Abstract
Coordinated migratory events by naïve and memory T cells are key to effective immunity. Naïve T cells predominantly recirculate through secondary lymphoid tissue until antigen encounter, while primed T cells efficiently localize to antigen-rich lymphoid and non-lymphoid tissue. Tissue-selective targeting by primed T cells is achieved by a combination of inflammatory signals and tissue-selective homing receptors acquired by T cells during activation and differentiation. A large number of molecular mediators and interactions promoting memory T cell migration to non-lymphoid sites of inflammation have been identified. Recently, additional antigen-driven mechanisms have been proposed, which orchestrate the targeted delivery of memory T cells to antigen-rich tissue. Importantly, recent studies have revealed that the T cell metabolic status influences their differentiation and homing patterns. We here summarize these key observations and discuss their relevance for the manipulation of immune anatomy in therapeutic settings.
Introduction
While extravasation of most leukocytes is mediated by cell-specific but non-tissue-selective inflammatory stimuli, specific adhesion and chemokine receptors have been associated with recirculation of naïve T cells and organ-selective trafficking of memory and effector T cells. Naïve T lymphocytes continually traffic from blood through specialized endothelium of secondary lymphoid organs (SLO) such as peripheral and mesenteric lymph nodes, spleen, and gut-associated lymphoid tissue including Peyer’s patches. Following priming, T cells leave SLO and accumulate at sites of inflammation or other tissue effector sites [1]. Primed T cells are capable of tissue-selective homing by expressing various combinations of integrins and chemokine receptors that provide a unique area code defining the final destination of the lymphocytes where they will exert their effector response [1]. Hence, expression of the CC-chemokine receptor 4 (CCR4), CCR8 and CCR10 along with the cell-surface carbohydrate epitope cutaneous lymphocyte antigen directs cells to the skin [1]. Similarly, effector/memory T cell trafficking into the lamina propria of the small intestine requires the interactions of α4β7 and chemokine receptor CCR9 on lymphocyte surfaces with MAdCAM-1 and CCL25 on endothelial cells (EC) of gut lamina propria venules, respectively [1].
Although the molecular interactions guiding T cell homing to the gut and skin are relatively well defined, mapping of T cell homing to other solid organs has revealed a great level of overlap and — perhaps with the exception of the liver [2•] — other organ-specific homing receptor/ligand pairs have not yet been identified. In addition, given the size of skin and gut, further mechanisms are likely to be in place which allow T cells to discriminate not only the area code for these tissues, but also the specific ‘address’ of antigen location.
TCR engagement regulates T cell migration
It is a longstanding question, to what extent the accumulation of specific T lymphocytes within the parenchymal tissue is directly influenced by antigen recognition. In principle, several mechanisms could lead to an accumulation of antigen-specific T cells at antigen-bearing sites: firstly, the trapping of antigen-reactive cells, e.g., upon T cell receptor (TCR)-triggered activation of integrin adhesion or effects on motility; secondly, local proliferation of antigen-specific cells; or thirdly, a direct effect of antigen recognition on the recruitment of T cells. While trapping or local expansion may be operative during primary T cell responses, it is unlikely that these mechanisms ‘perse’would be sufficient to sustain the efficacy and speed of specific T cell accumulation in target tissue in recall responses.
Antigen presentation by the endothelium has been repeatedly reported to directly contribute to the recruitment of primed specific T cells. Cognate recognition of human and murine EC was shown to enhance T cell trans-endothelial migration in vitro [3–5].
Indirect evidence that similar mechanisms may be in place to sustain the recruitment of specific T cells in vivo was first provided by the observation that major histocompatibility complex (MHC) class II molecule expression by microvascular endothelium in the central nervous system precedes and is required for the formation of T cell infiltrates in experimental autoimmune ence-phalomyelitis in guinea pigs [6]. Similarly, homing of insulin-specific CD8+ T cells to the islets of Langerhans during the onset of autoimmune diabetes in non-obese diabetic (NOD) mice in vivo was impaired in IFN-γ-deficient NOD mice [7].
Further in vivo studies provided direct evidence that antigen presentation by the endothelium contributes to the development and specificity of T cell infiltrates. Islet-specific homing d by insulin-specific H2-Kd-restricted CD8+ T cells was abrogated in mice lacking MHC class I expression, or in mice displaying impaired insulin peptide presentation by local endothelium due to deficient insulin secretion, suggesting that EC can cross-present tissue antigens [8]. In addition, upregulation of H2 molecules by local vessels led to peritoneal recruitment of HY (male)-specific H2-Db-restricted CD8+ lymphocytes T cells in male, but not female mice [9]. In line with previous studies [6], intravital microscopy revealed that antigen presentation by the endothelium selectively enhanced T cell diapedesis into the tissue, without affecting rolling and adhesion.
Regulation of T cell migration by costimulatory molecules
Costimulatory signals such as those mediated by CD28 delivered to T cells in conjunction with TCR engagement are required to sustain T cell division, differentiation and survival [10]. Negative costimulators (such as CTLA-4) counteract these effects thus promoting homeostatic mechanisms and preventing autoimmunity. These costimulators have been shown to regulate adhesion molecules activity and cytoskeletal rearrangement in vitro [11–14]. In vivo, CD28-mediated signals promote the localization of T cells to target tissue following priming. A prominent feature of CD28-deficient immune responses is the inefficient localization of primed T cells to non-lymphoid antigenic site [15–17] and intact CD28 signalling is required for primed T cells to leave lymphoid tissue and migrate to antigenic sites following priming [18]. TCR-transgenic T cells carrying a mutation in the cytoplasmic tail of CD28 (CD28Y170F) that abrogates phosphatidylinositol-3′-kinase (PI3K) recruitment without leading to defects in clonal expansion [19] failed to localize to target tissue following priming.
The mechanism by which CD28 facilitates migration of primed T cells to non-lymphoid tissue is unclear. CD28 does not appear to directly mediate adhesion [20], but may favour primed T cell migration to non-lymphoid tissue by inducing integrin mediated-adhesion [18]. The long-term effect of CD28-mediated signals on T cell migration [18] suggests that additional mechanisms, such as transcriptional regulation of chemokine receptor expression [21], are likely to be involved.
Despite sharing adhesion-inducing and pro-migratory properties in vitro [22], CTLA-4-mediated signals lead to effects antagonistic to those induced by CD28 on T cell migration in vivo. CTLA-4 ligation reduced conjugate formation with cognate DCs and their retention in lymph nodes in response to antigen [23], suggesting that CTLA-4 engagement may limit the expansion of specific T cells by reducing their cumulative interactions with cognate DCs. In addition, tissue infiltration by a murine HY-specific H2-Kk-restricted T cell clone was abrogated by CTLA-4 ligation [18], suggesting that CTLA-4 engagement can antagonize recruitment of primed T cells to target tissue mediated by antigen-induced signals.
Metabolic reprogramming regulates T activation and anergy
The regulation of energy metabolism is tightly coupled to T cell functions, including activation, proliferation, differentiation and anergy. Whereas resting T cells generate most of their energy by oxidative phosphorylation, T cell activation is characterized by a marked increase in glycolysis even in the presence of adequate oxygen supply, a mode of energy generation termed ‘aerobic glycolysis’. This metabolic switch enables activated T cells to generate ATP from glucose at a faster rate and, at the same time, efficiently utilize carbon sources in the form of amino acids and lipids for the biosynthesis of proteins and membranes, necessary for the expansion phase that characterize the immune response [24].
Downstream of TCR, PI3K leads to the activation of the serine-threonine kinase AKT through conversion of the membrane lipid PIP2 to PIP3. AKT promotes glucose metabolism by stimulating the localization of the glucose transporter Glut1 to the plasma membrane, thus facilitating increased glucose uptake. In addition, AKT increases glycolytic metabolism by stimulating the activity of hexokinase and phosphofructokinase, two rate-limiting enzymes of the glycolytic pathway. Interestingly, the effects of AKT on glucose metabolism in lymphocytes are antagonized by the inhibitory receptor CTLA-4, suggesting that antagonists of T cell activation may function in part by disrupting glucose metabolism [24].
T cell activation is not only accompanied by increased glycolytic metabolism but also by high rates of protein synthesis that support cell growth and effector functions. Downstream of TCR and CD28, AKT also controls the activation state of the mammalian target of rapamycin, mTOR, a sensor of nutritional and energetic status in cells [25,26]. mTOR modulates the rate of protein synthesis by regulating both the availability of amino acids and the process of cap-dependent translation through the control of the translational machinery (i.e., the translation inhibitor 4E-BP1, the translation initiation factor EIF2B, and the ribosomal p70 S6 kinase) [27].
Recent data have highlighted the importance of the metabolic machinery in the induction of anergy in T cells [28••]. Anergy was shown to be critically due to a failure of the AKT–mTOR pathway to upregulate nutrient transporters and activate glycolytic pathways in the absence of an appropriate costimulus [29]. Furthermore, interfering with leucine, glucose and energy metabolism via the use of N-acetyl-leucine amide, 2-deoxyglucose and 5-aminoimidazole-4-carboxamide ribonucleoside was sufficient to induce anergy even in the presence of an appropriate costimulatory signal [28••].
In addition, mTOR has been shown to serve a crucial function in determining the differentiation of CD4+T cells into inflammatory and regulatory subsets, the development of CD8+ memory T cells, and the regulation of T cell trafficking [25,26]. mTOR-deficient CD4+ T cells fail to differentiate into Th1, Th2 and Th17 subsets upon activation. Rather they differentiate towards a Foxp3+ regulatory phenotype [30•], in line with other studies showing that rapamycin, an immune suppressive agent targeting mTOR, prevents the differentiation of Th17 cells while favouring the development of Foxp3+ T regulatory (Treg) cells, which promote immune tolerance [31].
The control of T cell migration by the PI3K–AKT–mTOR pathway
It is well documented that both TCR and CD28 ligation elicit strong PI3K signalling [32,33] and T cell responses to chemokines are also partly mediated by PI3K [34]. The p110δ is the primary PI3K catalytic isoform coupled to TCR and CD28 [32,33,35]. It has recently been shown that both TCR-driven and CD28-driven T cell migration rely upon PI3K p110δ activity [18,36]. Studies using T cells from mice expressing a catalytically inactive p110δ isoform or treated with the p110δ-selective inhibitor, IC87114, revealed an essential role for this molecule in TCR-dependent localization of both CD4+ and CD8+ T cells in a male antigen-specific transplantation model [36]. Interestingly, and in support of previous findings [37], there was no defect in the p110δ mutant mice of either normal constitutive trafficking or migratory response to chemokines. Finally, genetic and pharmacologic inactivation of p110δ was recently shown to inhibit the development of chronic rejection of murine heart allografts by preventing activated T cell access to the transplant, while not inducing T cell tolerance. PI3K p110δ targeting was effective even when initiated post-grafting [38•].
Recent evidence also strongly correlates CD28-induced migration with PI3K (likely p110δ) signalling. TCR-transgenic mice carrying an ovalbumin-specific T cell receptor (OT-II) and a mutation in the cytoplasmic tail of CD28 that abrogates class I PI3K recruitment without leading to defects in clonal expansion (CD28Y170F) [19] were generated to allow discrimination of conventional costimulation-driven clonal expansion from their ability to infiltrate antigenic tissue (OT-II/CD28Y170F). OT-II and OT-II/CD28Y170F naïve T cells proliferated equivalently following immunization with OVA323–339 peptide. However, OT-II/CD28Y170F CD8+ memory T cells failed to localize to target tissue upon antigen challenge. It has to be borne in mind that the PI3K binding motif in CD28 is also required for binding of the adaptors Grb2 and Gads and the involvement of PI3K is implied but not proven.
Expression of the adhesion molecule CD62L (also known as L-selectin) and the chemokine receptors CCR7 and sphingosine-1-phosphate receptor 1 (S1P1) on the surface of naïve T cells facilitates their trafficking to SLOs. Upon TCR engagement, the PI3K–AKT–mTOR axis promotes the transcriptional downregulation of CD62L, CCR7 and S1P1 via the inhibition of the transcription factor KLF2 [39,40•]. Rapamycin-mediated inhibition of mTOR causes effector T cells to re-express KLF2, CD62L and CCR7 and home to SLOs where they are retained preventing elimination of target cells in the periphery [39,40•]. These evidences reveal yet another way of achieving immune tolerance with rapamycin.
The well-established control of T cell migration by TCR and costimulators implies that metabolic changes induced by these receptors can influence the efficiency and topography of T cell trafficking. The metabolic machinery is also likely to directly affect and be affected by T cell migratory events, as T cells continuously recirculate between different microenvironments (e.g., blood, lymphoid tissues, and non-lymphoid tissues) in which they must adapt to different oxygen and nutrient availability. Importantly we have highlighted that the PI3K–AKT–mTOR axis is a common denominator of these events. This field, however, is mostly unexplored at present, and represents a fascinating area of research for the forth-coming years to gain a better understanding of the physiology of T cell trafficking and the pathological mechanisms leading to T cell-mediated inflammation.
Conclusions
Therapeutic immunomodulation is commonly associated with the induction of changes in T cell expansion and effector function. On the basis of the recent observations summarized here, these functions are inextricably linked to the T cell metabolic status and trafficking patterns (Figure 1). Thus, the manipulation of costimulatory signals in therapeutic strategies aiming at the enhancement (cancer) or inhibition (autoimmunity or transplantation) of immune reactivity are bound to also affect T cell migration. Clinical trials inducing dominant CD28 signalling (i.e., use of anti-CTLA-4 antibodies in cancer patients or a phase I trial testing a CD28 super-agonist in healthy human subjects) have been burdened by side effects associated with T cell-mediated inflammation of non-lymphoid tissue accompanied by lymphocyte depletion from the blood [41,42].
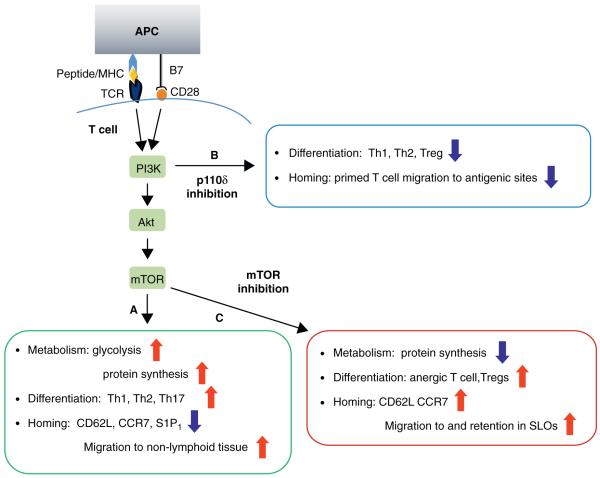
Figure 1.
Metabolic status regulates T cell activation, differentiation and homing. (A) Downstream of TCR and CD28, PI3K leads to activation of AKT, which subsequently controls mTOR activity. This signalling cascade promotes glucose metabolism and protein synthesis necessary for T cell activation, proliferation and differentiation into Th1, Th2 and Th17 subsets. The PI3K–AKT–mTOR axis also promotes downregulation of SLOs homing receptor CD62L, CCR7 and S1P1. Antigen experienced T cells, therefore, home to their respective non-lymphoid tissues. (B) Inhibition of PI3K via p110δ-selective inhibitor, IC87114, or genetic mutation reduces the capacity of T cells to differentiate along the Th1 and Th2 lineages. It also reduces the number and function of Tregs in peripheral organs, and compromises TCR-dependent migration of T effector cells into antigenic sites. (C) Inhibition of mTOR via rapamycin or genetic deletion reduces protein synthesis, and promotes T cell differentiation towards anergic and Treg subsets. Rapamycin-mediated inhibition of mTOR causes T effector cells to re-express CD62L and CCR7 and home to SLOs.
On the other hand, the ability of mTOR manipulation to regulate T cell metabolism and homing has led to the identification of novel mechanisms of action by rapamycin, an immunosuppressive, tolerogenic agent well established in clinical practice. These new properties will hopefully open new possibilities in its clinical indications and disease-targeting.
Finally, the recently discovered role of PI3K p110δ in regulating primed T cell migration to antigenic sites provides an additional pharmacological target to control of T cell-mediated pathologies including autoimmunity and transplantation.
Acknowledgements
F.M.M-B. is supported by the British Heart Foundation, the Medical Research Council of the UK and the Gates Foundation. C.M. is supported by the British Heart Foundation.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Marelli-Berg FM, Cannella L, Dazzi F, Mirenda V. The highway code of T cell trafficking. J Pathol. 2008;214:179–189. doi: 10.1002/path.2269. [DOI] [PubMed] [Google Scholar]
- 2•.Shetty S, Weston CJ, Oo YH, Westerlund N, Stamataki Z, Youster J, Hubscher SG, Salmi M, Jalkanen S, Lalor PF, Adams DH. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J Immunol. 2011;186:4147–4155. doi: 10.4049/jimmunol.1002961. This study identifies a key role for CLEVER-1/stabilin-1 and VAP-1 in facilitating Treg recruitment to the inflamed liver and to hepatocellular carcinoma, thus defining a molecular code for liver-selective T cell trafficking. Interestingly, 60% of transmigrating Tregs underwent transcellular migration through endothelial ICAM-1-rich and VAP-1-rich transcellular pores.
- 3.Marelli-Berg FM, Frasca L, Weng L, Lombardi G, Lechler RI. Antigen recognition influences transendothelial migration of CD4+ T cells. J Immunol. 1999;162:696–703. [PubMed] [Google Scholar]
- 4.Greening JE, Tree TI, Kotowicz KT, van Halteren AG, Roep BONJK, Peakman M. Processing and presentation of the islet autoantigen gad by vascular endothelial cells promotes transmigration of autoreactive T-cells. Diabetes. 2003;52:717–725. doi: 10.2337/diabetes.52.3.717. [DOI] [PubMed] [Google Scholar]
- 5.Manes TD, Pober JS. Antigen presentation by human microvascular endothelial cells triggers ICAM-1-dependent transendothelial protrusion by, and fractalkine-dependent transendothelial migration of, effector memory CD4(+) T cells. J Immunol. 2008;180:8386–8392. doi: 10.4049/jimmunol.180.12.8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobel RA, Blanchette BW, Bhan AK, Colvin RB. The immunopathology of experimental allergic encephalomyelitis. II. Endothelial cell Ia increases prior to inflammatory cell infiltration. J. Immunol. 1984;132:2402–2407. [PubMed] [Google Scholar]
- 7.Savinov AY, Wong FS, Chervonsky AV. IFN-gamma affects homing of diabetogenic T cells. J Immunol. 2001;167:6637–6643. doi: 10.4049/jimmunol.167.11.6637. [DOI] [PubMed] [Google Scholar]
- 8.Savinov AY, Wong FS, Stonebraker AC, Chervonsky AV. Presentation of antigen by endothelial cells and chemoattraction are required for homing of insulin-specific CD8+ T cells. J Exp Med. 2003;197:643–656. doi: 10.1084/jem.20021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marelli-Berg FM, James MJ, Dangerfield J, Dyson J, Millrain M, Scott D, Simpson E, Nourshargh S, Lechler RI. Cognate recognition of the endothelium induces + HY-specific CD8 T-lymphocyte transendothelial migration (diapedesis) in vivo. Blood. 2004;103:3111–3116. doi: 10.1182/blood-2003-08-2717. [DOI] [PubMed] [Google Scholar]
- 10.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu Y, van Seventer GA, Ennis E, Newman W, Horgan KJ, Shaw S. Crosslinking of the T cell-specific accessory molecules CD7 and CD28 modulates T cell adhesion. J Exp Med. 1992;175:577–582. doi: 10.1084/jem.175.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turcovski-Corrales SM, Fenton RG, Peltz G, Taub DD. CD28:B7 interactions promote T cell adhesion. Eur J Immunol. 1995;25:3087–3093. doi: 10.1002/eji.1830251115. [DOI] [PubMed] [Google Scholar]
- 13.Zell JI, Zell T, Warden CS, Chan AS, Cook ME, Dell CL, Hunt SWr, Shimizu Y. Regulation of beta 1-integrin-mediated cell adhesion by the CBL adaptor protein. Curr Biol. 1998;8:814–822. doi: 10.1016/s0960-9822(98)70323-9. [DOI] [PubMed] [Google Scholar]
- 14.Zell T, Hunt SWr, Mobley JL, Finkelstein LD, Shimizu Y. CD28-mediated up-regulation of beta 1-integrin adhesion involves phosphatidylinositol 3-kinase. J Immunol. 1996;156:883–886. [PubMed] [Google Scholar]
- 15.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. J Exp Med. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 17.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu I, Lechler RI, Okkenhaug K, Marelli-Berg FM. Physiological and aberrant regulation of memory T cell trafficking by the costimulatory molecule CD28. Blood. 2007;109:2968–2977. doi: 10.1182/blood-2006-10-050724. [DOI] [PubMed] [Google Scholar]
- 19.Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 20.Bromley S, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 21.Walker LSK, et al. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXCR-5 positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei B, da Rocha Dias S, Wang H, Rudd CE. CTL-associated antigen-4 ligation induces rapid T cell polarization that depends on phosphatidylinositol 3-kinase, VAV-1, CDC42, and myosin light chain kinase 1. J Immunol. 2007;179:400–408. doi: 10.4049/jimmunol.179.1.400. [DOI] [PubMed] [Google Scholar]
- 23.Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the tcr stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 24.Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter C, Waldmann H, Cobbold SP. mTOR signalling and metabolic regulation of T cell differentiation. Curr Opin Immunol. 2010;22:655–661. doi: 10.1016/j.coi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. Using a number of inhibitors of metabolic pathways, the authors show that not only are anergic T cells unable to upregulate metabolic machinery in response to TCR engagement (signal 1) in the context of appropriate costimulation (signal 2), but also that inhibition of metabolism can lead to T cell anergy.
- 29.Wells AD. New insights into the molecular basis of T cell anergy: anergy factors, avoidance sensors, and epigenetic imprinting. J Immunol. 2009;182:7331–7341. doi: 10.4049/jimmunol.0803917. [DOI] [PubMed] [Google Scholar]
- 30•.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. In this very interesting report the authors show that mTOR-deficient T cells display normal activation and IL-2 production upon initial stimula-tion. However, they fail to differentiate into Th1, Th2, or Th17 effector cells. Rather, under normally activating + conditions, T cells lacking mTOR differentiate into Foxp3 regulatory T cells, associated with hyperactive Smad3 activation in the absence of exogenous TGF-β.
- 31.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+Foxp3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 32.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 33.Parry RV, Whittaker GC, Sims M, Edmead CE, Welham MJ, Ward SG. Ligation of CD28 stimulates the formation of a multimeric signaling complex involving Grb-2-associated binder 2 (GAB2), src homology phosphatase-2, and phosphatidylinositol 3-kinase: evidence that negative regulation of CD28 signaling requires the GAB2 pleckstrin homology domain. J Immunol. 2006;176:594–602. doi: 10.4049/jimmunol.176.1.594. [DOI] [PubMed] [Google Scholar]
- 34.Ward SG, Marelli-Berg FM. Mechanisms of chemokine and antigen-dependent T-lymphocyte navigation. Biochem J. 2009;15:13–27. doi: 10.1042/BJ20081969. [DOI] [PubMed] [Google Scholar]
- 35.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of TH cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 36.Jarmin SJ, David R, Ma L, Chai J-G, Dewchand H, Takesono A, Ridley AJ, Okkenhaug K, Marelli-Berg FM. Targeting T cell receptor-induced phosphoinositide-3-kinase p110delta activity prevents T cell localization to antigenic tissue. J Clin Invest. 2008;118:1154–1164. doi: 10.1172/JCI33267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 38•.Ying H, Fu H, Rose ML, McCormack AM, Sarathchandra P, Okkenhaug K, Marelli-Berg FM. Genetic or pharmaceutical blockade of phosphoinositide 3-kinase p110delta prevents chronic rejection of heart allografts. Plos One. doi: 10.1371/journal.pone.0032892. in press. This study highlights the therapeutic potential of pharmacologic inhibi-tion of PI3K p110δ in a model of murine chronic allograft rejection. Notably, only activated T cell trafficking to the graft is affected, while their responsiveness to antigen challenge and constitutive trafficking remain intact.
- 39.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, Okkenhaug K, Hagenbeek TJ, Spits H, Cantrell DA. Phosphatidylinositol-3-kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–521. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Finlay D, Cantrell DA. Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. 2011;11:109–117. doi: 10.1038/nri2888. This review article discusses the emerging view that AKT, mTOR and possibly AMPK pathways combine the control of T cell metabolism to the regulation of T cell effector function and migratory capacity.
- 41.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, Panoskaltsis N. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody tgn1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]