heightened activity of extrinsic death receptor pathways or intrinsic mitochondrial and/or endoplasmic reticulum-linked pathways (52). Emerging evidence supports an important role for mitochondria as arbiters of cell fate in the failing heart. Intrinsic pathways converge on the mitochondria to induce mitochondrial remodeling and dysfunction, which in turn leads to the release of apoptogenic proteins, such as cytochrome c, into the cytosol and the activation of terminal caspase cascades (52). Preconditioning hCSCs with CoPP produced cytoprotection through a significant inhibition of cell apoptosis after oxidative stress and an increase in anti-apoptotic gene and protein expression.
Our data show that HO-1 is important for the cytoprotective activity of CoPP because shRNA-mediated knockdown of HO-1 abrogated CoPP-induced protection from cell death after oxidative stress (H2O2). The gene HMOX1 encoding HO-1 is a known target of NRF2, a transcription factor that plays an important role in the expression of phase 2 detoxifying and antioxidant enzymes as well as in the activation of other stress-inducible genes, including GSH S-transferase quinine reductase (NQQ1), and HMOX1 (53). When activated, NRF2 undergoes translocation from the cytosol to the nucleus. NRF2 transcriptional activity was elevated in CoPP-preconditioned hCSCs, and partial reduction in NRF2 achieved by shRNA resulted in a corresponding partial reduction in HO-1 expression. Thus, NRF2 is a candidate upstream factor in the induction of HO-1 in this pathway. Perhaps most importantly, the functional data showed that down-regulation of NRF2, HO-1, or COX-2 with shRNA gene silencing abrogated the cytoprotective effect of CoPP preconditioning on hCSCs. The exact interrelationship between HO-1 and COX-2 is unclear. HO-1 and COX-2 could regulate parallel pathways affecting cell death, such that each contributes independently to resistance to oxidative stress-induced cell death in an additive manner.
Previous studies have demonstrated that stem cells contribute to tissue repair and regeneration by releasing various growth factors (12–18). Emerging evidence suggests that resident CSCs can also function in a paracrine/autocrine manner (54, 55). Conditioned media from human cardiospheres and cardiosphere-derived cells have been shown to enhance the survival of cardiomyocyte against hypoxia by secreting two growth factors (VEGF and hepatocyte growth factor) (56). Likewise, intracoronary administration of c-kit+ CSCs into rat hearts induced proliferation of resident c-kit+ CSCs in the infarct zone presumably through a paracrine mechanism (6). However, despite the well known role of growth factors in orchestrating stem cell-based tissue repair and regeneration, the relationship between the secretion of potentially cardioprotective growth factors by c-kit+ CSCs and tissue repair has not been examined.
Therefore, we studied whether the preconditioned hCSCs have a cardioprotective secretory profile. Our data show that cytokine expression was globally up-regulated in CoPP-preconditioned cells. Specifically, compared with non-preconditioned hCSCs, preconditioned hCSCs expressed greater mRNA levels of CSF1/2/3, LIF, FGF2/3/18, EGF, EPO, BMP3 (bone morphogenetic protein 3), and CXCL2/3/13. Soluble progenitor-active cytokines, including colony-stimulating factors (CSF1/2/3) (57) and EPO (58), promote angiogenesis through mobilization of stem cells. Leukemia inhibitory factor regulates the commitment of CSCs to the endothelial cell lineage, contributing to revascularization in the process of tissue remodeling and/or regeneration (59). The CXCL family chemokines play important roles in promoting stem cell migration and homing through JNK and NFκB signaling pathways (60, 61). Among them, EGF, BMP3, and the FGF family cytokines are essential for stem cell survival and proliferation (62, 63). To assess the functional role of the secreted cytokines, conditioned medium was harvested from hCSCs pretreated with CoPP for 12 h followed by different time periods of recovery, and then used to culture a new batch of naive hCSCs. Use of conditioned medium from hCSCs pretreated with 10 μm CoPP for 12 h, followed by a 24–48-h recovery, was associated with a significant decrease in LDH release, indicating that the conditioned medium conferred protection against apoptosis induced by oxidative stress. Knockdown of HO-1, NRF2, or COX-2 in the CoPP-preconditioned hCSCs from which the conditioned medium was obtained diminished this cytokine effect. These results suggest that the salubrious effects of CoPP preconditioning may be due in part to the secretion of protective cytokines as a result of HO-1-, NRF2-, and COX-2-dependent pathways.
Over the past few years, it has become increasingly clear that exogenous CO exposure and the endogenous production of CO by HO-1 promote the activation of redox-sensitive transcription factors and protein kinases, which, in turn, induce antioxidant enzyme systems, including HO-1 itself (64). HO induction and endogenous CO have been found to be protective in numerous experimental models of vascular, cardiac, and pulmonary injury and against damage from various inflammatory conditions. In the present study, we observed that CoPP activates NRF2-mediated HO-1 expression through post-transcriptional NRF2 up-regulation, resulting in a high nuclear protein ratio of NRF2. Our findings suggest that CoPP up-regulates phosphorylation of NRF2, which is then translocated into the nucleus, where it activates the HO-1 expression. An increase in HO-1 protein leads to production of bilirubin and CO, which might be responsible, at least partially, for the cytoprotective effects of HO-1 and, thus, of CoPP.
It has been shown that NRF2 is phosphorylated by ERK under conditions of oxidative stress, resulting in its nuclear translocation and the subsequent enhancement in the expression of antioxidant genes (65, 66). These findings suggest the involvement of the ERK/NRF2 signaling pathway in eliciting the CoPP-induced cellular anti-apoptotic response and thus protecting against apoptosis. This hypothesis is supported by the observation that shRNA-mediated inhibition of NRF2 expression suppressed cytoprotection against apoptosis in CoPP-treated cells. The critical role of ERK in mediating the CoPP-induced cytoprotective response was then confirmed by the finding that inhibition of ERK abrogated the cytoprotection afforded by CoPP against H2O2-induced apoptosis and led to decreased phosphorylation of NRF2 and expression of HO-1 and COX-2 in hCSCs. Taken together, these results indicate that CoPP activates the ERK/NRF2 signaling pathway, which then elicits a cellular anti-apoptotic response and protects against oxidative stress-induced apoptosis in hCSCs.
In summary, we found that in vitro preconditioning of hCSCs with a small molecule, CoPP, enhances resistance to apoptosis through activation of the ERK/NRF2 signaling pathway and release of various cytokines. These results suggest that the HO-1 inducer CoPP may be a promising candidate as a protective agent against oxidative stress and apoptosis that could potentially improve the efficacy of CSC-based therapies for heart disease. In the future, it will be interesting to examine the in vivo survival of hCSCs enhanced by HO-1 induction and conduct a thorough analysis for the molecular mechanisms elucidated here in the immunodeficient mouse hearts following the acute myocardial infarction.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R21 HL104315 and R01 HL114951 (to C. C.); P20 RR024489 (to A. B.); and R01 HL55757, HL-70897, HL-76794, and P01HL78825 (to R. B.). This work was also supported by American Heart Association Grant 12BGIA9090005 (to C. C.) and Kentucky Science and Engineering Foundation Grant 2134-RDE-013 (to C. C.).

This article contains supplemental Figs. S1 and S2.
- CSC
- cardiac stem cell
- hCSC
- human cardiac stem cell
- CoPP
- cobalt protoporphyrin
- CSF
- colony-stimulating factor
- CXCL
- chemokine (CXC motif) ligand
- HO
- heme oxygenase
- LDH
- lactate dehydrogenase
- PI
- propidium iodide
- PC
- preconditioning
- RC
- recovery.
REFERENCES
- 1. Dawn B., Zuba-Surma E. K., Abdel-Latif A., Tiwari S., Bolli R. (2005) Cardiac stem cell therapy for myocardial regeneration. A clinical perspective. Minerva Cardioangiol. 53, 549–564 [PubMed] [Google Scholar]
- 2. Halkos M. E., Zhao Z. Q., Kerendi F., Wang N. P., Jiang R., Schmarkey L. S., Martin B. J., Quyyumi A. A., Few W. L., Kin H., Guyton R. A., Vinten-Johansen J. (2008) Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res. Cardiol. 103, 525–536 [DOI] [PubMed] [Google Scholar]
- 3. Schuh A., Liehn E. A., Sasse A., Schneider R., Neuss S., Weber C., Kelm M., Merx M. W. (2009) Improved left ventricular function after transplantation of microspheres and fibroblasts in a rat model of myocardial infarction. Basic Res. Cardiol. 104, 403–411 [DOI] [PubMed] [Google Scholar]
- 4. Li Q., Guo Y., Ou Q., Chen N., Wu W. J., Yuan F., O'Brien E., Wang T., Luo L., Hunt G. N., Zhu X., Bolli R. (2011) Intracoronary administration of cardiac stem cells in mice. A new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 106, 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dawn B., Stein A. B., Urbanek K., Rota M., Whang B., Rastaldo R., Torella D., Tang X. L., Rezazadeh A., Kajstura J., Leri A., Hunt G., Varma J., Prabhu S. D., Anversa P., Bolli R. (2005) Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl. Acad. Sci. U.S.A. 102, 3766–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang X. L., Rokosh G., Sanganalmath S. K., Yuan F., Sato H., Mu J., Dai S., Li C., Chen N., Peng Y., Dawn B., Hunt G., Leri A., Kajstura J., Tiwari S., Shirk G., Anversa P., Bolli R. (2010) Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bearzi C., Rota M., Hosoda T., Tillmanns J., Nascimbene A., De Angelis A., Yasuzawa-Amano S., Trofimova I., Siggins R. W., Lecapitaine N., Cascapera S., Beltrami A. P., D'Alessandro D. A., Zias E., Quaini F., Urbanek K., Michler R. E., Bolli R., Kajstura J., Leri A., Anversa P. (2007) Human cardiac stem cells. Proc. Natl. Acad. Sci. U.S.A. 104, 14068–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hansson E. M., Lindsay M. E., Chien K. R. (2009) Regeneration next. Toward heart stem cell therapeutics. Cell Stem Cell 5, 364–377 [DOI] [PubMed] [Google Scholar]
- 9. Laflamme M. A., Murry C. E. (2005) Regenerating the heart. Nat. Biotechnol. 23, 845–856 [DOI] [PubMed] [Google Scholar]
- 10. Müller-Ehmsen J., Whittaker P., Kloner R. A., Dow J. S., Sakoda T., Long T. I., Laird P. W., Kedes L. (2002) Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J. Mol. Cell Cardiol. 34, 107–116 [DOI] [PubMed] [Google Scholar]
- 11. Laflamme M. A., Chen K. Y., Naumova A. V., Muskheli V., Fugate J. A., Dupras S. K., Reinecke H., Xu C., Hassanipour M., Police S., O'Sullivan C., Collins L., Chen Y., Minami E., Gill E. A., Ueno S., Yuan C., Gold J., Murry C. E. (2007) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 25, 1015–1024 [DOI] [PubMed] [Google Scholar]
- 12. Haider H., Ashraf M. (2010) Preconditioning and stem cell survival. J. Cardiovasc. Transl. Res. 3, 89–102 [DOI] [PubMed] [Google Scholar]
- 13. Mirotsou M., Jayawardena T. M., Schmeckpeper J., Gnecchi M., Dzau V. J. (2011) Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell Cardiol. 50, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou P., Wirthlin L., McGee J., Annett G., Nolta J. (2009) Contribution of human hematopoietic stem cells to liver repair. Semin. Immunopathol. 31, 411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L., Tredget E. E., Wu P. Y., Wu Y. (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3, e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gnecchi M., Zhang Z., Ni A., Dzau V. J. (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burchfield J. S., Dimmeler S. (2008) Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair 1, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shintani S., Kusano K., Ii M., Iwakura A., Heyd L., Curry C., Wecker A., Gavin M., Ma H., Kearney M., Silver M., Thorne T., Murohara T., Losordo D. W. (2006) Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat. Clin. Pract. Cardiovasc. Med. 3, S123–S128 [DOI] [PubMed] [Google Scholar]
- 19. Dzau V. J., Gnecchi M., Pachori A. S. (2005) Enhancing stem cell therapy through genetic modification. J. Am. Coll Cardiol. 46, 1351–1353 [DOI] [PubMed] [Google Scholar]
- 20. Elmadbouh I., Haider H., Jiang S., Idris N. M., Lu G., Ashraf M. (2007) Ex vivo delivered stromal cell-derived factor-1α promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J. Mol. Cell Cardiol. 42, 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumoto R., Omura T., Yoshiyama M., Hayashi T., Inamoto S., Koh K. R., Ohta K., Izumi Y., Nakamura Y., Akioka K., Kitaura Y., Takeuchi K., Yoshikawa J. (2005) Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 25, 1168–1173 [DOI] [PubMed] [Google Scholar]
- 22. Yau T. M., Kim C., Ng D., Li G., Zhang Y., Weisel R. D., Li R. K. (2005) Increasing transplanted cell survival with cell-based angiogenic gene therapy. Ann. Thorac. Surg. 80, 1779–1786 [DOI] [PubMed] [Google Scholar]
- 23. Sikorski E. M., Hock T., Hill-Kapturczak N., Agarwal A. (2004) The story so far. Molecular regulation of the heme oxygenase-1 gene in renal injury. Am J. Physiol. Renal Physiol. 286, F425–F441 [DOI] [PubMed] [Google Scholar]
- 24. Ryter S. W., Morse D., Choi A. M. (2007) Carbon monoxide and bilirubin. Potential therapies for pulmonary/vascular injury and disease. Am. J. Respir. Cell Mol. Biol. 36, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abraham N. G., Kappas A. (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 60, 79–127 [DOI] [PubMed] [Google Scholar]
- 26. McCoubrey W. K., Jr., Huang T. J., Maines M. D. (1997) Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur. J. Biochem. 247, 725–732 [DOI] [PubMed] [Google Scholar]
- 27. McCoubrey W. K., Jr., Eke B., Maines M. D. (1995) Multiple transcripts encoding heme oxygenase-2 in rat testis. Developmental and cell-specific regulation of transcripts and protein. Biol. Reprod. 53, 1330–1338 [DOI] [PubMed] [Google Scholar]
- 28. Alam J., Stewart D., Touchard C., Boinapally S., Choi A. M., Cook J. L. (1999) Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 274, 26071–26078 [DOI] [PubMed] [Google Scholar]
- 29. Bolli R., Stein A. B., Guo Y., Wang O. L., Rokosh G., Dawn B., Molkentin J. D., Sanganalmath S. K., Zhu Y., Xuan Y. T. (2011) A murine model of inducible, cardiac-specific deletion of STAT3. Its use to determine the role of STAT3 in the upregulation of cardioprotective proteins by ischemic preconditioning. J. Mol. Cell. Cardiol. 50, 589–597 [DOI] [PubMed] [Google Scholar]
- 30. Kawamoto S., Flynn J. P., Shi Q., Sakr S. W., Luo J., Allen M. D. (2011) Heme oxygenase-1 induction enhances cell survival and restores contractility to unvascularized three-dimensional adult cardiomyocyte grafts implanted in vivo. Tissue Eng. Part A 17, 1605–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. L'Abbate A., Neglia D., Vecoli C., Novelli M., Ottaviano V., Baldi S., Barsacchi R., Paolicchi A., Masiello P., Drummond G. S., McClung J. A., Abraham N. G. (2007) Beneficial effect of heme oxygenase-1 expression on myocardial ischemia-reperfusion involves an increase in adiponectin in mildly diabetic rats. Am. J. Physiol. Heart Circ. Physiol. 293, H3532–H3541 [DOI] [PubMed] [Google Scholar]
- 32. Bolli R., Chugh A. R., D'Amario D., Loughran J. H., Stoddard M. F., Ikram S., Beache G. M., Wagner S. G., Leri A., Hosoda T., Sanada F., Elmore J. B., Goichberg P., Cappetta D., Solankhi N. K., Fahsah I., Rokosh D. G., Slaughter M. S., Kajstura J., Anversa P. (2011) Cardiac stem cells in patients with ischemic cardiomyopathy (SCIPIO). Initial results of a randomized phase 1 trial. Lancet 378, 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. He J. Q., Vu D. M., Hunt G., Chugh A., Bhatnagar A., Bolli R. (2011) Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS One 6, e27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai C., Masumiya H., Weisleder N., Matsuda N., Nishi M., Hwang M., Ko J. K., Lin P., Thornton A., Zhao X., Pan Z., Komazaki S., Brotto M., Takeshima H., Ma J. (2009) MG53 nucleates assembly of cell membrane repair machinery. Nat. Cell Biol. 11, 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai C., Weisleder N., Ko J. K., Komazaki S., Sunada Y., Nishi M., Takeshima H., Ma J. (2009) Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 284, 15894–15902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun B., Sun Z., Jin Q., Chen X. (2008) CO-releasing molecules (CORM-2)-liberated CO attenuates leukocyte infiltration in the renal tissue of thermally injured mice. Int. J. Biol. Sci. 4, 176–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shirai T., Rao V., Weisel R. D., Ikonomidis J. S., Li R. K., Tumiati L. C., Merante F., Mickle D. A. (1998) Preconditioning human cardiomyocytes and endothelial cells. J. Thorac. Cardiovasc. Surg. 115, 210–219 [DOI] [PubMed] [Google Scholar]
- 38. Haider H., Ashraf M. (2008) Strategies to promote donor cell survival. Combining preconditioning approach with stem cell transplantation. J. Mol. Cell. Cardiol. 45, 554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Otterbein L. E., Choi A. M. (2000) Heme oxygenase. Colors of defense against cellular stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1029–L1037 [DOI] [PubMed] [Google Scholar]
- 40. Abraham N. G., Kappas A. (2005) Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 39, 1–25 [DOI] [PubMed] [Google Scholar]
- 41. Morita T., Kourembanas S. (1995) Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J. Clin. Invest. 96, 2676–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoshida T., Maulik N., Ho Y. S., Alam J., Das D. K. (2001) H(mox-1) constitutes an adaptive response to effect antioxidant cardioprotection. A study with transgenic mice heterozygous for targeted disruption of the heme oxygenase-1 gene. Circulation 103, 1695–1701 [DOI] [PubMed] [Google Scholar]
- 43. Yet S. F., Tian R., Layne M. D., Wang Z. Y., Maemura K., Solovyeva M., Ith B., Melo L. G., Zhang L., Ingwall J. S., Dzau V. J., Lee M. E., Perrella M. A. (2001) Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ. Res. 89, 168–173 [DOI] [PubMed] [Google Scholar]
- 44. Melo L. G., Agrawal R., Zhang L., Rezvani M., Mangi A. A., Ehsan A., Griese D. P., Dell'Acqua G., Mann M. J., Oyama J., Yet S. F., Layne M. D., Perrella M. A., Dzau V. J. (2002) Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation 105, 602–607 [DOI] [PubMed] [Google Scholar]
- 45. Liu X., Simpson J. A., Brunt K. R., Ward C. A., Hall S. R., Kinobe R. T., Barrette V., Tse M. Y., Pang S. C., Pachori A. S., Dzau V. J., Ogunyankin K. O., Melo L. G. (2007) Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 year after acute myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 293, H48–H59 [DOI] [PubMed] [Google Scholar]
- 46. Kim D. H., Burgess A. P., Li M., Tsenovoy P. L., Addabbo F., McClung J. A., Puri N., Abraham N. G. (2008) Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-α and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Pharmacol. Exp. Ther. 325, 833–840 [DOI] [PubMed] [Google Scholar]
- 47. Li M., Kim D. H., Tsenovoy P. L., Peterson S. J., Rezzani R., Rodella L. F., Aronow W. S., Ikehara S., Abraham N. G. (2008) Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 57, 1526–1535 [DOI] [PubMed] [Google Scholar]
- 48. Sambuceti G., Morbelli S., Vanella L., Kusmic C., Marini C., Massollo M., Augeri C., Corselli M., Ghersi C., Chiavarina B., Rodella L. F., L'Abbate A., Drummond G., Abraham N. G., Frassoni F. (2009) Diabetes impairs the vascular recruitment of normal stem cells by oxidant damage, reversed by increases in pAMPK, heme oxygenase-1, and adiponectin. Stem Cells 27, 399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Orozco-Ibarra M., Estrada-Sánchez A. M., Massieu L., Pedraza-Chaverrí J. (2009) Heme oxygenase-1 induction prevents neuronal damage triggered during mitochondrial inhibition. Role of CO and bilirubin. Int. J. Biochem. Cell Biol. 41, 1304–1314 [DOI] [PubMed] [Google Scholar]
- 50. Nicolai A., Li M., Kim D. H., Peterson S. J., Vanella L., Positano V., Gastaldelli A., Rezzani R., Rodella L. F., Drummond G., Kusmic C., L'Abbate A., Kappas A., Abraham N. G. (2009) Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53, 508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brouard S., Berberat P. O., Tobiasch E., Seldon M. P., Bach F. H., Soares M. P. (2002) Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-κB to protect endothelial cells from tumor necrosis factor-α-mediated apoptosis. J. Biol. Chem. 277, 17950–17961 [DOI] [PubMed] [Google Scholar]
- 52. Foo R. S., Mani K., Kitsis R. N. (2005) Death begets failure in the heart. J. Clin. Invest. 115, 565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frand A. R., Kaiser C. A. (1998) The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1, 161–170 [DOI] [PubMed] [Google Scholar]
- 54. Stastna M., Abraham M. R., Van Eyk J. E. (2009) Cardiac stem/progenitor cells, secreted proteins, and proteomics. FEBS Lett. 583, 1800–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stastna M., Chimenti I., Marbán E., Van Eyk J. E. (2010) Identification and functionality of proteomes secreted by rat cardiac stem cells and neonatal cardiomyocytes. Proteomics 10, 245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chimenti I., Smith R. R., Li T. S., Gerstenblith G., Messina E., Giacomello A., Marbán E. (2010) Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 106, 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Takahashi T., Kalka C., Masuda H., Chen D., Silver M., Kearney M., Magner M., Isner J. M., Asahara T. (1999) Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 [DOI] [PubMed] [Google Scholar]
- 58. Heeschen C., Aicher A., Lehmann R., Fichtlscherer S., Vasa M., Urbich C., Mildner-Rihm C., Martin H., Zeiher A. M., Dimmeler S. (2003) Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood 102, 1340–1346 [DOI] [PubMed] [Google Scholar]
- 59. Mohri T., Fujio Y., Maeda M., Ito T., Iwakura T., Oshima Y., Uozumi Y., Segawa M., Yamamoto H., Kishimoto T., Azuma J. (2006) Leukemia inhibitory factor induces endothelial differentiation in cardiac stem cells. J. Biol. Chem. 281, 6442–6447 [DOI] [PubMed] [Google Scholar]
- 60. Smith H., Whittall C., Weksler B., Middleton J. (2012) Chemokines stimulate bidirectional migration of human mesenchymal stem cells across bone marrow endothelial cells. Stem Cells Dev. 21, 476–486 [DOI] [PubMed] [Google Scholar]
- 61. Ha J., Choi H. S., Lee Y., Kwon H. J., Song Y. W., Kim H. H. (2010) CXC chemokine ligand 2 induced by receptor activator of NF-κB ligand enhances osteoclastogenesis. J. Immunol. 184, 4717–4724 [DOI] [PubMed] [Google Scholar]
- 62. Aghila Rani K. G., Kartha C. C. (2010) Effects of epidermal growth factor on proliferation and migration of cardiosphere-derived cells expanded from adult human heart. Growth Factors 28, 157–165 [DOI] [PubMed] [Google Scholar]
- 63. Stewart A., Guan H., Yang K. (2010) BMP-3 promotes mesenchymal stem cell proliferation through the TGF-β/activin signaling pathway. J. Cell. Physiol. 223, 658–666 [DOI] [PubMed] [Google Scholar]
- 64. Piantadosi C. A. (2002) Carbon monoxide poisoning. N. Engl. J. Med. 347, 1054–1055 [DOI] [PubMed] [Google Scholar]
- 65. Zipper L. M., Mulcahy R. T. (2003) Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 73, 124–134 [DOI] [PubMed] [Google Scholar]
- 66. Sun Z., Huang Z., Zhang D. D. (2009) Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One 4, e6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.