Background: Reduced K+-Cl− cotransporter-2 (KCC2) function in spinal cords contributes to diminished synaptic inhibition and neuropathic pain development.
Results: Nerve injury causes KCC2 protein breakdown by stimulating glutamate receptors and calcium-dependent calpain activity in spinal cords.
Conclusion: Increased KCC2 proteolysis diminishes synaptic inhibition to maintain chronic neuropathic pain.
Significance: Understanding mechanisms of diminished synaptic inhibition is essential for improving neuropathic pain treatments.
Keywords: Calcium; Calpain; Chloride Transport; Glutamate Receptors; Glutamate Receptors Ionotropic (AMPA, NMDA); Pain; Synaptic Plasticity
Abstract
Loss of synaptic inhibition by γ-aminobutyric acid and glycine due to potassium chloride cotransporter-2 (KCC2) down-regulation in the spinal cord is a critical mechanism of synaptic plasticity in neuropathic pain. Here we present novel evidence that peripheral nerve injury diminishes glycine-mediated inhibition and induces a depolarizing shift in the reversal potential of glycine-mediated currents (Eglycine) in spinal dorsal horn neurons. Blocking glutamate N-methyl-d-aspartate (NMDA) receptors normalizes synaptic inhibition, Eglycine, and KCC2 by nerve injury. Strikingly, nerve injury increases calcium-dependent calpain activity in the spinal cord that in turn causes KCC2 cleavage at the C terminus. Inhibiting calpain blocks KCC2 cleavage induced by nerve injury and NMDA, thereby normalizing Eglycine. Furthermore, calpain inhibition or silencing of μ-calpain at the spinal level reduces neuropathic pain. Thus, nerve injury promotes proteolytic cleavage of KCC2 through NMDA receptor-calpain activation, resulting in disruption of chloride homeostasis and diminished synaptic inhibition in the spinal cord. Targeting calpain may represent a new strategy for restoring KCC2 levels and tonic synaptic inhibition and for treating chronic neuropathic pain.
Introduction
Chronic neuropathic pain, induced by damage to the peripheral or central nervous system, is a major clinical problem and remains difficult to treat. The spinal cord dorsal horn is the first site of synaptic integration in the pain pathway. Activity-dependent synaptic plasticity at the spinal cord level is fundamentally important to the development of neuropathic pain (1). The mechanisms underlying neuropathic pain are complex and may include increased excitability of primary afferent nerves (2, 3), sprouting of primary afferent terminals in the spinal dorsal horn (4, 5), and increased glutamate-mediated input and N-methyl-d-aspartate receptor (NMDAR)3 activity in the spinal dorsal horn (6, 7). In addition, nerve injury transforms normally GABA-mediated synaptic inhibition to excitation of dorsal horn neurons, which constitutes another key mechanism for neuropathic pain (8). However, the reciprocal relationship and the potential link among the proposed mechanisms for neuropathic pain are still poorly defined.
Activation of GABAA and glycine receptors opens Cl− channels and normally inhibits mature neurons in the brain and spinal cord through Cl− influx to hyperpolarize the cell membrane because of low intracellular Cl− concentrations (9, 10). In spinal dorsal horn neurons, low intracellular Cl− concentration is maintained by K+-Cl− cotransporter-2 (KCC2) (8), which extrudes Cl−. Nerve injury reduces the level of KCC2 to cause a depolarizing shift in the reversal potential of GABA-mediated currents in dorsal horn neurons (8). Brain-derived neurotrophic factor (BDNF) released from activated microglia after nerve injury can reduce KCC2 function in the spinal cord (11). However, although acute BDNF treatment induces a transient reduction in KCC2, BDNF paradoxically up-regulates KCC2 in spinal cord neurons and corticospinal neurons after nerve tissue damage (12, 13). Also, spinally administered BDNF alleviates pain hypersensitivity induced by spinal nerve ligation (14), and overexpression of BDNF at the spinal level reduces neuropathic pain caused by sciatic nerve injury (15). At this time the mechanisms underlying prolonged KCC2 down-regulation in the spinal cord after nerve injury remain elusive.
In addition to GABA, glycine is another principal inhibitory neurotransmitter in the adult spinal cord. Nerve injury causes a greater reduction in the amplitude of glycine-mediated IPSCs than that of GABA-mediated IPSCs in the spinal cord (16). The impact of nerve injury-induced reduction in glycine-mediated inhibition on the excitability of spinal dorsal horn neurons has yet to be appreciated. We now show that nerve injury down-regulates KCC2 and diminishes glycine-mediated inhibition, thereby causing hyperexcitability of spinal dorsal horn neurons. We present evidence for a previously undiscovered mechanism underlying KCC2 down-regulation and synaptic disinhibition in the spinal dorsal horn induced by nerve injury; this mechanism involves proteolytic cleavage of KCC2 at the C terminus through the NMDAR and subsequent calpain activation, resulting in disruption of Cl− homeostasis and increased excitability of spinal dorsal horn neurons in neuropathic pain.
EXPERIMENTAL PROCEDURES
Animal Model and Implantation of Intrathecal Catheters
We used male Sprague-Dawley rats (180–200 g; Harlan, Indianapolis, IN) in this study. We used L5 and L6 spinal nerve ligation as an experimental model of neuropathic pain. In brief, we induced anesthesia with 2–3% isoflurane and then isolated the left L5-L6 spinal nerves and ligated them tightly with 5–0 silk suture. Sham animals were used as controls, and they underwent similar surgical procedures except nerve ligation. In some experiments we induced neuropathic pain by loose ligation of the sciatic nerve in rats. We isolated the left sciatic nerve under a surgical microscope and placed four ligatures (1 mm apart) around the nerve. The ligatures were tied so that the nerve trunk was only slightly constricted. Final electrophysiological recording and biochemical measurements were done 3–4 weeks after surgery. All of the surgical preparations and experimental protocols were approved by the Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines for the ethical use of animals.
We implanted intrathecal catheters in spinal nerve ligation (SNL) rats during isoflurane-induced anesthesia 2–3 weeks after surgery. Briefly, each animal was placed prone on a stereotaxic frame and a small incision was made at the back of the animal's neck. Then, a small puncture was made in the atlanto-occipital membrane of the cisterna magna, and a PE-10 catheter (8 cm) was inserted such that the caudal tip reached the lumbar enlargement of the spinal cord (17). We then exteriorized the rostral end of the catheter and closed the wound with sutures. We allowed the animals to recover for at least 5 days before intrathecal injections. Animals displaying signs of motor or neurological dysfunction were excluded from the study. Drugs and chitosan-siRNAs were injected intrathecally in a volume of 10 μl followed by a 10-μl flush with normal saline.
Spinal Cord Slice Preparation and Electrophysiological Recordings
Lumbar spinal cord slices at the L5-L6 level were prepared from adult rats as we described previously (18, 19). We removed the lumbar spinal cord through laminectomy during isoflurane-induced anesthesia. We sliced the spinal cord (400 μm) using vibratome and continually superfused the slices with artificial cerebrospinal fluid containing 117.0 mm NaCl, 3.6 mm KCl, 1.2 mm MgCl2, 2.5 mm CaCl2, 1.2 mm NaH2PO4, 11.0 mm glucose, and 25.0 mm NaHCO3 (bubbled with 95% O2, 5% CO2). Because lamina II outer neurons preferentially receive nociceptive afferent input (5, 19, 20), only neurons in the lamina II outer zone of the spinal cord were recorded. We obtained all perforated voltage-clamp recordings at 34 °C using glass pipettes filled with a solution containing 140 mm CsCl, 5 mm EGTA, 10 mm HEPES, pH 7.4, and 50 μg ml−1 gramicidin D (21). Gramicidin D was first dissolved in dimethylsulfoxide (DMSO) in a stock solution of 50 mg ml−1 before being added to the above pipette solution. Focal electrical stimulation (∼50 μm away from the recorded neuron) was used to evoke glycinergic IPSCs in the presence of 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (a specific AMPA receptor antagonist), 10 μm bicuculline (a selective GABAA receptor antagonist), and 50 μm AP-5 (a specific NMDAR antagonist), which were bath-applied during the recording period (∼6 min). A list of drugs used and their intended targets is provided in supplemental Table 1.
Neurons were voltage-clamped at −60 mV, and glycinergic IPSCs were recorded at different membrane potentials from −90 to +20 mV using 20-mV steps. The Eglycine was determined by using linear regression to calculate a best-fit line for the voltage dependence of glycine-mediated currents. The intercept of the current-voltage line with the abscissa was taken as Eglycine. In some experiments, glycine (300 μm) was directly applied to lamina II neurons using a puff pipette, and the membrane voltage responses were measured and quantified. Excitatory postsynaptic potentials (EPSPs) were recorded in the cell-attached mode using a pipette solution containing 0.9% NaCl under the current-clamp condition without using any glutamate, GABA, or glycine receptor antagonists. EPSPs were evoked by electrical stimulation (0.2 ms, 0.6 mA, and 0.1 Hz) through a bipolar stimulating electrode placed on the dorsal root. Data acquisition and analysis of postsynaptic currents and potentials were done as described previously (18, 19).
Western Blot Analysis
To determine the total protein levels in the dorsal spinal cord tissues, rats were anesthetized with sodium pentobarbital (60 mg/kg, intraperitoneally) and then decapitated. The L5-L6 spinal dorsal quadrants were dissected and homogenized in ice-cold buffer containing 20 mm Tris, pH 7.6, 0.5% Nonidet P-40, 250 mm NaCl, 3 mm EDTA, 3 mm EGTA, 2 mm DTT, and cocktails of protease and phosphatase inhibitors (Sigma). The homogenate was centrifuged at 12,000 × g for 10 min at 4 ºC. We collected the supernatant and measured the protein concentration with use of the Lowry protein assay. For immunoblots, 30 μg of lysates were separated by 15% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. We then probed the blots using anti-KCC2 (N terminus, 19419-R, Santa Cruz Biotechnology, Santa Cruz, CA), anti-NKCC1 (T4, Developmental Studies Hybridoma Bank at the University of Iowa), anti-β-actin (4967, Cell Signaling Technology, Beverly, MA), anti-GAPDH (MAB374, Millipore, Temecula, CA), anti-μ-calpain (2556, Cell Signaling Technology), anti-m-calpain (AB81023, Millipore), or anti-αII-spectrin (MAB1622, Millipore) antibodies. Immunoblots were developed with an enhanced chemiluminescence kit. The amounts of target proteins were quantified by normalizing the optical density of the protein bands to that of GAPDH or β-actin in the same samples.
To measure KCC2 protein levels in the plasma membrane and “crude” cytoplasmic fractions, subcellular fractionations were carried out according to the procedures described previously (22, 23). The L5-L6 spinal dorsal quadrants were homogenized in ice-cold buffer containing 10 mm Tris (pH 7.5), 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 0.32 m sucrose, and cocktails of protease and phosphatase inhibitors. Homogenate was centrifuged at 1,000 × g for 10 min at 4 ºC to remove nuclei and large debris. The supernatant was then centrifuged at 25,000 × g for 30 min at 4 ºC to separate the plasma membrane fraction (the pellet) and cytoplasmic fraction (the supernatant). The pellet was resuspended in lysis buffer containing 20 mm Tris, pH 7.6, 0.5% Nonidet P-40, 250 mm NaCl, 3 mm EDTA, 3 mm EGTA, 2 mm dithiothreitol, and cocktails of protease and phosphatase inhibitors and sonicated for 5 s at 4 ºC. Equal amounts of proteins in each sample were separated by 12% SDS-PAGE and transferred to a PVDF membrane. The blots were then probed with anti-KCC2 C terminus (07-432, Millipore) or anti-KCC2 N terminus (19419-R, KCC2b; Santa Cruz Biotechnology) antibody. GAPDH was used as a loading control because it is not only a cytosolic protein but is also present on the plasma membrane (24, 25). To determine whether the fractionation method of the spinal cord tissues employed in our study can effectively separate the proteins present in the plasma membrane and cytosolic fractions, equal amounts of proteins in the total tissue lysate, plasma membrane fraction, and cytosolic fraction from the dorsal spinal cord tissues were subjected to 8% SDS-PAGE and transferred to a PVDF membrane. The blots were then probed with anti-N-cadherin (4061, Cell Signaling) and anti-fragile X mental retardation protein (FMRP) (4524, Cell Signaling) antibodies. N-cadherin is a well known membrane protein marker, whereas fragile X mental retardation protein is an intracellular protein in neural tissues (26, 27).
Quantitative PCR Analysis
Total RNA was purified with the TRIzol reagent (Invitrogen). cDNA was synthesized from 1 μg of total RNA with use of the reverse transcription system (Promega, San Luis Obispo, CA) and oligo-dT primers. The cDNA was subjected to PCR amplification, detected with S18 rRNA primers (forward, 5′-AGAGGGCTGGGGAGCTCACG-3′; reverse, 5′-GCTCCAGGTCCTCACGCAGC-3′), KCC2 (forward, 5′-CGGATCTTCACCGTGGCGCA-3′; reverse, 5′-CGGAGCCGAGTGTTGGCTGG-3′), μ-calpain (forward, 5′-CTCCGGGGCAGGAGTAGGCA-3′; reverse, 5′-AACTGGCTGTGGGGCTCCCA-3′); m-calpain (forward, 5′-CTCCGGGGCAGGAGTAGGCA-3′; reverse, 5′-AACTGGCTGTGGGGCTCCCA-3′). Real-time PCR was performed on the iQ5 PCR system by using SYBR Green PCR core reagents kit (Bio-Rad). All samples were run in triplicate using an annealing temperature of 60 ºC. We confirmed the specificity of the PCR products by using melting curve analysis and agarose gel electrophoresis. The Ct value of each gene was normalized by using that of S18 rRNA, and the relative mRNA level of the target gene was quantified by using the comparative 2ΔΔCT method.
Small Interfering RNA (siRNA) Incorporated into Chitosan Nanoparticles
An efficient method of delivering siRNA to the spinal cord is by using chitosan nanoparticles (28). Briefly, chitosan (Sigma) was dissolved in 1% acetate buffer and was adjusted to pH 4.6 with 10 n NaOH. Sodium tripolyphosphate solution (0.25%, w/v) containing the siRNA (70 μg/ml) was added to the chitosan solution (5 mg/ml) with fast stirring. After 30 min, the mixture was centrifuged at 9000 × g for another 30 min at 4 ºC. The pellet was then diluted with sterile RNase-free water to obtain the final concentration of 1 μg/μl chitosan-siRNA nanoparticles. To knock down rat μ-calpain expression, we designed and selected two μ-calpain-specific siRNA (Integrated DNA Technologies, Coralville, IA). The two antisense sequences for the μ-calpain were as follows: GGAACTGGGTCCCAATTCCTT (#1) and TGGGTAGATGTGGTTGTGGATGATT (#2). A scrambled sequence (GGACCAGCCGAGATCTTCTTT) was selected as a mismatch control siRNA.
Behavioral Assessments of Nociception
To detect tactile allodynia, we applied von Frey filaments to the left hind paw (ipsilateral to SNL) of rats. Rats were individually placed in suspended chambers on a mesh floor. After an acclimation period for 30 min, a series of calibrated von Frey filaments (Stoelting, Wood Dale, IL) was applied perpendicularly to the plantar surface of both hind paws with sufficient force to bend the filament for 6 s. Brisk withdrawal or paw flinching was considered as a positive response. In the absence of a response, the filament of the next greater force was applied. After a response, the filament of the next lower force was applied. The tactile stimulus producing a 50% likelihood of withdrawal response was calculated by using the “up-down” method (29).
To quantify the mechanical nociceptive threshold, we conducted the paw pressure test on the left hind paw by using the Ugo Basil Analgesimeter (Varese, Italy). To activate the device, a foot pedal was pressed which activated a motor that applied a constantly increasing force on a linear scale. When the animal displayed pain by either withdrawing of the paw or vocalization, the pedal was immediately released, and the animal's nociceptive threshold was read on the scale. A maximum of 400 g was used as a cutoff to avoid potential tissue injury to the animals (17).
Data Analysis
We used Student's t test to compare two groups and one-way analysis of variance to compare more than two groups. We used corresponding nonparametric analysis (i.e. Mann-Whitney or Kruskal-Wallis test) when data were not normally distributed. The level of significance was set at p < 0.05, and all error bars represent S.E. Single, double, and triple asterisks denote statistical significance at the 0.05, 0.01, and 0.001, respectively.
RESULTS
Nerve Injury Switches Glycine-mediated Synaptic Inhibition to Excitation in the Spinal Dorsal Horn
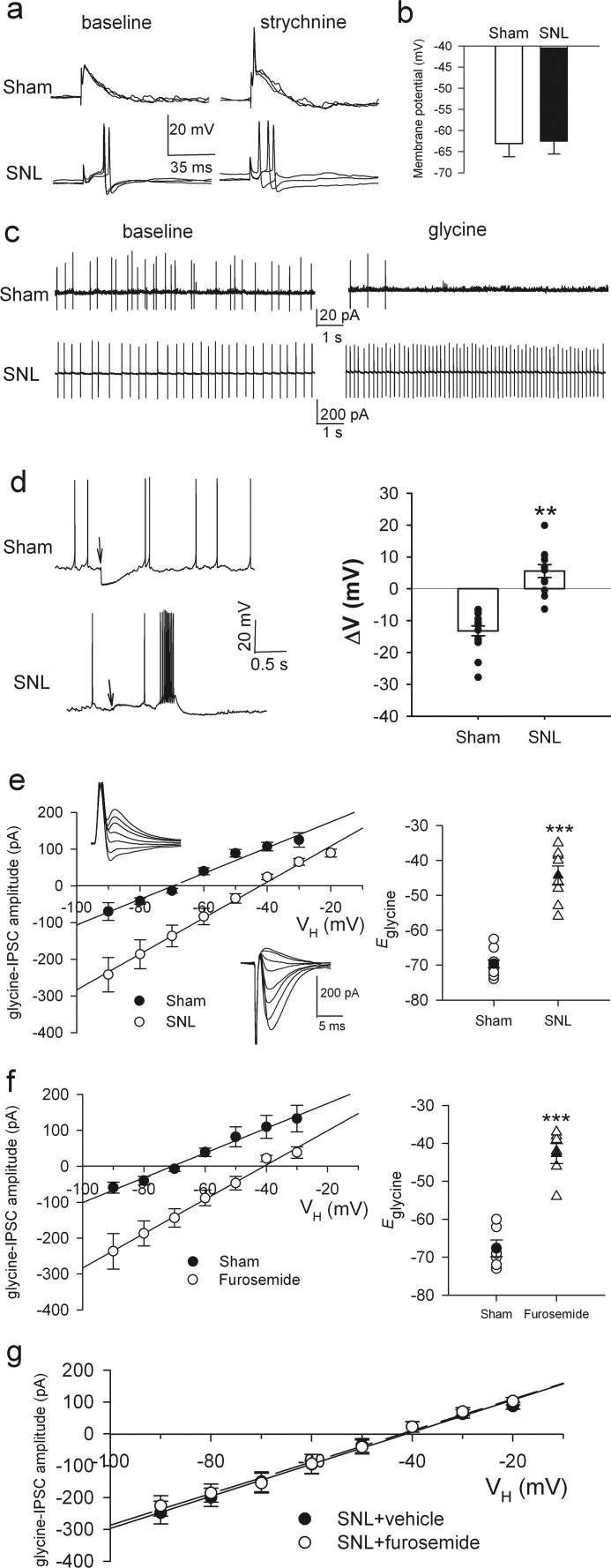
GABAA receptor-mediated inhibitory input to spinal lamina I neurons becomes excitatory after sciatic nerve injury (8). We used L5 and L6 SNL in adult rats, a commonly used neuropathic pain model (30), to determine whether the loss of synaptic inhibition occurs to glycine-mediated input to spinal lamina II neurons, which also receive nociceptive input (4, 5, 19, 31). We confirmed the presence of mechanical hyperalgesia and tactile allodynia in the hind paw of rats 2 weeks after SNL. SNL caused a large reduction in the paw withdrawal threshold in response to the pressure stimulus (pre-injury, 120.37 ± 2.71 g; SNL, 77.41 ± 2.02 g; n = 26, p < 0.01) and von Frey filaments (pre-injury, 22.16 ± 1.16 g; SNL, 2.03 ± 0.02 g; n = 26, p < 0.01). Using Cl−-impermeable gramicidin-perforated patch-clamp recordings (21) in adult spinal cord slices, we first examined the effects of the glycine receptor antagonist strychnine on neuronal excitability. Before strychnine application, electrical stimulation of the dorsal root evoked only excitatory postsynaptic potentials (EPSPs) without triggering action potentials. During strychnine (2 μm) application, dorsal root stimulation consistently increased EPSPs and triggered action potentials of neurons in sham control rats (n = 6; Fig. 1a). However, blocking glycine receptors with strychnine had no further effect on the EPSP-spiking activity of neurons already potentiated by SNL (n = 6; Fig. 1a). The resting membrane potential of neurons did not differ significantly between sham control and SNL rats (n = 15 in each group, Fig. 1b).
FIGURE 1.
Nerve injury causes diminished glycine-mediated synaptic inhibition, a depolarizing shift in Eglycine of lamina II neurons, and KCC2 down-regulation in the spinal cord. a, perforated recordings show EPSP-spike activity of lamina II neurons evoked by electrical stimulation of the dorsal root in sham control (top) and SNL (bottom) rats before and during bath application of 2 μm strychnine. Responses evoked by three consecutive stimulations (at 15-s intervals) were superimposed. b, mean resting membrane potentials of lamina II neurons in sham control (n = 15) and SNL (n = 15) rats (p = 0.92, Mann-Whitney test) are shown. c, cell-attached recordings show the spontaneous firing activity of lamina II neurons from sham control (top) and SNL (bottom) rats before and during bath application of 1 mm glycine. d, perforated recordings (left) and mean membrane potential changes (right) induced by puff application of 300 μm glycine to lamina II neurons in sham control (n = 15) and SNL (n = 12) rats are shown. The downward arrows indicate the time of puff glycine application. e, I-V plots show glycine-mediated IPSCs (left) and mean changes in Eglycine (right) in lamina II neurons from sham control (n = 10) and SNL (n = 8) rats. Inset, original traces of glycine-mediated IPSCs recorded using perforated patch-clamp at different holding potentials (VH) from −90 to +30 mV at 20-mV steps. f, I-V plots show glycine-mediated IPSCs (left) and changes in Eglycine (right) in lamina II neurons (n = 6) of control rats before and during bath application of 200 μm furosemide. g, I-V plots show Eglycine of lamina II neurons in spinal cord slices of SNL rats treated with furosemide (n = 6) or vehicle (n = 7). VH, holding potential. **, p < 0.01; ***, p < 0.001, Mann-Whitney test. Error bars represent S.E.
We then carried out tight-seal cell-attached recordings and assessed the effects of glycine on lamina II neurons with spontaneous firing activity. Bath application of 1 mm glycine rapidly inhibited the firing activity of neurons from 3.18 ± 0.45 to 0.14 ± 0.02 Hz (n = 6, p < 0.01) in sham rats but paradoxically increased the firing activity of neurons from 3.98 ± 0.37 to 7.81 ± 0.57 Hz in SNL rats (n = 6, p < 0.01; Fig. 1c). In the presence of 50 μm dl-2-amino-5-phosphonovaleric acid (AP-5), a specific NMDAR antagonist, glycine application still increased the firing activity of these neurons in SNL rats, thus ruling out the possibility that glycine activates NMDARs via their glycine binding site. Furthermore, puff application of 300 μm glycine hyperpolarized the membrane potential of all 15 dorsal horn neurons tested in sham rats. However, puff glycine application produced only a small hyperpolarization or paradoxically depolarized the membrane potential of dorsal horn neurons in SNL rats (n = 12, p < 0.01; Fig. 1d). These results indicate that glycine-mediated synaptic inhibition is lost and becomes excitatory in the spinal dorsal horn after nerve injury.
Nerve Injury Leads to the Depolarizing Shift in Eglycine through Down-regulation of KCC2
We next tested whether a depolarizing shift in the reversal potential of glycine-mediated currents (Eglycine) would explain the loss of synaptic inhibition in the spinal dorsal horn after nerve injury. We first used gramicidin-perforated patch-clamp recordings to determine SNL-induced changes in Eglycine of lamina II neurons. Glycine receptor-mediated IPSCs were evoked by focal stimulation through an electrode placed adjacent to the recorded neuron. Eglycine of neurons was depolarized by ∼25 mV in SNL rats compared with sham control rats (SNL, −44.3 ± 2.7 mV, n = 8; sham, −69.6 ± 1.1 mV, n = 10; p < 0.001, Fig. 1e).
To determine the contribution of KCC2 to nerve injury-induced depolarizing shift in Eglycine, we bath applied an inhibitor of KCC2, furosemide (200 μm, 20 min), to spinal cord slices taken from sham and SNL rats. Furosemide caused a depolarizing shift in Eglycine of lamina II neurons (control, −67.8 ± 2.1 mV; furosemide, −42.7 ± 2.6 mV; n = 6, p < 0.001; Fig. 1f) in sham rats. However, furosemide had no significant effect on Eglycine of neurons recorded from SNL rats (supplemental Fig. 1a). Furosemide at high concentrations may inhibit NKCC1 present in the spinal cord. We confirmed that bath application of bumetanide (20 μm, 20 min) at a concentration that selectively inhibits NKCC1 had no effect on Eglycine of neurons from sham rats (control, −67.8 ± 2.1 mV; bumetanide, −67.2 ± 2.6 mV; n = 6, p > 0.05).
To examine whether the protein level of KCC2 in the spinal cord is decreased in SNL rats, we analyzed the total protein level of KCC2 in the dorsal spinal quadrants using immunoblotting. The KCC2 protein band (140 kDa) in the dorsal spinal cord ipsilateral to SNL was significantly reduced (∼32% of control level) 4 weeks after SNL (Fig. 2a, p < 0.01). Both monomer and dimer forms of KCC2 in dorsal spinal cords were detected by using 6% polyacrylamide gel, and the dimer form of KCC2 proteins in the dorsal spinal cord was also diminished in SNL rats (Fig. 2b). However, the mRNA level of KCC2 in the spinal cord was not significantly changed by SNL (Fig. 2c). Sham operation had no effect on the KCC2 protein level in the spinal cord (Fig. 2d). Moreover, the protein level of NKCC1 and its subcellular distribution were not significantly affected by SNL (supplemental Fig. 1). Together, these data suggest that nerve injury causes the depolarizing shift in Eglycine of spinal dorsal horn neurons through down-regulation of KCC2.
FIGURE 2.
Nerve injury reduces the monomer and dimer KCC2 protein levels, but not KCC2 mRNA levels, in the dorsal spinal cord. a, Western blots (left) and quantification (right) of KCC2 (∼140 kDa) in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL (n = 8) are shown. b, Western blots (left) and quantification (right) of protein levels of the monomer and dimer KCC2 in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL (n = 6) are shown. The tissue sample was subjected to 6% polyacrylamide gel, and the KCC2 protein level was normalized to that of GAPDH in the same sample. c, nerve injury does not change the KCC2 mRNA level in the spinal cord. The histogram shows quantification of KCC2 mRNA levels in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL 4 weeks after SNL (p = 0.39, Mann-Whitney test). The KCC2 mRNA level was normalized by that of S18 rRNA (n = 6). d, shown are Western blots (inset) and quantification of KCC2 levels in the dorsal spinal cord from control and sham-operated rats (n = 6 in each group). * p < 0.05, ** p < 0.01, Mann-Whitney test. Error bars represent S.E.
NMDARs Contribute to Diminished Synaptic Inhibition and KCC2 Down-regulation in the Spinal Cord by Nerve Injury
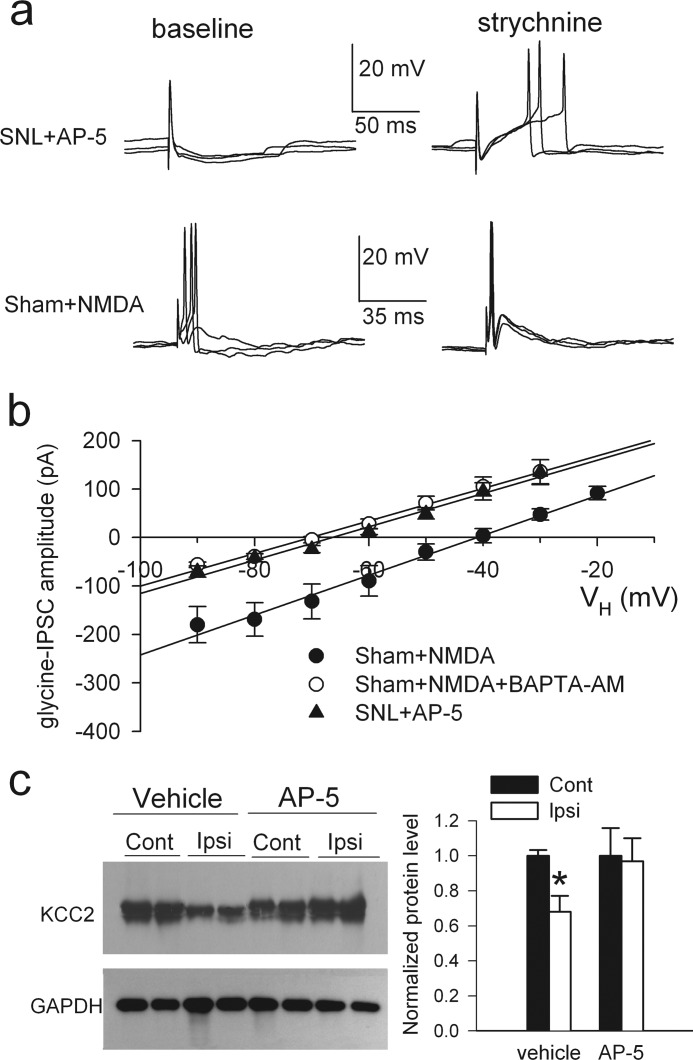
NMDARs in the spinal cord play a pivotal role in nerve injury-induced chronic pain. Glutamate-mediated synaptic input and NMDAR activity of lamina II neurons are increased in spinal cords of SNL rats (32, 33). We determined whether increased NMDAR activity contributes to synaptic disinhibition and the shift in Eglycine of lamina II neurons caused by nerve injury. In spinal cord slices taken from SNL rats, incubation with AP-5 (50 μm, 3 h) reduced the neuronal excitability to the control level and restored the excitatory effect of strychnine (n = 7; Fig. 3a). Also, AP-5 treatment normalized Eglycine of neurons in SNL rats (−67.2 ± 1.9 mV, n = 7; Fig. 3b).
FIGURE 3.
Nerve injury diminishes synaptic inhibition and the depolarizing shift in Eglycine of lamina II neurons through NMDAR activation. a, perforated recordings show the effects of bath application of 2 μm strychnine on EPSP-spike activity of lamina II neurons evoked by dorsal root stimulation in slices taken from control rats incubated with NMDA (30 μm, 2.5–3 h) or in slices taken from SNL rats incubated with AP-5 (50 μm, 3 h). b, I-V plots show changes in Eglycine of lamina II neurons from slices of control rats incubated with NMDA alone (n = 7) or NMDA plus BAPTA-AM (50 μm, n = 6) and from slices taken from SNL rats incubated with AP-5 (n = 7). VH, holding potential. c) shown are Western blots (left) and quantification (right) of KCC2 protein (∼140 kDa) amounts in the dorsal spinal cords ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with intrathecal administration of AP-5 (n = 7) or vehicle (saline, n = 8). KCC2 was detected by using KCC2 N terminus antibody. * p < 0.05, Kruskal-Wallis test. Error bars represent S.E.
We next examined the effect of NMDAR stimulation on the excitability of lamina II neurons from sham control rats. In spinal cord slices treated with NMDA (30 μm, 2.5–3 h), dorsal root stimulation evoked EPSPs and triggered spike activity of neurons. Moreover, strychnine no longer had any stimulatory effect on the spike activity of these neurons (n = 5, Fig. 3a). In addition, NMDA treatment caused a depolarizing shift in Eglycine of neurons from control rats (−43.1 ± 3.1 mV; n = 7, p < 0.05, Fig. 3b) similar to that seen in SNL rats. However, in the presence of the membrane-permeable Ca2+ chelator BAPTA-AM (50 μm), NMDA treatment failed to shift Eglycine (−69.2 ± 2.2 mV; n = 6, p > 0.05, Fig. 3b).
To determine whether NMDARs contribute to down-regulation of KCC2 in the spinal cord by nerve injury, we treated SNL rats with intrathecal injection of AP-5 (10 μg, twice a day for 3 consecutive days) or vehicle 4 weeks after surgery. AP-5 reversed tactile allodynia (supplemental Fig. 2, a and b) and prevented the decrease in the total protein level of KCC2 in the dorsal spinal cord ipsilateral to SNL (Fig. 3c). In addition, AP-5 treatment blocked the depolarizing shift in the reversal potential of GABA-mediated currents in dorsal horn neurons from SNL rats (supplemental Fig. 2c). These results suggest that nerve injury reduces KCC2 protein levels and synaptic inhibition in the spinal cord through activation of NMDARs and subsequent Ca2+ influx.
Nerve Injury Induces Proteolysis of KCC2 at the C Terminus through NMDAR Activation
The KCC2 protein is present in both the plasma membrane and cytoplasmic fractions (12, 34). To determine whether nerve injury alters the subcellular distribution of KCC2 in the spinal cord, we analyzed the protein level of KCC2 in the plasma membrane and cytosolic fractions in the dorsal spinal cords of SNL rats. The protein level of KCC2 in the plasma membrane fraction was significantly reduced in the ipsilateral side compared with the contralateral side (Fig. 4, a and b). Strikingly, an ∼20-kDa fragment band appeared in the cytosolic, but not the plasma membrane, fraction of spinal tissues ipsilateral to SNL when an antibody against the C terminus of KCC2 was used (n = 8, Fig. 4a). Moreover, we detected an ∼120-kDa fragment band in the cytosolic, but not the plasma membrane, fraction in the tissues ipsilateral to SNL using an antibody against the N terminus of KCC2 (n = 8, Fig. 4a). The molecular mass of intact KCC2 is 140 kDa, which equals to the combined molecular weights of two fragment bands detected by N- and C-terminal KCC2 antibodies.
FIGURE 4.
Nerve injury induces truncation of KCC2 at the C terminus in the spinal cord through NMDAR activation. a, shown are Western blots of KCC2 proteins in the plasma membrane and cytosolic fractions of dorsal spinal cord in ipsilateral (Ipsi) and contralateral (Cont) to SNL detected with the KCC2 C terminus (top) and N terminus (middle) antibodies. GAPDH (bottom) was used as a loading control. Note that the C-terminal (∼20 kDa, CT) and N-terminal (∼120 kDa, NT) fragments of KCC2 were detected in the cytosolic fraction. b, shown are Western blots (left) and quantification (right) of the amount and fragment (∼120 kDa) of KCC2 in dorsal spinal cords ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with intrathecal administration of AP-5 (n = 7 rats) or vehicle (saline, n = 8 rats). *, p < 0.05, Kruskal-Wallis test. Error bars represent S.E.
Sciatic nerve constriction injury, another commonly used rat model of neuropathic pain, also significantly reduced the KCC2 level in the plasma membrane and induced KCC2 truncation in the cytosolic fraction of dorsal spinal cords (supplemental Fig. 3a). We confirmed that the fractionation method can effectively separate the proteins present in the plasma membrane and cytosolic fractions of the spinal cord tissues (supplemental Fig. 3b). Thus, KCC2 in the spinal cord is cleaved at a single site in the C terminus after peripheral nerve injury.
We subsequently determined whether increased NMDAR activity plays a role in the breakdown of KCC2 in the spinal cord by nerve injury. In spinal cord slices taken from SNL rats, incubation with AP-5 (50 μm, 3 h) prevented the truncation of KCC2 and reversed the decrease in the protein level of KCC2 on the plasma membrane (Fig. 4b). These data indicate that peripheral nerve injury causes proteolytic cleavage of KCC2 through NMDAR activation at the spinal level.
Nerve Injury Increases Calpain Activity Leading to KCC2 Proteolysis and Synaptic Disinhibition in the Spinal Cord
How does peripheral nerve injury cause proteolysis of KCC2 at the spinal cord level? Calpains are Ca2+-dependent neutral proteases that determine the fate of proteins through regulated proteolytic activity (35, 36). Because NMDAR activation is involved in KCC2 proteolysis after nerve injury, we hypothesized that nerve injury increases calpain activity through NMDAR activation, which cleaves KCC2 and diminishes synaptic inhibition in the spinal cord. Both μ- and m-calpain are widely expressed in the central nervous system (36). We first measured the protein level of μ- and m-calpain in the spinal cord of SNL rats using immunoblotting. The protein level of μ-calpain, but not m-calpain, was significantly increased in the spinal tissues ipsilateral to SNL compared with that in the contralateral side (n = 6, p < 0.01; Fig. 5a).
FIGURE 5.
Nerve injury increases calpain activity in the spinal cord through NMDAR activation. a, shown are Western blots (left) and quantification (right) of μ-calpain (80 kDa) and m-calpain (80 kDa) protein amounts in the dorsal spinal cords ipsilateral (Ipsi) and contralateral (Cont) to SNL (n = 6 in each group). b, shown are immunoblots (left) and quantification (right) of the αII-spectrin breakdown product (spectrin BD, 150 kDa) in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL of rats treated with intrathecal injection of vehicle (saline), AP-5, or calpeptin (n = 6 in each group. *, p < 0.05; **, p < 0.01, Kruskal-Wallis test with Dunn's post test. Error bars represent S.E.
Spectrin is highly sensitive to proteolysis by calpain, and the stable αII-spectrin breakdown product (150 kDa) is commonly used as an immunological assay for calpain activation (37). We thus analyzed the protein level of the αII-spectrin breakdown product, which was significantly greater in the dorsal spinal cord at the ipsilateral than at the contralateral side of SNL (n = 6, p < 0.01; Fig. 5b). This increase was abolished by intrathecal treatment with AP-5 (10 μg, twice a day for three consecutive days) in SNL rats (Fig. 5b). Moreover, intrathecal treatment with calpeptin (100 μg, twice a day for 3 consecutive days), a specific cell-permeable calpain inhibitor (38), also completely blocked the increase in the protein level of αII-spectrin breakdown product in the spinal cord of SNL rats (Fig. 5b). These data indicate that nerve injury increases calpain activity in the spinal cord through NMDAR activation.
We next investigated whether increased calpain activity contributes to diminished synaptic inhibition and KCC2 cleavage by nerve injury and NMDAR stimulation. Incubation of the spinal cord slices of SNL rats with calpeptin (30 μm, 3 h) normalized the excitability of lamina II neurons to the control level and restored the excitatory effect of strychnine on EPSP-spiking activity evoked by dorsal root stimulation (n = 7, Fig. 6a). Calpeptin treatment also normalized Eglycine of neurons from SNL rats (−69.8 ± 3.8 mV, n = 7, Fig. 6b) to the sham control level. Moreover, NMDA treatment failed to shift Eglycine of neurons from control rats in the presence of calpeptin (−70.4 ± 2.1 mV, n = 6, Fig. 6b). AP-5 abolished the effect of NMDA on KCC2 cleavage and the reduction in plasma membrane KCC2 protein levels in the dorsal spinal cords (Fig. 6c). In addition, intrathecal treatment with calpeptin also abolished KCC2 cleavage and normalized the total and plasma membrane protein levels of KCC2 in the spinal cord of SNL rats (Fig. 6, d and e). These results collectively indicate that nerve injury leads to proteolytic cleavage of KCC2 and synaptic disinhibition through NMDAR-calpain activation in the spinal cord.
FIGURE 6.
Nerve injury causes down-regulation and cleavage of KCC2 and changes in synaptic inhibition and Eglycine of lamina II neurons through calpain activation. a, perforated recordings show the EPSP spike activity of lamina II neurons before and during bath application of 2 μm strychnine in spinal slices of SNL rats preincubated with calpeptin (30 μm, 3 h; n = 7). b, I-V plots show changes in Eglycine of lamina II neurons from slices of SNL rats incubated with calpeptin (n = 7) or vehicle (1% DMSO, n = 9) and slices of control rats incubated with NMDA plus calpeptin (n = 6). VH, holding potential. c, shown are Western blots (left) and quantification (right) of the KCC2 protein levels in dorsal spinal cord slices of control rats treated with NMDA (30 μm for 3 h) or NMDA plus AP5 (50 μm for 3 h) (n = 5 in each group). NT, N-terminal. d, shown are Western blots (top) and quantification (bottom) of the total KCC2 amount in dorsal spinal cords ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with intrathecal administration of calpeptin (n = 7) or vehicle (1% DMSO, n = 8). e, shown are immunoblots (top) and quantification (bottom) of the KCC2 amount and fragment (∼120 kDa) in the plasma membrane and cytosol fractions of dorsal spinal cords ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with intrathecal administration of calpeptin (n = 6) or vehicle (1% DMSO, n = 6). KCC2 was detected by using KCC2 N terminus antibody. *, p < 0.05, Kruskal-Wallis test. Error bars represent S.E.
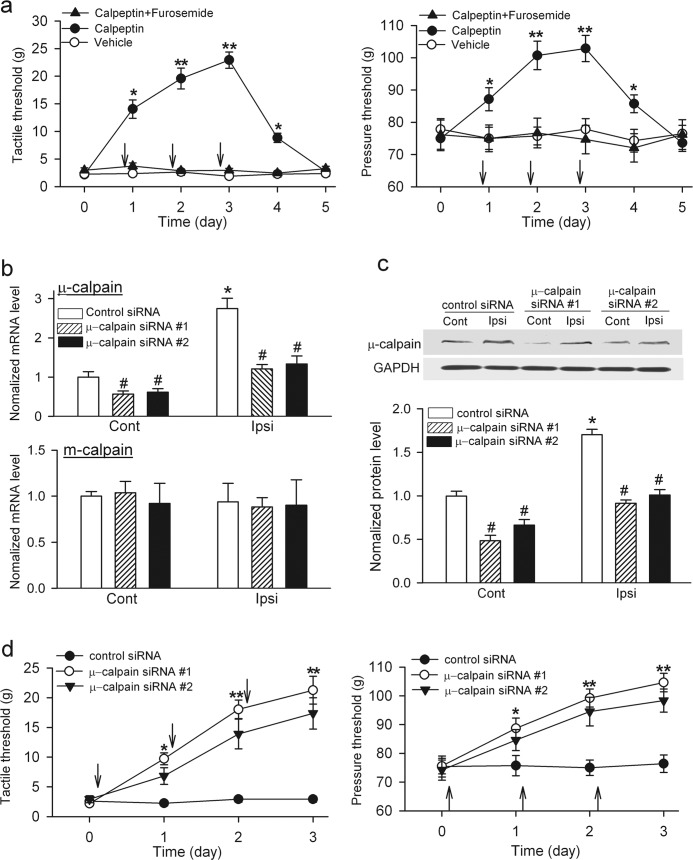
Increased Calpain Activity in the Spinal Cord Contributes to Pain Hypersensitivity Induced by Nerve Injury
To determine the contribution of spinal calpain to the maintenance of neuropathic pain, we examined the effects of calpeptin on pain hypersensitivity 4 weeks after SNL. Intrathecal administration of calpeptin (100 μg, twice a day for 3 consecutive days), not vehicle, significantly increased the tactile and nociceptive withdrawal thresholds in a time-dependent manner (Fig. 7a). Its effect lasted for another day after the last injection. Also, a single intrathecal calpeptin (100 μg) injection gradually and significantly increased tactile threshold, an effect lasting for more than 4 h (supplemental Fig. 4). However, intrathecal co-treatment with calpeptin and furosemide failed to significantly reverse the pain hypersensitivity in SNL rats (Fig. 7a).
FIGURE 7.
Inhibition of calpain or knockdown of μ-calpain at the spinal level reduces SNL-induced pain hypersensitivity. a, shown are mean effects of intrathecal administration of calpeptin (100 μg, twice a day for 3 consecutive days, n = 10 rats), vehicle (1% DMSO, n = 7 rats), or calpeptin plus furosemide (100 μg twice a day for 3 consecutive days, n = 7 rats) on the withdrawal thresholds in response to von Frey filaments (left) or noxious pressure (right) applied to the left hind paw of rats 4 weeks after SNL. Each measurement was conducted 2 h after intrathecal injection (indicated by arrows). b, shown is quantification of the mRNA levels of μ-calpain (top) and m-calpain (bottom) in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with control siRNA or two different μ-calpain-specific siRNA for 3 days (n = 6 in each group). c, shown are Western blots and quantification of μ-calpain proteins in the dorsal spinal cord ipsilateral (Ipsi) and contralateral (Cont) to SNL in rats treated with control siRNA or two different μ-calpain-specific siRNA for 3 days (n = 6 in each group). d, shown are the mean effects of intrathecal administration of scrambled control siRNA (n = 8 rats) and two different μ-calpain-specific siRNA (5 μg, once a day for 3 consecutive days; #1 sRNA, n = 9 rats; #2 sRNA, n = 7 rats) on the withdrawal thresholds in response to von Frey filaments (left) or noxious pressure (right) applied to the left hind paw of rats 4 weeks after SNL. Measurements were performed before siRNA injection each day (indicated by arrows). #, p < 0.05 compared with the control siRNA group; * p < 0.05; **, p < 0.01, compared with the baseline or contralateral side, one-way analysis of variance test. Error bars represent S.E.
Because nerve injury preferentially increased μ-calpain, but not m-calpain, protein levels in the spinal cord, we next used μ-calpain-specific siRNA to determine the contribution of spinal μ-calpain to pain hypersensitivity in SNL rats. We designed and selected two specific siRNA sequences targeting the μ-calpain and conjugated them to chitosan nanoparticles for intrathecal administration. We have shown that this approach effectively knocks down specific targets in the spinal cord (28). Quantitative PCR analysis revealed that the mRNA level of μ-calpain, but not of m-calpain, in the dorsal spinal cord was significantly greater in the ipsilateral than in the contralateral side of SNL (Fig. 7b). Intrathecal injection of either μ-calpain-specific siRNA (5 μg/day for 3 consecutive days) in SNL rats caused a large reduction in the mRNA level of μ-calpain, but not of m-calpain, in the dorsal spinal cord (Fig. 7b). Furthermore, treatment with each μ-calpain-specific siRNA reduced the μ-calpain protein level by ∼50% in the dorsal spinal cord of SNL rats (Fig. 7c). Intrathecal injection of either μ-calpain-specific siRNA, but not the scrambled control siRNA, significantly increased tactile and nociceptive withdrawal thresholds in a time-dependent fashion (Fig. 7d). These data indicate that increased μ-calpain activity at the spinal level contributes to pain hypersensitivity caused by nerve injury.
DISCUSSION
Loss of synaptic inhibition at the spinal level is a key mechanism for neuropathic pain (8, 39). In addition to GABA, glycine is another principal inhibitory neurotransmitter in the spinal dorsal horn. Glycine normally inhibits neuronal excitability by hyperpolarizing the cell membrane and by activation of a shunting conductance, which inhibits propagation of EPSPs along the dendrites of neurons (12, 40). We show that EPSP spike activity of lamina II neurons evoked by primary afferent stimulation is potentiated after blocking glycine receptors. Nerve injury causes an ∼25-mV depolarizing shift of Eglycine in lamina II neurons, and glycine is either less effective to inhibit action potential firing or paradoxically increases the firing activity of dorsal horn neurons after nerve injury. The depolarizing shift of Eglycine can exert a facilitative action on excitatory input such that subthreshold excitations can generate action potentials. Hence, peripheral nerve injury transforms normally glycine-mediated synaptic inhibition to excitation and causes a net excitation of dorsal horn neurons that contributes to central sensitization in neuropathic pain.
KCC2 plays a key role in regulating the transmembrane Cl− gradient and in shaping synaptic inhibition (10). Down-regulation of KCC2 in the spinal cord has been demonstrated in various animal models of neuropathic pain (8, 39). Impaired KCC2 activity can disrupt neuronal Cl− homeostasis in dorsal horn neurons, which plays a pivotal role in diminished synaptic inhibition because of a reduced capacity for Cl− extrusion. We show that inhibition of KCC2 with furosemide causes a depolarizing shift in Eglycine of dorsal horn neurons in control rats, as is observed in SNL rats. However, furosemide has no effect on altered Eglycine of lamina II neurons in SNL rats. Thus, down-regulation of KCC2 plays a major role in the loss of glycine inhibition and the depolarizing shift in Eglycine of dorsal horn neurons by nerve injury. Increased NMDAR activity in the spinal dorsal horn is crucially involved in neuropathic pain caused by nerve injury (6, 41). We show that stimulation of NMDARs mimics nerve injury-induced changes in spinal lamina II neurons: diminished glycine-mediated inhibition and a depolarizing shift in Eglycine. Moreover, blocking of NMDARs completely normalizes synaptic inhibition, Eglycine shift, and KCC2 down-regulation in the spinal cord caused by nerve injury. Of note, increased NMDAR activity and loss of synaptic inhibition at the spinal level have been previously considered independent events in neuropathic pain. Our findings show that glutamate-mediated excitatory input is directly linked to the loss of synaptic inhibition in the spinal dorsal horn, thus providing a new understanding to the mechanisms of synaptic plasticity in neuropathic pain (Fig. 8).
FIGURE 8.
Diagram depicting the relationship between increased NMDAR activity and diminished synaptic inhibition at the spinal level in chronic neuropathic pain. Peripheral nerve injury increases NMDAR activity in the spinal dorsal horn, which stimulates calcium-dependent calpain activity and up-regulates μ-calpain. Increased calpain activity induces KCC2 proteolytic cleavage and impairs KCC2 activity, thereby leading to an increase in intracellular chloride level and diminished synaptic inhibition normally maintained by GABA and glycine. Conversely, diminished synaptic inhibition can also increase synaptic glutamate release and subsequent NMDAR activation. Both diminished synaptic inhibition and increased NMDAR activity contribute to augmented nociceptive input and persistent neuropathic pain.
Neurons in lamina II (and all other laminae) are heterogeneous, and paired neuronal recordings suggest that the vast majority of lamina II neurons are glutamate-releasing interneurons (42). We show that nerve injury or NMDAR stimulation consistently induced a depolarizing shift in Eglycine in lamina II neurons. Because nerve injury converts GABA- and glycine-mediated inhibition to excitation in the spinal dorsal horn, stimulation of GABAA and glycine receptors can promote neuronal excitation after nerve injury. Still, GABA release may limit the excitability of the network by causing primary afferent depolarization (43, 44) and by activating GABAB receptors. Diminished synaptic inhibition of dorsal horn neurons causes neuronal hyperexcitability and increases glutamate release to facilitate the network excitation and nociceptive transmission in neuropathic pain. This reciprocal interaction between increased NMDAR activity and reduced KCC2 function may form a vicious cycle to maintain central sensitization and lasting neuropathic pain.
We found unexpectedly that nerve injury causes KCC2 truncation at the C terminus in the cytosolic fraction in the spinal cord. This surprising finding prompted us to search for the specific mechanisms involved in proteolytic cleavage of KCC2 after nerve injury. The Ca2+ chelator BAPTA-AM abolishes NMDA-induced changes in Eglycine of dorsal horn neurons, suggesting that the signaling molecules activated by Ca2+ influx through NMDARs are involved in KCC2 down-regulation. Increased NMDAR activity leads to Ca2+ influx, and subsequent increases in intracellular Ca2+ concentrations can activate calpain, a cytoplasmic cysteine protease that is activated by Ca2+ at neutral pH (45). Calpain has numerous substrates including membrane, cytosolic, and structural proteins such as spectrin (46). By analyzing αII-spectrin breakdown product, we show that nerve injury increases calpain activity in the spinal cord through NMDAR activation. Both μ-calpain and m-calpain (also known as calpain 1 and calpain 2, respectively) are highly expressed in the central nervous system with different sensitivity to intracellular Ca2+ (36). Whereas m-calpain requires 0.4–0.8 mm Ca2+ for half-maximal proteolytic activity, μ-calpain requires only 3–50 μm for its activity (47) and is the form most concentrated at synaptic sites (48). Of note, the mRNA and protein levels of μ-calpain, but not of m-calpain, in the dorsal spinal cord are increased by peripheral nerve injury. Moreover, we show that inhibition of calpain with calpeptin or blocking of NMDARs prevents KCC2 cleavage and restores changes in KCC2 protein levels, glycine synaptic inhibition, and Eglycine of spinal dorsal horn neurons by nerve injury. The biochemical evidence that KCC2 is a direct substrate of calpain is still lacking. A structural feature of KCC2 is an expanded domain of ∼100 amino acids at the C terminus (49) that is rich in prolines, serines, and charged residues and consists of the predicted PEST (proline/glutamate/serine/threonine) sequences (50). PEST sequences within the protein structure are suggested as the recognition site of substrates by calpain (35). The C-terminal domain of KCC2 is essential for constitutive activity of KCC2 (49). Thus, cleavage of KCC2 at the C terminus and the subsequent down-regulation of plasmalemma KCC2 account for impaired KCC2 function in the spinal dorsal horn after nerve injury.
Oligomerization also may be important for KCC2 function (51), and the KCC2 C terminus is likely a key site of dimer interaction. Consistent with this concept, we show that nerve injury diminishes the dimer form of KCC2 in the spinal cord. The KCC2 protein fragment is detected primarily in the cytoplasmic fraction, suggesting that cleavage of KCC2 occurs predominantly in the cytoplasm. Because of the high turnover rate of KCC2 (52), increased KCC2 degradation in the cytoplasm and reduced trafficking of KCC2 to the plasma membrane could account for consequent reduction in plasmalemma KCC2 levels in the spinal cord. Protein phosphatase 1-mediated KCC2 dephosphorylation plays a role in reduced KCC2 function by NMDAR activation (53), whereas protein kinase C-mediated KCC2 phosphorylation increases KCC2 stability on the cell surface (54). It is not yet clear whether KCC2 dephosphorylation is involved in KCC2 down-regulation in the spinal dorsal horn by nerve injury, because protein kinase C activity in the spinal cord is increased and associated with neuropathic pain (55, 56). It seems that protein phosphatase 1-mediated KCC2 dephosphorylation cannot fully explain the profound reduction in the KCC2 protein level in the spinal cord by nerve injury. It has been shown that NR2 subunits are substrates of calpain and that activation of NMDARs can activate calpain, which in turn down-regulates the NMDAR function (57, 58). However, PSD-95 clustering and direct association of NR2 and PSD-95 may prevent calpain cleavage of NMDARs (59). Our study uncovers an important post-translational mechanism through which increased calpain activity induces proteolytic cleavage of KCC2 and the loss of synaptic inhibition in the spinal dorsal horn in neuropathic pain. Regulated KCC2 proteolysis by fine-tuning of μ-calpain activity serves as a unique signaling mechanism that alters integrity and activity of KCC2 and neuronal function.
We show that inhibiting calpain activity with calpeptin at the spinal level reverses tactile allodynia and hyperalgesia induced by SNL. Moreover, specific knockdown of spinal μ-calpain with siRNA also attenuates SNL-induced pain hypersensitivity. Because calpeptin fails to reverse pain hypersensitivity in the presence of the KCC2 inhibitor, inhibiting abnormal calpain activity can restore synaptic inhibition and reduce neuropathic pain by preventing KCC2 cleavage. Blocking NMDARs or calpain at the spinal level attenuates nerve injury-induced pain hypersensitivity within 30 min, suggesting that these treatments can prevent further cleavage of KCC2 to allow new KCC2 being synthesized and trafficked to the plasma membrane to extrude sufficient amounts of Cl−. Our findings provide a framework for an integrated view about the signaling network involved in maintaining chronic neuropathic pain (Fig. 8).
To conclude, nerve injury promotes proteolytic cleavage of KCC2 through NMDAR-calpain activation, resulting in disruption of Cl− homeostasis and diminished synaptic inhibition by GABA and glycine in the spinal cord. The signaling cascade NMDAR-Ca2+-calpain-KCC2 proteolysis plays a critical role in the loss of KCC2 function and synaptic inhibition in spinal cords induced by increased glutamatergic input after nerve injury. Our study not only reveals a new post-translational mechanism underlying sustained KCC2 down-regulation in the spinal cord after nerve injury but also opens new avenues for research into signaling mechanisms of synaptic plasticity and neuropathic pain. Normalizing calpain activity may represent an important mechanism through which NMDAR antagonists produce long-lasting analgesic effects in patients with neuropathic pain caused by nerve injury (60, 61). On the basis of our evidence, reducing abnormal calpain activity could represent a new strategy for restoring Cl− homeostasis and for treatment of chronic neuropathic pain.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants GM064830, NS045602, and NS073935 (to H.-L. P.), GM092599 (to A. K. S.), and CA151668 (to G. L.-B.). This work was also supported by the N. G. and Helen T. Hawkins endowment (to H.-L. P).

This article contains supplemental Table 1 and Figs. 1–4.
- NMDAR
- N-methyl-d-aspartate receptor
- EPSP
- excitatory postsynaptic potential
- Eglycine
- reversal potential of glycine-mediated current
- GABA
- γ-aminobutyric acid
- IPSC
- inhibitory postsynaptic current
- KCC2
- K+-Cl− cotransporter-2
- NKCC1
- Na+-K+-2Cl− cotransporter-1
- SNL
- spinal nerve ligation
- BDNF
- Brain-derived neurotrophic factor
- AP-5
- dl-2-amino-5-phosphonovaleric acid.
REFERENCES
- 1. Woolf C. J., Salter M. W. (2000) Neuronal plasticity. Increasing the gain in pain. Science 288, 1765–1769 [DOI] [PubMed] [Google Scholar]
- 2. Campbell J. N., Raja S. N., Meyer R. A., Mackinnon S. E. (1988) Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain 32, 89–94 [DOI] [PubMed] [Google Scholar]
- 3. Waxman S. G. (1999) The molecular pathophysiology of pain. Abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain 6, S133–S140 [DOI] [PubMed] [Google Scholar]
- 4. Woolf C. J., Shortland P., Coggeshall R. E. (1992) Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 355, 75–78 [DOI] [PubMed] [Google Scholar]
- 5. Pan H. L., Khan G. M., Alloway K. D., Chen S. R. (2003) Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats. Mechanism of action. J. Neurosci. 23, 2911–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu X. J., Gingrich J. R., Vargas-Caballero M., Dong Y. N., Sengar A., Beggs S., Wang S. H., Ding H. K., Frankland P. W., Salter M. W. (2008) Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat. Med. 14, 1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X. L., Zhang H. M., Chen S. R., Pan H. L. (2007) Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J. Physiol. 579, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coull J. A., Boudreau D., Bachand K., Prescott S. A., Nault F., Sík A., De Koninck P., De Koninck Y. (2003) Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature 424, 938–942 [DOI] [PubMed] [Google Scholar]
- 9. Ganguly K., Schinder A. F., Wong S. T., Poo M. (2001) GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell 105, 521–532 [DOI] [PubMed] [Google Scholar]
- 10. Hübner C. A., Stein V., Hermans-Borgmeyer I., Meyer T., Ballanyi K., Jentsch T. J. (2001) Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron 30, 515–524 [DOI] [PubMed] [Google Scholar]
- 11. Coull J. A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M. W., De Koninck Y. (2005) BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 12. Boulenguez P., Liabeuf S., Bos R., Bras H., Jean-Xavier C., Brocard C., Stil A., Darbon P., Cattaert D., Delpire E., Marsala M., Vinay L. (2010) Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 16, 302–307 [DOI] [PubMed] [Google Scholar]
- 13. Shulga A., Thomas-Crusells J., Sigl T., Blaesse A., Mestres P., Meyer M., Yan Q., Kaila K., Saarma M., Rivera C., Giehl K. M. (2008) Posttraumatic GABA(A)-mediated [Ca2+]i increase is essential for the induction of brain-derived neurotrophic factor-dependent survival of mature central neurons. J. Neurosci. 28, 6996–7005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lever I., Cunningham J., Grist J., Yip P. K., Malcangio M. (2003) Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 18, 1169–1174 [DOI] [PubMed] [Google Scholar]
- 15. Eaton M. J., Blits B., Ruitenberg M. J., Verhaagen J., Oudega M. (2002) Amelioration of chronic neuropathic pain after partial nerve injury by adeno-associated viral (AAV) vector-mediated overexpression of BDNF in the rat spinal cord. Gene Ther. 9, 1387–1395 [DOI] [PubMed] [Google Scholar]
- 16. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2011) Functional plasticity of group II metabotropic glutamate receptors in regulating spinal excitatory and inhibitory synaptic input in neuropathic pain. J. Pharmacol. Exp. Ther. 336, 254–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen S. R., Pan H. L. (2006) Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia. J Neurophysiol 95, 3086–3096 [DOI] [PubMed] [Google Scholar]
- 18. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan Y. Z., Pan H. L. (2004) Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J. Neurophysiol. 91, 2413–2421 [DOI] [PubMed] [Google Scholar]
- 20. Woodbury C. J., Ritter A. M., Koerber H. R. (2000) On the problem of lamination in the superficial dorsal horn of mammals. A reappraisal of the substantia gelatinosa in postnatal life. J. Comp. Neurol. 417, 88–102 [DOI] [PubMed] [Google Scholar]
- 21. Ye Z. Y., Li D. P., Byun H. S., Li L., Pan H. L. (2012) NKCC1 up-regulation disrupts chloride homeostasis in the hypothalamus and increases neuronal activity-sympathetic drive in hypertension. J. Neurosci. 32, 8560–8568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hallett P. J., Collins T. L., Standaert D. G., Dunah A. W. (2008) Biochemical fractionation of brain tissue for studies of receptor distribution and trafficking. Curr. Protoc. Neurosci., Chapter 1, 1.16 [DOI] [PubMed] [Google Scholar]
- 23. Park J. S., Voitenko N., Petralia R. S., Guan X., Xu J. T., Steinberg J. P., Takamiya K., Sotnik A., Kopach O., Huganir R. L., Tao Y. X. (2009) Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J. Neurosci. 29, 3206–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laschet J. J., Minier F., Kurcewicz I., Bureau M. H., Trottier S., Jeanneteau F., Griffon N., Samyn B., Van Beeumen J., Louvel J., Sokoloff P., Pumain R. (2004) Glyceraldehyde-3-phosphate dehydrogenase is a GABAA receptor kinase linking glycolysis to neuronal inhibition. J. Neurosci. 24, 7614–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu K., Aoki C., Elste A., Rogalski-Wilk A. A., Siekevitz P. (1997) The synthesis of ATP by glycolytic enzymes in the postsynaptic density and the effect of endogenously generated nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 94, 13273–13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mazroui R., Huot M. E., Tremblay S., Filion C., Labelle Y., Khandjian E. W. (2002) Trapping of messenger RNA by fragile X mental retardation protein into cytoplasmic granules induces translation repression. Hum. Mol. Genet. 11, 3007–3017 [DOI] [PubMed] [Google Scholar]
- 27. Park J. S., Yaster M., Guan X., Xu J. T., Shih M. H., Guan Y., Raja S. N., Tao Y. X. (2008) Role of spinal cord α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund's adjuvant-induced inflammatory pain. Mol. Pain 4, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai Y. Q., Chen S. R., Han H. D., Sood A. K., Lopez-Berestein G., Pan H. L. (2009) Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J. Neurochem. 111, 1000–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 [DOI] [PubMed] [Google Scholar]
- 30. Kim S. H., Chung J. M. (1992) An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 50, 355–363 [DOI] [PubMed] [Google Scholar]
- 31. Cervero F., Iggo A. (1980) The substantia gelatinosa of the spinal cord. A critical review. Brain 103, 717–772 [DOI] [PubMed] [Google Scholar]
- 32. Zhang H. M., Chen S. R., Pan H. L. (2009) Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain. Neuroscience 158, 875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isaev D., Gerber G., Park S. K., Chung J. M., Randik M. (2000) Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5-L6 spinal nerves. Neuroreport 11, 4055–4061 [DOI] [PubMed] [Google Scholar]
- 34. Khirug S., Ahmad F., Puskarjov M., Afzalov R., Kaila K., Blaesse P. (2010) A single seizure episode leads to rapid functional activation of KCC2 in the neonatal rat hippocampus. J. Neurosci. 30, 12028–12035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tompa P., Buzder-Lantos P., Tantos A., Farkas A., Szilágyi A., Bánóczi Z., Hudecz F., Friedrich P. (2004) On the sequential determinants of calpain cleavage. J. Biol. Chem. 279, 20775–20785 [DOI] [PubMed] [Google Scholar]
- 36. Wu H. Y., Lynch D. R. (2006) Calpain and synaptic function. Mol. Neurobiol. 33, 215–236 [DOI] [PubMed] [Google Scholar]
- 37. Roberts-Lewis J. M., Savage M. J., Marcy V. R., Pinsker L. R., Siman R. (1994) Immunolocalization of calpain I-mediated spectrin degradation to vulnerable neurons in the ischemic gerbil brain. J. Neurosci. 14, 3934–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gellerman D. M., Bi X., Baudry M. (1997) NMDA receptor-mediated regulation of AMPA receptor properties in organotypic hippocampal slice cultures. J. Neurochem. 69, 131–136 [DOI] [PubMed] [Google Scholar]
- 39. Jolivalt C. G., Lee C. A., Ramos K. M., Calcutt N. A. (2008) Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium chloride co-transporters. Pain 140, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeilhofer H. U. (2005) The glycinergic control of spinal pain processing. Cell. Mol. Life Sci. 62, 2027–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaplan S. R., Malmberg A. B., Yaksh T. L. (1997) Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J. Pharmacol. Exp. Ther. 280, 829–838 [PubMed] [Google Scholar]
- 42. Santos S. F., Rebelo S., Derkach V. A., Safronov B. V. (2007) Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J. Physiol. 581, 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rudomin P., Schmidt R. F. (1999) Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 129, 1–37 [DOI] [PubMed] [Google Scholar]
- 44. Willis W. D., Jr. (1999) Dorsal root potentials and dorsal root reflexes. A double-edged sword. Exp. Brain Res. 124, 395–421 [DOI] [PubMed] [Google Scholar]
- 45. Croall D. E., Ersfeld K. (2007) The calpains. Modular designs and functional diversity. Genome Biol. 8, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vosler P. S., Brennan C. S., Chen J. (2008) Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38, 78–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goll D. E., Thompson V. F., Li H., Wei W., Cong J. (2003) The calpain system. Physiol. Rev. 83, 731–801 [DOI] [PubMed] [Google Scholar]
- 48. Perlmutter L. S., Siman R., Gall C., Seubert P., Baudry M., Lynch G. (1988) The ultrastructural localization of calcium-activated protease “calpain” in rat brain. Synapse 2, 79–88 [DOI] [PubMed] [Google Scholar]
- 49. Mercado A., Broumand V., Zandi-Nejad K., Enck A. H., Mount D. B. (2006) A C-terminal domain in KCC2 confers constitutive K+-Cl− cotransport. J. Biol. Chem. 281, 1016–1026 [DOI] [PubMed] [Google Scholar]
- 50. Rechsteiner M., Rogers S. W. (1996) PEST sequences and regulation by proteolysis. Trends Biochem. Sci 21, 267–271 [PubMed] [Google Scholar]
- 51. Blaesse P., Guillemin I., Schindler J., Schweizer M., Delpire E., Khiroug L., Friauf E., Nothwang H. G. (2006) Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J. Neurosci. 26, 10407–10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rivera C., Voipio J., Thomas-Crusells J., Li H., Emri Z., Sipilä S., Payne J. A., Minichiello L., Saarma M., Kaila K. (2004) Mechanism of activity-dependent down-regulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 24, 4683–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee H. H., Deeb T. Z., Walker J. A., Davies P. A., Moss S. J. (2011) NMDA receptor activity down-regulates KCC2 resulting in depolarizing GABAA receptor-mediated currents. Nat. Neurosci. 14, 736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee H. H., Walker J. A., Williams J. R., Goodier R. J., Payne J. A., Moss S. J. (2007) Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784 [DOI] [PubMed] [Google Scholar]
- 55. Malmberg A. B., Chen C., Tonegawa S., Basbaum A. I. (1997) Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science 278, 279–283 [DOI] [PubMed] [Google Scholar]
- 56. Mao J., Price D. D., Mayer D. J., Hayes R. L. (1992) Pain-related increases in spinal cord membrane-bound protein kinase C following peripheral nerve injury. Brain Res 588, 144–149 [DOI] [PubMed] [Google Scholar]
- 57. Guttmann R. P., Sokol S., Baker D. L., Simpkins K. L., Dong Y., Lynch D. R. (2002) Proteolysis of the N-methyl-d-aspartate receptor by calpain in situ. J. Pharmacol. Exp. Ther. 302, 1023–1030 [DOI] [PubMed] [Google Scholar]
- 58. Wu H. Y., Yuen E. Y., Lu Y. F., Matsushita M., Matsui H., Yan Z., Tomizawa K. (2005) Regulation of N-methyl-d-aspartate receptors by calpain in cortical neurons. J. Biol. Chem. 280, 21588–21593 [DOI] [PubMed] [Google Scholar]
- 59. Dong Y. N., Waxman E. A., Lynch D. R. (2004) Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor. J. Neurosci. 24, 11035–11045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kiefer R. T., Rohr P., Ploppa A., Dieterich H. J., Grothusen J., Koffler S., Altemeyer K. H., Unertl K., Schwartzman R. J. (2008) Efficacy of ketamine in anesthetic dosage for the treatment of refractory complex regional pain syndrome. an open-label phase II study. Pain Med. 9, 1173–1201 [DOI] [PubMed] [Google Scholar]
- 61. Sigtermans M. J., van Hilten J. J., Bauer M. C., Arbous M. S., Marinus J., Sarton E. Y., Dahan A. (2009) Ketamine produces effective and long-term pain relief in patients with complex regional pain syndrome type 1. Pain 145, 304–311 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.