Background: The role of IL-7 in human pre-T cell development is controversial.
Results: Using IL-7Rα-modified Molt3, we demonstrate a synergistic effect of pre-TCR and IL-7 involving STAT5, Akt, and Erk1/2.
Conclusion: There is a stringent requirement for down-regulation of IL-7/IL-7Rα signaling during human T cell development.
Significance: Understanding how IL-7 regulates human T cell development will help future development of T cell therapeutics.
Keywords: Interleukin, Lentivirus, Signaling, T Cell Biology, T Cell Receptor, IL-7, T Cell Development, Lentiviral Vector, Pre-T Cell
Abstract
The role of IL-7 in pre-T cell receptor (TCR) signaling during human T cell development is poorly understood. To study this, we engineered Molt3, a T cell progenitor T-acute lymphoblastic leukemia cell line, using lentiviral IL-7 receptor α (IL-7Rα) to serve as a model system. IL-7 promoted pre-TCR activation in IL-7Rαhi Molt3 as illustrated by CD25 up-regulation after anti-CD3 stimulation. Anti-CD3 treatment activated Akt and Erk1/2 signaling pathways as proven using specific inhibitors, and IL-7 further enhanced both signaling pathways. The close association of IL-7Rα with CD3ζ in the pre-TCR complex was illustrated through live imaging confocal fluorescence microscopy. These results demonstrate a direct and cooperative role of IL-7 in pre-TCR signaling.
Introduction
Maturation of T cell receptor (TCR)2 αβ or γδ T cells from hematopoietic progenitor cells is a progressive and multistep process. T cell development has been characterized by the sequential expression of CD4 and CD8 coreceptors. The CD8−CD4− double-negative (DN) thymocytes differentiate through the CD8 immature single-positive (SP) stage in mice and the CD4 immature SP stage in humans, followed by CD8+CD4+ double-positive (DP) cells and then CD4+ and CD8+ SP cells (1–4). IL-7, pre-TCR, and Notch signaling plays a crucial role in early T cell development. The transition from DN to DP is regulated by pre-TCR (5, 6) and Notch (7–11) signaling. IL-7 supports DN cell survival and proliferation; however, the role of IL-7 during DN-to-DP transition is not well characterized (12–17).
IL-7 is thought to play a cooperative role in pre-TCR signaling (18, 19). However, there are contradicting reports of both inhibitory and supportive roles of IL-7 during DN-to-DP transition. An IL-7 dose-dependent inhibition of DN-to-DP transition in vivo has been reported (20), and IL-7 blocks differentiation at the DN3 stage in fetal thymus organ culture (21). In addition, IL-7 suppresses anti-CD3 antibody-mediated DN-to-DP transition in RAG-1 (recombination-activating gene-1)-deficient pre-T cells (22), and furthermore, IL-7 inhibits DN-to-DP transition in fetal thymus organ culture derived from IL-7 receptor α (IL-7Rα) transgenic mice by inhibiting transcription of T cell factor, lymphoid enhancer factor, and retinoic acid-related orphan receptor γt (23). As pre-TCR signaling is central in DN-to-DP transition (24, 25), the above studies suggest that IL-7 signaling inhibits DN-to-DP transition by inhibiting pre-TCR signaling. On the other hand, IL-7Rα−/− mice display γδ T cell deficiency and a reduced amount of αβ T cells (26, 27). In humans, mutation in IL-7Rα is associated with immunodeficiency (28). Anti-IL-7Rα antibody inhibits DP transition and TCRαβ expression in human CD34 cells in fetal thymus organ culture (29, 30). The latter studies suggest that IL-7/IL-7Rα signaling plays a supportive role in DN-to-DP transition, which contradicts some other studies.
Insights into direct interaction of IL-7 and pre-TCR signaling pathways could help resolve the controversy. During T cell development, pre-TCR signals in an autonomous fashion independent of extracellular ligands (5, 31, 32); however, anti-CD3 antibody can activate pre-TCR signaling (33, 34). A TCRαβ-deficient Jurkat cell line has been used to study pre-TCR signaling (35–37). However, Jurkat cells are considered to be mature T cells and do not recapitulate T cell precursor activities during pre-T cell development. Therefore, we evaluated different T cell progenitor T-acute lymphoblastic leukemia (T-ALL) cell lines and engineered an IL-7Rα-expressing Molt3 cell line as a model pre-T cell system. Our results indicate that IL-7 enhances pre-TCR signaling through Erk1/2 and Akt pathways. This is consistent with the report that receptors with cytoplasmic domain-associated kinases aggregate with TCR and promote TCR signaling. On the other hand, the receptors with phosphatase-associated cytoplasmic domains move away from TCR (38). Further examination of the membrane compartmentalization of IL-7Rα and CD3ζ in live cells using confocal microscopy indicated that IL-7Rα co-localizes with CD3ζ, confirming a cooperative role of IL-7Rα in pre-TCR signaling.
EXPERIMENTAL PROCEDURES
Flow Cytometry and Antibodies
The antibodies used for surface and intracellular staining were as follows: phycoerythrin-Cy7-conjugated anti-CD3 (clone SK7), Pacific Blue-conjugated anti-CD4 (clone RPA-T4), allophycocyanin-Cy7-conjugated anti-CD8 (clone SK1), FITC-conjugated anti-phospho-Tyr-694 STAT5 (clone 47), phycoerythrin-conjugated anti-phospho-Ser-473 Akt (clone M89-61), and Alexa Fluor 647-conjugated anti-phospho-Thr-202/phospho-Tyr-204 Erk1/2 (clone 20A) (BD Biosciences); anti-CD127 (clone 40131; R&D Systems, Minneapolis, MN); and anti-pre-Tα (G-14; Santa Cruz Biotechnology). For flow cytometry staining, cells were first washed with PBS plus 2% FBS and blocked with mouse and human serum at 4 °C for 30 min. Cells were incubated with antibodies following the manufacturers' instructions. For each fluorochrome-labeled antibody used, an appropriate isotype control was included. After antibody staining, the cells were washed twice with FACS buffer and fixed with 2% paraformaldehyde. Intracellular staining was performed using a BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer's protocol. For detection of phosphorylated proteins, cells were stimulated using the indicated stimulus for 15 min. The cells were then fixed by adding an equal amount of prewarmed 2% paraformaldehyde and permeabilized with 90% methanol for 30 min on ice or at −20 °C overnight. Next, the cells were washed and incubated with the indicated antibodies for 1 h at room temperature, followed by two washes with FACS buffer. Data were acquired using BD FACSDiva software (Version 5.0.1) on a BD LSR II flow cytometer and analyzed using FlowJo software (Version 7.1.3.0, Tree Star Inc., Pasadena, TX).
Lentiviral Vector Construction and Transduction
Lentiviral vectors were generated using the NHP/TYF lentiviral vector system as described previously (39, 40). IL-7Rα cDNA was cloned into the pTYF transducing vector behind the human EF1α promoter.
Inhibitors
Jak3 inhibitor V was purchased from Calbiochem. MEK1/2 inhibitor U0126 and PI3K inhibitor LY294002 were purchased from Cell Signaling Technology, Inc. (Danvers, MA).
RNA Extraction and Semiquantitative RT-PCR
RNA was harvested from cells using TRI Reagent (Sigma-Aldrich). 1 μg of RNA was reverse-transcribed into cDNA using a two-step avian myeloblastosis virus RT-PCR kit (GeneChoice, Frederick, MD). The following primers were used for PCR: GAPDH, 5′-CCG ATG GCA AAT TCG ATG GC-3′ (forward) and 5′-GAT GAC CCT TTT GGC TCC CC-3′ (reverse); pre-Tα, 5′AGT ACA CAG CCC ATG CAT CTG TCA-3′ (forward) and 5′-AAT GCT CCA AGA CTG GAG GAA GGA-3′ (reverse); CD3ϵ, 5′-TGA AGC ATC ATC AGT AGT CAC AC-3′ (forward) and 5′-GGC CTC TGT CAA CAT TTA CC-3′ (reverse); and IL-7Rα, 5′-TCG CAG CAC TCA CTG ACC-3′ (forward) and 5′-GTC ATT GGC TCC TTC CCG-3′ (reverse). After 30 cycles of amplification (95 °C for 30 s, 55 °C for 30 s, and 72 °C for 60 s), PCR products were separated on a 2% agarose gel.
Intracellular Free Ca2+ Measurements
Molt3 and Jurkat cells were loaded with the Ca2+ indicator dye Indo-1/AM (Molecular Probes) and stimulated with HIT3a (5 μg/ml), anti-mouse IgG (0.15 mg/ml), or ionomycin (1 μg/ml). A 1-ml volume of cells at a density of 2 × 106 cells/ml was warmed to 37 °C and placed onto the BD LSR II flow cytometer. Base-line measurements were collected for 1 min, anti-CD3 antibody and anti-mouse IgG were added, and measurement was continued for an additional 5 min, followed by the addition of ionomycin and measurement for an additional 1–2 min. Stimulus-induced changes in the intracellular Ca2+ concentration were determined over time by monitoring the fluorescence emission ratio of the Ca2+-bound versus free form of Indo-1 at 405 and 495 nm, respectively; data were analyzed and plotted using FlowJo software.
Western Blotting
Antibodies for phospho-Erk1/2 (Thr-202/Tyr-204; D13.14.4E and 137F5) were obtained from Cell Signaling Technology, Inc. For Western blot analysis, cells were collected at specific time points post-treatment and washed with cold PBS before being lysed in Blue loading buffer with DTT (Cell Signaling Technology, Inc.) and supplemented with protease inhibitor mixture (Roche Diagnostics). Protein was separated on a 10% gel and then transferred to PVDF membrane (Millipore). The membrane was blocked and probed with antibodies as indicated and then incubated with peroxidase-conjugated secondary antibody (Cell Signaling Technology, Inc.). Bound proteins were visualized by SuperSignal West Pico chemiluminescent substrate (Thermo Scientific, Waltham, MA).
Statistical Analysis
Statistical analysis was performed by applying the Wilcoxon matched-pairs signed-rank test using GraphPad Prism 5 software.
RESULTS
IL-7R Expression and Pre-TCR Signaling in T-ALL Cell Lines
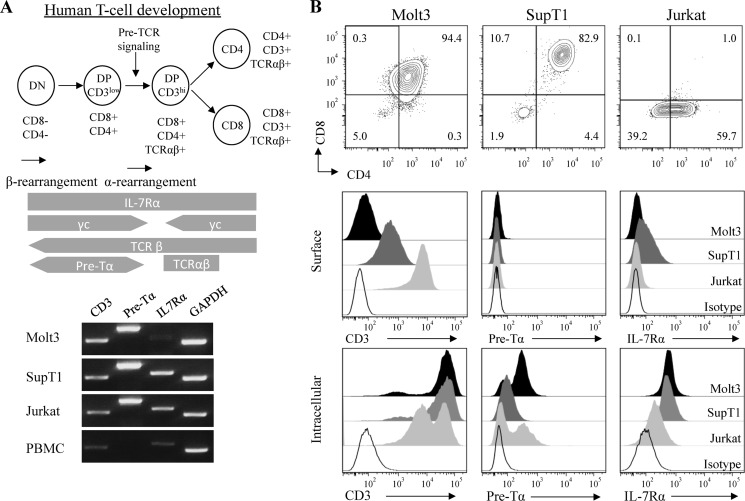
Key stages of human T cell development have been characterized as DN, CD3low DP, CD3hi TCRαβ+ DP, CD8 SP, or CD4 SP as shown at top of Fig. 1. Full-length transcripts of TCRβ and pre-Tα have been detected in the CD3low DP stage, and pre-TCR signaling drives progression to CD3hi DP and induces allelic exclusion at the TCRβ locus, followed by TCRα locus rearrangement (41). We have recently established that concomitant IL-7/IL-7Rα signaling and pre-TCR activation play a central role in promoting DN-to-DP transition as well as T cell maturation during human T cell development in vitro (73). To investigate the direct effect of IL-7 on pre-TCR signaling, we examined several T-ALL cell lines, including Jurkat, SupT1, and Molt3.
FIGURE 1.
Analysis of pre-T signaling receptor molecules in Molt3, SupT1, and Jurkat T-ALL cell lines. A, schematic illustration of human T cell development. Results from the RT-PCR analysis of transcripts of CD3ϵ, pre-Tα, and IL-7Rα using GAPDH as a control are also shown. PBMC, peripheral blood mononuclear cells. B, flow cytometry analysis of surface expression of CD8, CD4, CD3, pre-Tα, and IL-7Rα and intracellular expression of CD3, pre-Tα, and IL-7Rα.
In mice, pre-TCR signaling is initiated at the DN3 stage in the absence of coreceptors. In humans, pre-TCR signaling is initiated at the CD4 immature SP and early DP stages of development. Molt3 resembles an early stage of DP T cells characterized by low CD3 and low CD4/CD8 coreceptor expression; SupT1 resembles a later stage of DP T cell development with high CD3 and coreceptor expression. As Molt3 has a rearranged TCRβ chain and is positive for intracellular TCRβ expression (42, 43), we further evaluated the intracellular and extracellular expression of CD3, pre-Tα, and IL-7Rα.
RT-PCR analysis of RNA showed comparable levels of CD3 and pre-Tα but reduced IL-7Rα transcripts in Molt3 compared with SupT1 and Jurkat cells (Fig. 1A). Analysis of surface markers showed that Molt3 is CD4lowCD8low DP with low surface CD3 expression (Fig. 1B, upper panels), but intracellular staining revealed that cytoplasmic CD3 expression in Molt3 was similar to that in SupT1 and Jurkat cells (Fig. 1B, lower panels). Intracellular staining of IL-7Rα and pre-Tα showed comparable expression of IL-7Rα but higher pre-Tα in Molt3 compared with SupT1 and Jurkat cells (Fig. 1B). In addition, Molt3 showed CD7+CD1alow expression (data not shown). Together, these results suggest that Molt3 is arrested in the CD3low DP pre-T cell development stage, during which pre-TCR signaling occurs (44). However, it lacks surface IL-7Rα expression, a critical requirement for IL-7 signaling (45–47).
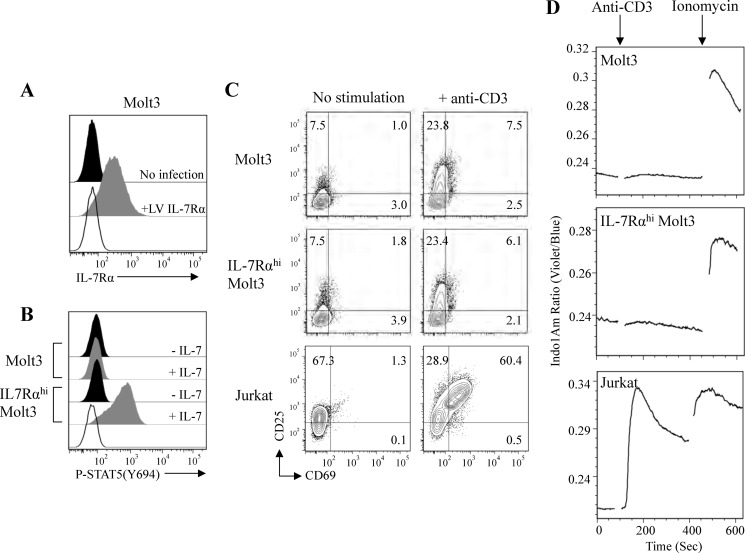
Ectopic Up-regulation of IL-7Rα in Molt3 and Response to IL-7 and Anti-CD3 Stimulation
SupT1 expresses high levels of CD3 and IL-7Rα and would be an ideal choice to study the effect of IL-7 on pre-TCR signaling. However, we found that SupT1 failed to respond to both IL-7 and anti-CD3 stimulation as assessed by the lack of STAT5 activation, lacked up-regulation of T cell activation markers CD25 and CD69, and did not show induction of calcium flux. Hence, we constructed a lentiviral vector encoding IL-7Rα and established an IL-7Rαhi Molt3 cell line. Flow cytometry analysis confirmed surface IL-7Rα expression in the lentiviral IL-7Rα-transduced Molt3 cell line (Fig. 2A, +LV IL-7Rα). IL-7 responsiveness was then assessed by the induction of STAT5 phosphorylation; the result illustrated that IL-7 induced STAT5 (Tyr-694) activation in IL-7Rαhi Molt3 but not in unmodified Molt3 (Fig. 2B). We further evaluated the response to anti-CD3 stimulation by the analysis of T cell activation markers CD25 and CD69 (48). Whereas anti-CD3 antibody activated CD25 and CD69 in Jurkat cells, both the unmodified and IL-7Rαhi Molt3 cell lines up-regulated CD25, but only induced low levels of CD69 in response to anti-CD3 stimulation (Fig. 2C). TCR stimulation directly corresponds to calcium flux, which can be measured by Indo-1/AM violet/blue ratio. Thus, we evaluated induction of calcium flux, and the results showed a low level of response to anti-CD3 stimulation of both unmodified and IL-7Rαhi Molt3 compared with control ionomycin treatment or anti-CD3 antibody-stimulated Jurkat cells (Fig. 2D). Therefore, the anti-CD3 signaling consequence differed between the early T cells (Molt3) and the mature T cells (Jurkat).
FIGURE 2.
Analysis of IL-7 and anti-CD3 signaling in lentiviral vector-modified IL-7Rαhi Molt3. A, surface expression of IL-7Rα on Molt3 (no infection) and lentiviral vector-modified IL-7Rαhi Molt3 (+LV IL-7Rα). B, STAT5 activation in response to 100 ng/ml IL-7 by Phosflow antibody staining and flow cytometry analysis. C, surface expression of activation markers CD25 and CD69 in Molt3, IL-7Rαhi Molt3, and Jurkat cells after overnight incubation with anti-CD3/CD28 beads. D, intracellular free calcium measurement in Indo-1/AM-loaded Molt3, IL-7Rαhi Molt3, and Jurkat cells in response to anti-CD3 antibody (clone HIT3a) and ionomycin.
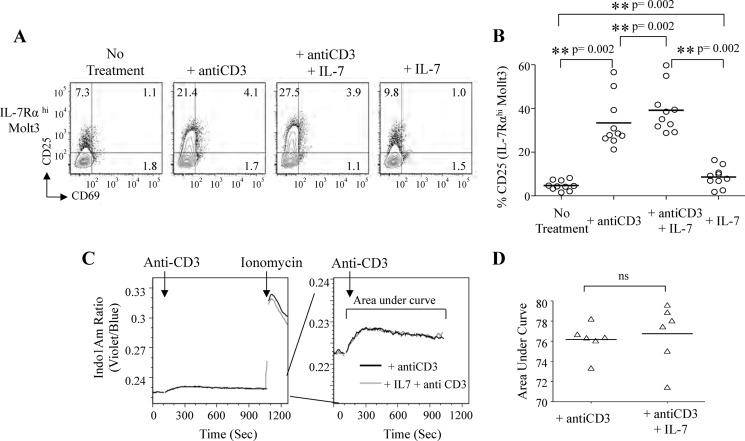
Effect of IL-7 on Anti-CD3 Antibody-induced Pre-TCR Signaling
To more precisely assess the effect of IL-7 on anti-CD3 antibody-mediated pre-TCR activation, we tested four conditions( no treatment, anti-CD3 Ab, anti-CD3 Ab plus IL-7, and IL-7) using IL-7Rαhi Molt3 cells (Fig. 3A). The results showed a consistent increase (n = 10, p = 0.002) in the expression of CD25 after anti-CD3 Ab plus IL-7 treatment (Fig. 3B). Interestingly, IL-7 alone also induced a slight increase in CD25 (p = 0.002) (Fig. 3, A and B). The intensity and duration of TCR stimulation directly correspond to calcium flux, which can be measured by Indo-1/AM violet/blue ratio over time. Evaluation of the effect of IL-7 on anti-CD3 antibody-induced calcium flux showed no change in both the intensity and duration of calcium flux (Fig. 3, C and D).
FIGURE 3.
IL-7 enhances anti-CD3 antibody-induced pre-TCR signaling but not calcium flux induction in IL-7Rαhi Molt3. A, flow cytometry analysis of activation markers CD25 and CD69 in IL-7Rαhi Molt3 after overnight treatment as indicated. B, statistical analysis using the Wilcoxon matched-pairs signed-rank test (n = 10). C, intracellular free calcium measurement in Indo-1/AM-loaded IL-7Rαhi Molt3 in response to anti-CD3 antibody (clone HIT3a) and IL-7 using ionomycin as a control. D, the area under curve was quantified using FlowJo software, and data were analyzed using GraphPad Prism 5 (n = 6). ns, not significant.
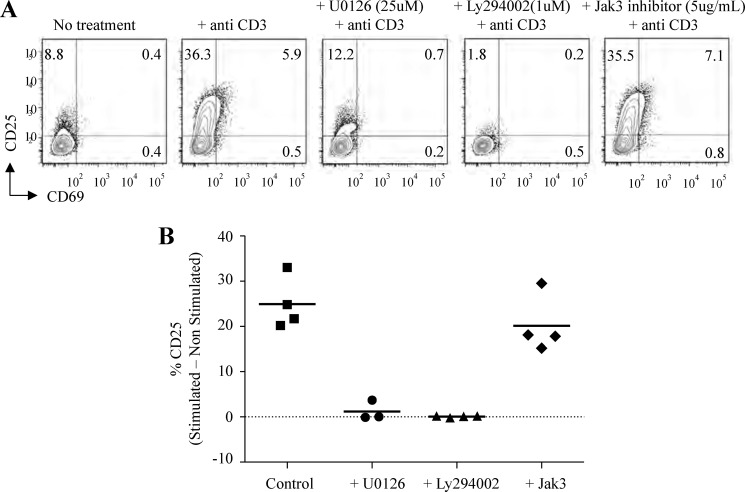
Identification of Multiple Pre-TCR Signaling Pathways
Pre-TCR signaling drives DN-to-DP transition, but the signaling partners of the cytoplasmic tail of the human pre-TCR complex have not yet been identified (6). Pre-TCR signaling occurs through pathways reminiscent of signaling after TCR ligation in mature T cells based on knock-out and transgenic mouse studies (6, 31, 49–53). However, critical differences exist between the mouse and human cytoplasmic domains of the pre-Tα chain (5, 25, 54, 55).
There are three potential pathways for human pre-TCR signaling: Erk1/2-, PI3K-, and γc-mediated signaling pathways. To understand how IL-7 enhances pre-TCR signaling, we first examined the candidate pathways involved in pre-TCR signaling in IL-7Rαhi Molt3. For the PI3K pathway, we tested the inhibitor LY294002. Erk1/2 signaling pathway activation is important for DN-to-DP transition, so we tested the inhibitor U0126 (56). To evaluate the γc signaling pathway, we utilized a Jak3 inhibitor, as γc chain signal transduction begins with the binding of Jak3 (57–59). IL-7Rαhi Molt3 was pretreated with the indicated inhibitors for 2 h, followed by anti-CD3 stimulation and overnight incubation. Analysis of CD25 and CD69 by surface staining and flow cytometry showed that the Erk1/2 and PI3K-Akt pathways were required for pre-TCR signaling, yet the γc signaling pathway was dispensable (Fig. 4, A and B). Similar results were obtained using unmodified Molt3 (data not shown).
FIGURE 4.
Analysis of pre-TCR signaling pathways in IL-7Rαhi Molt3 using specific kinase inhibitors. A, analysis of pre-TCR activation markers in IL-7Rαhi Molt3 after overnight activation and inhibitor treatment as indicated. B, percentage of CD25 expression in inhibitor-treated versus control anti-CD3 antibody-stimulated cells after subtraction of the non-stimulated control. Data represent at least three independent experiments.
Effect of IL-7 on Pre-TCR-mediated Erk1/2 and Akt Activation
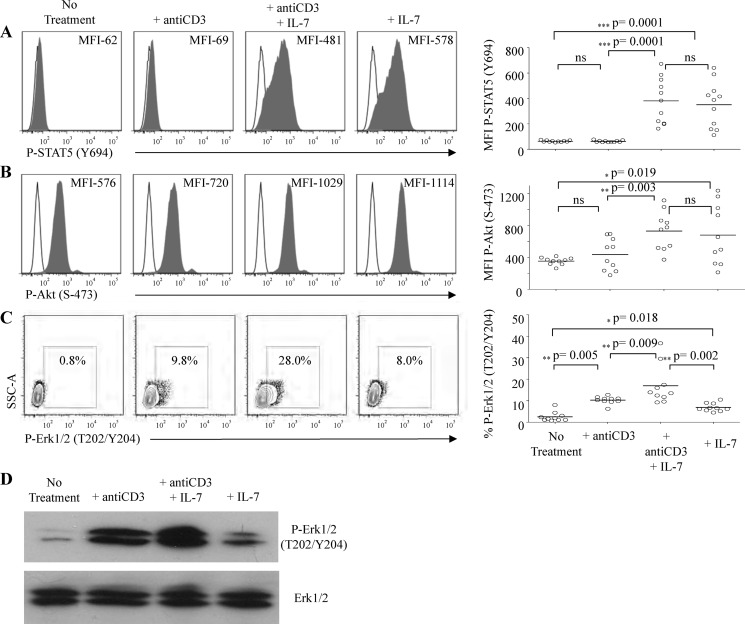
To further characterize the effect of IL-7/IL-7Rα signaling on the Erk1/2 and PI3K-Akt pathways in pre-TCR signaling, we treated IL-7Rαhi Molt3 with IL-7 and anti-CD3 Ab. Activation of STAT5 served as a control for IL-7 signaling. We used phosphorylation-specific antibodies and flow cytometry analysis for quantitative assessment of STAT5, Akt, and Erk1/2 activation. Preliminary flow cytometry analyses of these target proteins at 5, 15, 30, and 60 min post-stimulation established that all three proteins were optimally phosphorylated at 15 min, and thus, we chose this time point for further experiments. The results showed no STAT5 activation in response to anti-CD3 stimulation, but a large population of cells exhibited phospho-STAT5 in response to IL-7 treatment (Fig. 5A). Molt3 is a γ-secretase inhibitor-resistant cell line and has PTEN deficiency and therefore has constitutively active Akt (60). We found that pre-TCR signaling enhanced Akt activation on some occasions, but the effect lacked statistical significance. On the other hand, IL-7 alone significantly increased Akt activation based on mean fluorescence index (Fig. 5B). Anti-CD3 stimulation activated Erk1/2 (from 0.8 to 9.8%) (Fig. 5C); interestingly, IL-7 alone was able to activate Erk1/2 (8%) and showed a synergistic effect upon anti-CD3 stimulation (28%, n = 10) (Fig. 5C), which was further confirmed by Western blotting (Fig. 5D).
FIGURE 5.
Synergy of IL-7 with pre-TCR signaling through activating Akt and Erk1/2. IL-7Rαhi Molt3 cells treated with 100 ng/ml IL-7 and anti-CD3/CD28 beads for 15 min. Cells were fixed; permeabilized; stained with antibodies against activated STAT5 (A), Akt (B), and Erk1/2 (C); and subjected to flow cytometry analysis. The mean fluorescence index (MFI) for STAT5 and Akt and the percentage for Erk1/2 are shown and are summarized on the right via the Wilcoxon matched-pairs signed-rank test (n = 10). D, Western analysis of Erk1/2 activation in response to the indicated treatment. ns, not significant.
Dynamic Interaction of IL-7Rα and CD3ζ
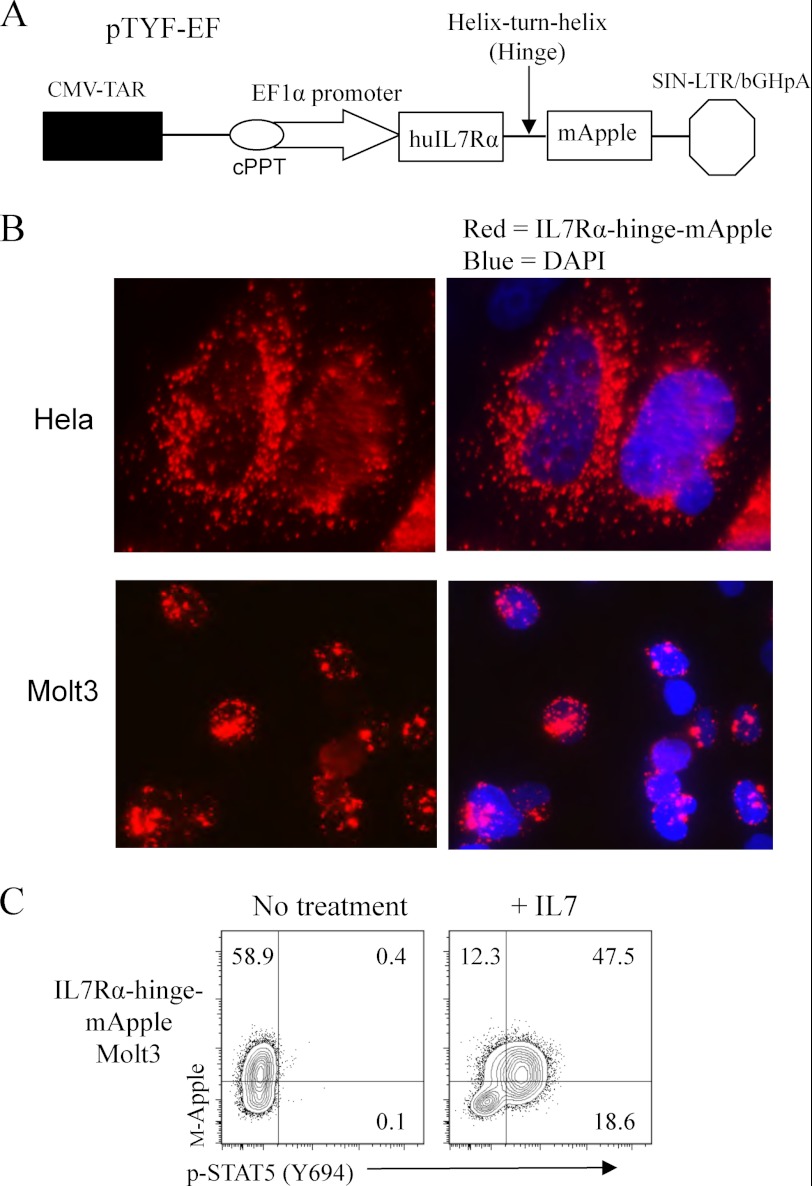
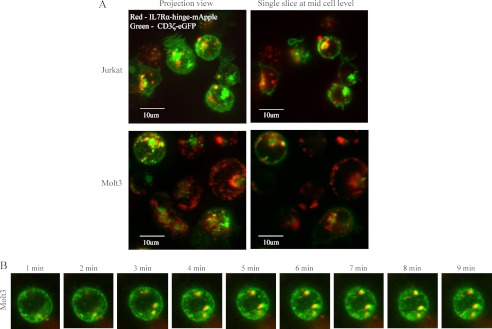
As IL-7 enhanced pre-TCR signaling, we further evaluated a possible direct interaction between IL-7Rα and CD3ζ, the latter being a central component of the pre-TCR signaling complex. We engineered an IL-7Rα-mApple (monomeric red fluorescent protein) fusion to track IL-7Rα in live cells. Lentiviral IL-7Rα-mApple gene-transduced cells expressed a membrane form of the IL-7Rα fusion but lacked STAT5 activation function in response to IL-7 (data not shown). To overcome this defect, we inserted a helix-turn-helix spacer between IL-7Rα and mApple as illustrated in Fig. 6A. HeLa and Molt3 cells were transduced with the lentiviral IL-7Rα-hinge-mApple fusion gene. Immunofluorescence detection showed abundant expression of the fusion protein in the perinuclear compartment (Fig. 6B). We treated IL-7Rα-hinge-mApple Molt3 cells with IL-7 and examined STAT5 activation through phospho-STAT5 intracellular Ab staining and flow cytometry. The result showed that IL-7 treatment markedly induced phospho-STAT5 in IL-7Rα-hinge-mApple Molt3 cells (Fig. 6C). To evaluate the localization of IL-7Rα with respect to CD3ζ, a CD3ζ-eGFP fusion gene (kindly provided by Dr. M. Krummel, University of California, San Francisco) was cloned into a lentiviral expression vector. The functional analysis of the CD3ζ-eGFP fusion protein has been reported previously (61). Jurkat and Molt3 cells were cotransduced with lentiviral vectors encoding IL-7Rα-hinge-mApple and CD3ζ-eGFP, and the transduced cells were imaged live under a confocal microscope. We found that IL-7Rα (red) and CD3ζ (green) tended to co-localize (yellow) in microclusters formed intracellularly or on the cell surface (Fig. 7A). Time-lapse images illustrated that IL-7Rα was moving in close proximity with CD3ζ in Molt3 (Fig. 7B), suggesting a cooperative role of IL-7Rα in pre-TCR signaling.
FIGURE 6.
Live imaging and functional analysis of an IL-7Rα-red fluorescent protein fusion. A, functional IL-7Rα-mApple lentiviral vector construct with a helix-turn-helix hinge domain. CMV-TAR, cytomegalovirus-TAT transactivation response element; cPPT, central polypurine tract; hu, human; SIN-LTR/bGHpA, self-inactivating long terminal repeat/bovine growth hormone polyadenylation signal. B, fluorescence images of HeLa and Molt3 cells infected with IL-7Rα-hinge-mApple. C, STAT5 activation in response to IL-7 in Molt3 cells infected with lentiviral IL-7Rα-hinge-mApple.
FIGURE 7.
Dynamic intracellular interaction of IL-7Rα and CD3ζ. A, confocal images of Molt3 and Jurkat cells infected with lentiviral IL-7Rα-hinge-mApple and CD3ζ-eGFP. B, time-lapse confocal fluorescence images of Molt3 cells co-infected with lentiviral IL-7Rα-hinge-mApple and CD3ζ-eGFP.
DISCUSSION
IL-7 inhibits pre-TCR signaling both in vitro and in transgenic mice (20–23). However, studies of in vitro human T cell development and patients with mutations in IL-7Rα suggest an important role of IL-7 signaling in early T cell development (28, 29). Because of the complexity of these systems, the involvement of IL-7 in pre-TCR signaling has not been characterized. The goal of this study was to decipher the role of IL-7 in pre-TCR signaling. After extensive analysis of existing human T-ALL cell lines, we established an IL-7Rα-modified Molt3 cell line as a DP pre-T model system and demonstrated a synergistic role of IL-7 in pre-TCR signaling. Additionally, we reported that, in addition to STAT5 and Akt, IL-7 activated the Erk1/2 pathway in IL-7Rαhi Molt3. It has been shown that the cytoplasmic tail of IL-7Rα is associated with Lck, the kinase normally associated with T cell coreceptors CD4 and CD8. Thus, the observation that IL-7 suppresses DP transition could be a result of indirect transcriptional regulation of the coreceptor CD4 gene rather than a direct effect on pre-TCR signaling; in fact, IL-7 is known to enhance the expression and activity of CD4 silencer-binding factors such as c-Myb (62).
There is discordance in the RNA and intracellular and surface protein expression of the CD3, pre-Tα, and IL-7Rα genes in the three T-ALL cell lines analyzed here. The levels of CD3 RNA and intracellular protein expression in Molt3 were comparable with those in SupT1 and Jurkat cells, but surface CD3 expression was markedly different (highest in Jurkat and lowest in Molt3 cells), suggesting possible post-translational regulation of surface CD3 expression. Molt3 was previously characterized as CD3-negative; however, this could be an artifact of the anti-CD3 antibody clones used (63). Many studies use antibody clones specifically recognizing CD3ϵ paired with CD3δ or CD3γ; the anti-CD3 antibody clone used in our study does not require such pairing. Moreover, pre-TCR does not require CD3δ for its function (64). Unlike murine pre-Tα, human pre-Tα has an endoplasmic reticulum retention signal in its cytoplasmic domain and is strongly associated with the CD3ζ chain (54, 55). We detected abundant pre-Tα RNA expression in all three T-ALL cell lines, yet surface pre-Tα was barely detected, and only Molt3 showed increased intracellular pre-Tα expression. Interestingly, IL-7Rα transcripts were present at extremely low levels in Molt3 compared with SupT1 and Jurkat cells, but the levels of intracellular protein expression were comparable. However, surface IL-7Rα expression was detected only in SupT1. Thus, the modified Molt3 cell line expressing high surface IL-7Rα (IL-7Rαhi Molt3) represents a rational model system for the study of pre-TCR and IL-7/IL-7Rα signaling in pre-T cells.
IL-7 has been shown to activate the Erk1/2 pathway in pre-B cells but not in mature T cells (65, 66). Activation of the PI3K and Erk1/2 pathways in pre-T cells in response to IL-7 has not been previously evaluated (67). We found that IL-7 activated Akt and STAT5, as well as Erk1/2, which is in line with the finding that IL-7 activates Erk1/2 in an IL-7-dependent immature T cell line, TAIL-7 (68, 69). Unlike STAT5 and Akt activation, both of which showed substantial increases upon IL-7 treatment, IL-7 activated Erk1/2 only in a small percentage of cells (∼8%), which correlated well with the marginal increase in CD25 expression in response to IL-7. Our findings suggest that IL-7 activates Erk1/2 in pre-T cells through a separate pathway in parallel to the canonical γc-dependent Jak-STAT activation pathway.
During mouse T cell development, IL-7Rα is expressed in DN cells, shut down in DP cells, and then re-expressed in mature T cells (70, 71). Different from mouse thymocytes, IL-7Rα is expressed in all stages of developing T cells in humans, but its binding partner γc is down-regulated during the CD3low DP and CD3hi DP stages, resulting in a loss of STAT5 activation (72). During human T cell development, we found that there is a stringent requirement for down-regulation of IL-7/IL-7Rα signaling in pre-TCR signaling (73). Here, we reported that IL-7 delivers additional signals similar to pre-TCR signals in a T-ALL cell line. The lack of down-regulation of IL-7 may aberrantly allow the precursors to bypass TCRβ selection and progress toward the next stage with an improperly rearranged TCRβ chain. Our finding provides a plausible explanation for the necessary down-regulation of IL-7 signaling during the pre-TCR stage of T cell development.
Acknowledgments
We thank Starlyn Okada and Shuhong Han for discussion, Yingchi Chen and Yuling Yeh for technical assistance, and Neil Benson and Daniel Silver for help with flow cytometry and confocal microscopy analyses, respectively.
This work was supported by amfAR Grant 107768-47-RGNT and the Yongling Foundation.
- TCR
- T cell receptor
- DN
- double-negative
- SP
- single-positive
- DP
- double-positive
- IL-7Rα
- IL-7 receptor α
- T-ALL
- T-acute lymphoblastic leukemia
- eGFP
- enhanced GFP.
REFERENCES
- 1. Takahama Y. (2006) Journey through the thymus: stromal guides for T cell development and selection. Nat. Rev. Immunol. 6, 127–135 [DOI] [PubMed] [Google Scholar]
- 2. Rothenberg E. V., Moore J. E., Yui M. A. (2008) Launching the T cell lineage developmental program. Nat. Rev. Immunol. 8, 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carpenter A. C., Bosselut R. (2010) Decision checkpoints in the thymus. Nat. Immunol. 11, 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dervovi D., Zúñiga-Pflücker J. C. (2010) Positive selection of T cells, an in vitro view. Semin. Immunol. 22, 276–286 [DOI] [PubMed] [Google Scholar]
- 5. von Boehmer H., Fehling H. J. (1997) Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 15, 433–452 [DOI] [PubMed] [Google Scholar]
- 6. von Boehmer H. (2005) Unique features of the pre-T cell receptor α-chain: not just a surrogate. Nat. Rev. Immunol. 5, 571–577 [DOI] [PubMed] [Google Scholar]
- 7. Huang E. Y., Gallegos A. M., Richards S. M., Lehar S. M., Bevan M. J. (2003) Surface expression of Notch1 on thymocytes: correlation with the double-negative to double-positive transition. J. Immunol. 171, 2296–2304 [DOI] [PubMed] [Google Scholar]
- 8. Ciofani M., Schmitt T. M., Ciofani A., Michie A. M., Cuburu N., Aublin A., Maryanski J. L., Zúñiga-Pflücker J. C. (2004) Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J. Immunol. 172, 5230–5239 [DOI] [PubMed] [Google Scholar]
- 9. Awong G., La Motte-Mohs R. N., Zúñiga-Pflücker J. (2008) In vitro human T cell development directed by Notch-ligand interactions. Methods Mol. Biol. 430, 135–142 [DOI] [PubMed] [Google Scholar]
- 10. Zúñiga-Pflücker J. C. (2004) T cell development made simple. Nat. Rev. Immunol. 4, 67–72 [DOI] [PubMed] [Google Scholar]
- 11. Schmitt T. M., Zúñiga-Pflücker J. C. (2006) T cell development, doing it in a dish. Immunol. Rev. 209, 95–102 [DOI] [PubMed] [Google Scholar]
- 12. Bolotin E., Smogorzewska M., Smith S., Widmer M., Weinberg K. (1996) Enhancement of thymopoiesis after bone marrow transplant by in vivo interleukin-7. Blood 88, 1887–1894 [PubMed] [Google Scholar]
- 13. Andrew D., Aspinall R. (2001) IL-7 and not stem cell factor reverses both the increase in apoptosis and the decline in thymopoiesis seen in aged mice. J. Immunol. 166, 1524–1530 [DOI] [PubMed] [Google Scholar]
- 14. Fry T. J., Mackall C. L. (2002) Interleukin-7: from bench to clinic. Blood 99, 3892–3904 [DOI] [PubMed] [Google Scholar]
- 15. Chu Y. W., Memon S. A., Sharrow S. O., Hakim F. T., Eckhaus M., Lucas P. J., Gress R. E. (2004) Exogenous IL-7 increases recent thymic emigrants in peripheral lymphoid tissue without enhanced thymic function. Blood 104, 1110–1119 [DOI] [PubMed] [Google Scholar]
- 16. Barata J. T., Cardoso A. A., Boussiotis V. A. (2005) Interleukin-7 in T cell acute lymphoblastic leukemia: an extrinsic factor supporting leukemogenesis? Leuk. Lymphoma 46, 483–495 [DOI] [PubMed] [Google Scholar]
- 17. Mackall C. L., Fry T. J., Gress R. E. (2011) Harnessing the biology of IL-7 for therapeutic application. Nat. Rev. Immunol. 11, 330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fehling H. J., Krotkova A., Saint-Ruf C., von Boehmer H. (1995) Crucial role of the pre-T cell receptor α gene in development of αβ but not γδ T cells. Nature 375, 795–798 [DOI] [PubMed] [Google Scholar]
- 19. Di Santo J. P., Aifantis I., Rosmaraki E., Garcia C., Feinberg J., Fehling H. J., Fischer A., von Boehmer H., Rocha B. (1999) The common cytokine receptor γ-chain and the pre-T cell receptor provide independent but critically overlapping signals in early α/β T cell development. J. Exp. Med. 189, 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Kassar N., Lucas P. J., Klug D. B., Zamisch M., Merchant M., Bare C. V., Choudhury B., Sharrow S. O., Richie E., Mackall C. L., Gress R. E. (2004) A dose effect of IL-7 on thymocyte development. Blood 104, 1419–1427 [DOI] [PubMed] [Google Scholar]
- 21. Plum J., De Smedt M., Leclercq G. (1993) Exogenous IL-7 promotes the growth of CD3-CD4-CD8-CD44+CD25+/− precursor cells and blocks the differentiation pathway of TCRαβ cells in fetal thymus organ culture. J. Immunol. 150, 2706–2716 [PubMed] [Google Scholar]
- 22. Yasuda Y., Kaneko A., Nishijima I., Miyatake S., Arai K. (2002) Interleukin-7 inhibits pre-T cell differentiation induced by the pre-T cell receptor signal, and the effect is mimicked by hGM-CSF in hGM-CSF receptor transgenic mice. Immunology 106, 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu Q., Erman B., Park J. H., Feigenbaum L., Singer A. (2004) IL-7 receptor signals inhibit expression of transcription factors TCF-1, LEF-1, and RORγt: impact on thymocyte development. J. Exp. Med. 200, 797–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shinkai Y., Alt F. W. (1994) CD3ϵ-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/− mice in the absence of TCR β-chain expression. Int. Immunol. 6, 995–1001 [DOI] [PubMed] [Google Scholar]
- 25. Cheng A. M., Negishi I., Anderson S. J., Chan A. C., Bolen J., Loh D. Y., Pawson T. (1997) The Syk and ZAP-70 SH2-containing tyrosine kinases are implicated in pre-T cell receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 94, 9797–9801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maki K., Sunaga S., Komagata Y., Kodaira Y., Mabuchi A., Karasuyama H., Yokomuro K., Miyazaki J. I., Ikuta K. (1996) Interleukin-7 receptor-deficient mice lack γδ T cells. Proc. Natl. Acad. Sci. U.S.A. 93, 7172–7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peschon J. J., Morrissey P. J., Grabstein K. H., Ramsdell F. J., Maraskovsky E., Gliniak B. C., Park L. S., Ziegler S. F., Williams D. E., Ware C. B., Meyer J. D., Davison B. L. (1994) Early lymphocyte expansion is severely impaired in interleukin-7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Puel A., Ziegler S. F., Buckley R. H., Leonard W. J. (1998) Defective IL-7R expression in T−B+NK+ severe combined immunodeficiency. Nat. Genet. 20, 394–397 [DOI] [PubMed] [Google Scholar]
- 29. Pallard C., Stegmann A. P., van Kleffens T., Smart F., Venkitaraman A., Spits H. (1999) Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity 10, 525–535 [DOI] [PubMed] [Google Scholar]
- 30. Plum J., De Smedt M., Leclercq G., Verhasselt B., Vandekerckhove B. (1996) Interleukin-7 is a critical growth factor in early human T cell development. Blood 88, 4239–4245 [PubMed] [Google Scholar]
- 31. Aifantis I., Gounari F., Scorrano L., Borowski C., von Boehmer H. (2001) Constitutive pre-TCR signaling promotes differentiation through Ca2+ mobilization and activation of NF-κB and NFAT. Nat. Immunol. 2, 403–409 [DOI] [PubMed] [Google Scholar]
- 32. Yamasaki S., Ishikawa E., Sakuma M., Ogata K., Sakata-Sogawa K., Hiroshima M., Wiest D. L., Tokunaga M., Saito T. (2006) Mechanistic basis of pre-T cell receptor-mediated autonomous signaling critical for thymocyte development. Nat. Immunol. 7, 67–75 [DOI] [PubMed] [Google Scholar]
- 33. Levelt C. N., Mombaerts P., Iglesias A., Tonegawa S., Eichmann K. (1993) Restoration of early thymocyte differentiation in T cell receptor β-chain-deficient mutant mice by transmembrane signaling through CD3ϵ. Proc. Natl. Acad. Sci. U.S.A. 90, 11401–11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacobs H., Vandeputte D., Tolkamp L., de Vries E., Borst J., Berns A. (1994) CD3 components at the surface of pro-T cells can mediate pre-T cell development in vivo. Eur. J. Immunol. 24, 934–939 [DOI] [PubMed] [Google Scholar]
- 35. Ramiro A. R., Navarro M. N., Carreira A., Carrasco Y. R., de Yébenes V. G., Carrillo G., San Millán J. L., Rubin B., Toribio M. L. (2001) Differential developmental regulation and functional effects on pre-TCR surface expression of human pTαa and pTαb spliced isoforms. J. Immunol. 167, 5106–5114 [DOI] [PubMed] [Google Scholar]
- 36. Navarro M. N., Nusspaumer G., Fuentes P., González-García S., Alcain J., Toribio M. L. (2007) Identification of CMS as a cytosolic adaptor of the human pTα chain involved in pre-TCR function. Blood 110, 4331–4340 [DOI] [PubMed] [Google Scholar]
- 37. Arnaud J., Huchenq A., Vernhes M. C., Caspar-Bauguil S., Lenfant F., Sancho J., Terhorst C., Rubin B. (1997) The interchain disulfide bond between TCRαβ heterodimers on human T cells is not required for TCR-CD3 membrane expression and signal transduction. Int. Immunol. 9, 615–626 [DOI] [PubMed] [Google Scholar]
- 38. Kabouridis P. S. (2006) Lipid rafts in T cell receptor signaling. Mol. Membr. Biol. 23, 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chang L. J., Liu X., He J. (2005) Lentiviral siRNAs targeting multiple highly conserved RNA sequences of human immunodeficiency virus type 1. Gene Ther. 12, 1133–1144 [DOI] [PubMed] [Google Scholar]
- 40. Wang B., He J., Liu C., Chang L. J. (2006) An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens. Vaccine 24, 3477–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ramiro A. R., Trigueros C., Márquez C., San Millán J. L., Toribio M. L. (1996) Regulation of pre-T cell receptor (pTα-TCRβ) gene expression during human thymic development. J. Exp. Med. 184, 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burger R., Hansen-Hagge T. E., Drexler H. G., Gramatzki M. (1999) Heterogeneity of T-acute lymphoblastic leukemia (T-ALL) cell lines: suggestion for classification by immunophenotype and T cell receptor studies. Leukemia Res. 23, 19–27 [DOI] [PubMed] [Google Scholar]
- 43. Hu H. Z., de Weger R. A., Bosboom-Kalsbeek K., Tilanus M. G., Rozing J., Schuurman H. J. (1992) T cell receptor Vβ variable gene family expression in human peripheral blood lymphocytes at the mRNA and membrane protein levels. Clin. Exp. Immunol. 88, 335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Plum J., De Smedt M., Leclercq G., Taghon T., Kerre T., Vandekerckhove B. (2008) Human intrathymic development: a selective approach. Semin. Immunopathol. 30, 411–423 [DOI] [PubMed] [Google Scholar]
- 45. Jiang Q., Li W. Q., Aiello F. B., Mazzucchelli R., Asefa B., Khaled A. R., Durum S. K. (2005) Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 16, 513–533 [DOI] [PubMed] [Google Scholar]
- 46. Mazzucchelli R., Durum S. K. (2007) Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7, 144–154 [DOI] [PubMed] [Google Scholar]
- 47. Henriques C. M., Rino J., Nibbs R. J., Graham G. J., Barata J. T. (2010) IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Rα in T cells. Blood 115, 3269–3277 [DOI] [PubMed] [Google Scholar]
- 48. Biselli R., Matricardi P. M., D'Amelio R., Fattorossi A. (1992) Multiparametric flow cytometric analysis of the kinetics of surface molecule expression after polyclonal activation of human peripheral blood T lymphocytes. Scand. J. Immunol. 35, 439–447 [DOI] [PubMed] [Google Scholar]
- 49. Cantrell D. A. (2002) Transgenic analysis of thymocyte signal transduction. Nat. Rev. Immunol. 2, 20–27 [DOI] [PubMed] [Google Scholar]
- 50. Juntilla M. M., Koretzky G. A. (2008) Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol. Lett. 116, 104–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue L., Chiang L., Kang C., Winoto A. (2008) The role of the PI3K-Akt kinase pathway in T cell development beyond the β checkpoint. Eur. J. Immunol. 38, 3200–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang L., Qiao G., Ying H., Zhang J., Yin F. (2010) TCR-induced Akt serine 473 phosphorylation is regulated by protein kinase Cα. Biochem. Biophys. Res. Commun. 400, 16–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mao C., Tili E. G., Dose M., Haks M. C., Bear S. E., Maroulakou I., Horie K., Gaitanaris G. A., Fidanza V., Ludwig T., Wiest D. L., Gounari F., Tsichlis P. N. (2007) Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J. Immunol. 178, 5443–5453 [DOI] [PubMed] [Google Scholar]
- 54. Del Porto P., Bruno L., Mattei M. G., von Boehmer H., Saint-Ruf C. (1995) Cloning and comparative analysis of the human pre-T cell receptor α-chain gene. Proc. Natl. Acad. Sci. U.S.A. 92, 12105–12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Carrasco Y. R., Navarro M. N., de Yébenes V. G., Ramiro A. R., Toribio M. L. (2002) Regulation of surface expression of the human pre-T cell receptor complex. Semin. Immunol. 14, 325–334 [DOI] [PubMed] [Google Scholar]
- 56. Crompton T., Gilmour K. C., Owen M. J. (1996) The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell 86, 243–251 [DOI] [PubMed] [Google Scholar]
- 57. Baird A. M., Thomis D. C., Berg L. J. (1998) T cell development and activation in Jak3-deficient mice. J. Leukoc. Biol. 63, 669–677 [DOI] [PubMed] [Google Scholar]
- 58. Suzuki K., Nakajima H., Saito Y., Saito T., Leonard W. J., Iwamoto I. (2000) Janus kinase 3 (JAK3) is essential for common cytokine receptor γ-chain (γc)-dependent signaling: comparative analysis of γc, Jak3, and γc and Jak3 double-deficient mice. Int. Immunol. 12, 123–132 [DOI] [PubMed] [Google Scholar]
- 59. Hofmann S. R., Lam A. Q., Frank S., Zhou Y. J., Ramos H. L., Kanno Y., Agnello D., Youle R. J., O'Shea J. J. (2004) Jak3-independent trafficking of the common γ-chain receptor subunit: chaperone function of Jaks revisited. Mol. Cell. Biol. 24, 5039–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Medyouf H., Gao X., Armstrong F., Gusscott S., Liu Q., Gedman A. L., Matherly L. H., Schultz K. R., Pflumio F., You M. J., Weng A. P. (2010) Acute T cell leukemias remain dependent on Notch signaling despite PTEN and INK4A/ARF loss. Blood 115, 1175–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yudushkin I. A., Vale R. D. (2010) Imaging T cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3ζ in the endosomal compartment. Proc. Natl. Acad. Sci. U.S.A. 107, 22128–22133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Qin J. Z., Zhang C. L., Kamarashev J., Dummer R., Burg G., Döbbeling U. (2001) Interleukin-7 and interleukin-15 regulate the expression of the bcl-2 and c-myb genes in cutaneous T cell lymphoma cells. Blood 98, 2778–2783 [DOI] [PubMed] [Google Scholar]
- 63. Salmerón A., Sánchez-Madrid F., Ursa M. A., Fresno M., Alarcón B. (1991) A conformational epitope expressed upon association of CD3α with either CD3δ or CD3γ is the main target for recognition by anti-CD3 monoclonal antibodies. J. Immunol. 147, 3047–3052 [PubMed] [Google Scholar]
- 64. Berger M. A., Davé V., Rhodes M. R., Bosma G. C., Bosma M. J., Kappes D. J., Wiest D. L. (1997) Subunit composition of pre-T cell receptor complexes expressed by primary thymocytes: CD3δ is physically associated but not functionally required. J. Exp. Med. 186, 1461–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hofmeister R., Khaled A. R., Benbernou N., Rajnavolgyi E., Muegge K., Durum S. K. (1999) Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 10, 41–60 [DOI] [PubMed] [Google Scholar]
- 66. Fleming H. E., Paige C. J. (2002) Cooperation between IL-7 and the pre-B cell receptor: a key to B cell selection. Semin. Immunol. 14, 423–430 [DOI] [PubMed] [Google Scholar]
- 67. Johnson S. E., Shah N., Bajer A. A., LeBien T. W. (2008) IL-7 activates the phosphatidylinositol 3-kinase/Akt pathway in normal human thymocytes but not normal human B cell precursors. J. Immunol. 180, 8109–8117 [DOI] [PubMed] [Google Scholar]
- 68. Barata J. T., Boussiotis V. A., Yunes J. A., Ferrando A. A., Moreau L. A., Veiga J. P., Sallan S. E., Look A. T., Nadler L. M., Cardoso A. A. (2004) IL-7-dependent human leukemia T cell line as a valuable tool for drug discovery in T-ALL. Blood 103, 1891–1900 [DOI] [PubMed] [Google Scholar]
- 69. Barata J. T., Silva A., Brandao J. G., Nadler L. M., Cardoso A. A., Boussiotis V. A. (2004) Activation of PI3K is indispensable for interleukin-7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. J. Exp. Med. 200, 659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Akashi K., Kondo M., Weissman I. L. (1998) Role of interleukin-7 in T cell development from hematopoietic stem cells. Immunol. Rev. 165, 13–28 [DOI] [PubMed] [Google Scholar]
- 71. Munitic I., Williams J. A., Yang Y., Dong B., Lucas P. J., El Kassar N., Gress R. E., Ashwell J. D. (2004) Dynamic regulation of IL-7 receptor expression is required for normal thymopoiesis. Blood 104, 4165–4172 [DOI] [PubMed] [Google Scholar]
- 72. Marino J. H., Tan C., Taylor A. A., Bentley C., Van De Wiele C. J., Ranne R., Paliotta M., Broughan T. A., Teague T. K. (2010) Differential IL-7 responses in developing human thymocytes. Hum. Immunol. 71, 329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patel E. S., Okada S., Hachey K., Yang L.-J., Durum S. K., Moreb J. S., Chang L.-J. (2012) Regulation of in vitro human T cell development through interleukin-7 deprivation and anti-CD3 stimulation. BMC Immunol. 13, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]