Abstract
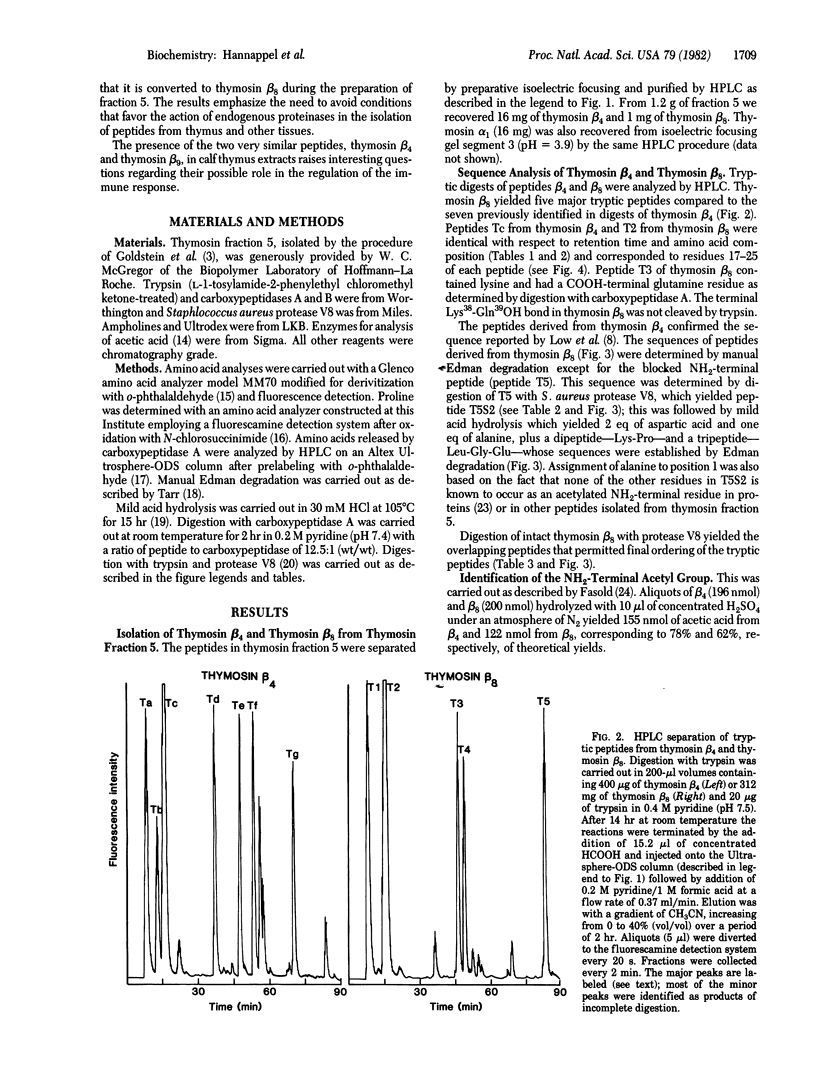
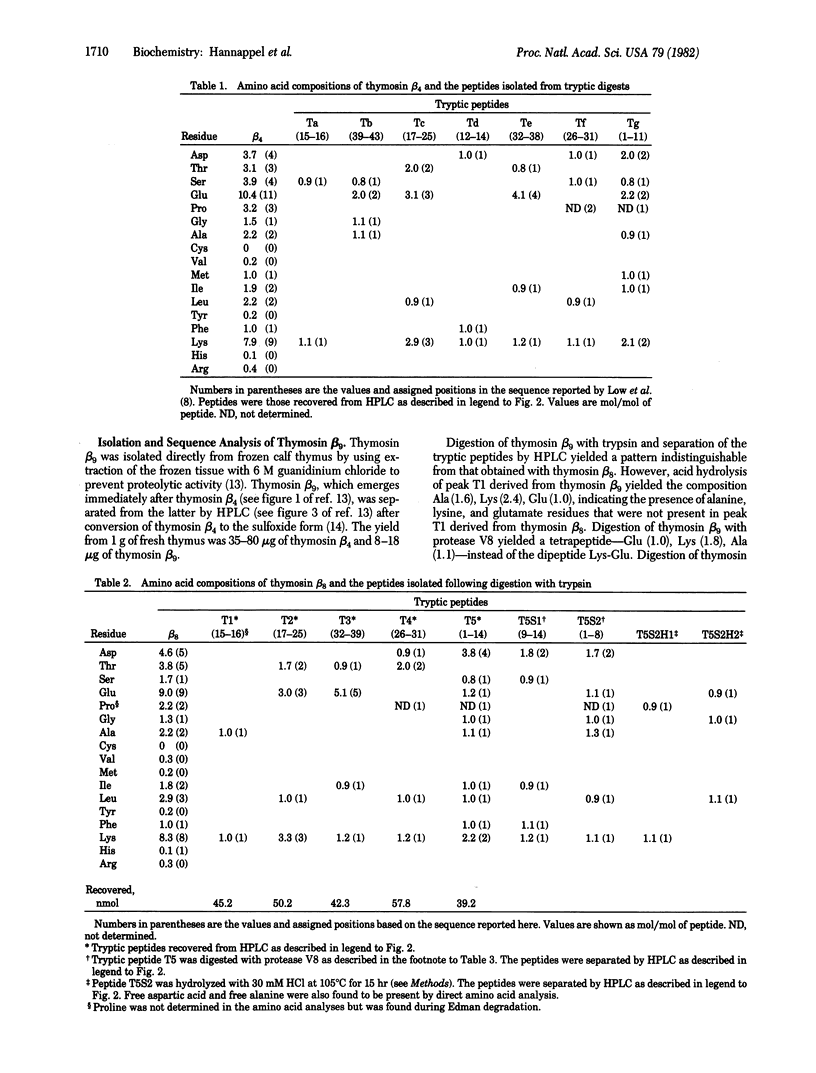
Two new peptides, designated thymosin beta 8 and thymosin beta 9, respectively, have been isolated and their amino acid sequences established. Thymosin beta 8, isolated from calf thymus fraction 5, has a mass of 4518 daltons and contains 39 amino acid residues, of which 31 are identical to the corresponding amino acid residues in thymosin beta 4 isolated from the same source. The NH2 terminus of thymosin beta 8 is acetylalanine, compared with acetylserine in thymosin beta 4. Thymosin beta 9, isolated from fresh-frozen calf thymus by a procedure that minimizes proteolysis, is identical to thymosin beta 8 except for the presence of an additional dipeptide, -Ala-LysOH, at the COOH terminus. It has a mass of 4717 daltons and 32 of its 41 amino acids are identical to those of thymosin beta 4. The similarity in structures of thymosin beta 4 and thymosin beta 9 suggests that they may have related functions.
Full text
PDF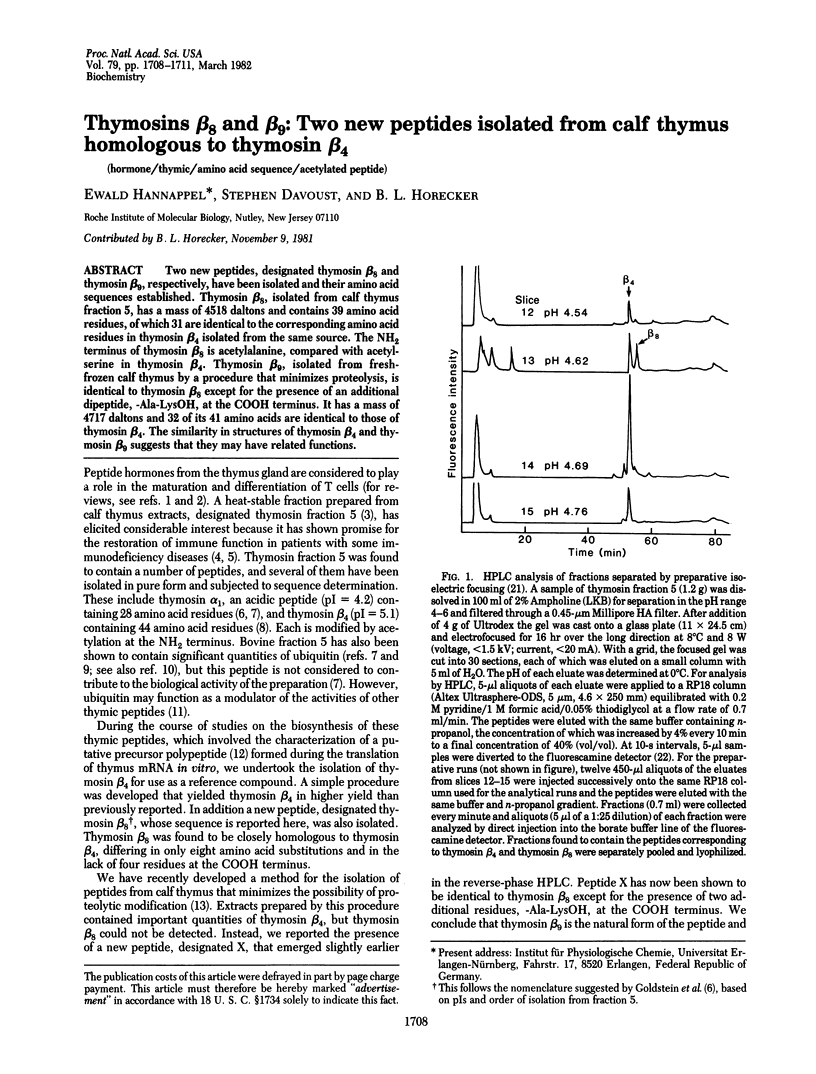

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach J. F., Bach M. A., Charreire J., Dardenne M., Pleau J. M. The mode of action of thymic hormones. Ann N Y Acad Sci. 1979;332:23–32. doi: 10.1111/j.1749-6632.1979.tb47094.x. [DOI] [PubMed] [Google Scholar]
- Benson J. R., Hare P. E. O-phthalaldehyde: fluorogenic detection of primary amines in the picomole range. Comparison with fluorescamine and ninhydrin. Proc Natl Acad Sci U S A. 1975 Feb;72(2):619–622. doi: 10.1073/pnas.72.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H. The vertebrate eye lens. Science. 1977 Jul 8;197(4299):127–138. doi: 10.1126/science.877544. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. Cleavage at glutamic acid with staphylococcal protease. Methods Enzymol. 1977;47:189–191. doi: 10.1016/0076-6879(77)47023-x. [DOI] [PubMed] [Google Scholar]
- Freire M., Crivellaro O., Isaacs C., Moschera J., Horecker B. L. Translation of mRNA from calf thymus in the wheat germ system: evidence for a precursor of thymosin alpha1. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6007–6011. doi: 10.1073/pnas.75.12.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Guha A., Zatz M. M., Hardy M. A., White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1800–1803. doi: 10.1073/pnas.69.7.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. L., Low T. L., McAdoo M., McClure J., Thurman G. B., Rossio J., Lai C. Y., Chang D., Wang S. S., Harvey C. Thymosin alpha1: isolation and sequence analysis of an immunologically active thymic polypeptide. Proc Natl Acad Sci U S A. 1977 Feb;74(2):725–729. doi: 10.1073/pnas.74.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannappel E., Davoust S., Horecker B. L. Isolation of peptides from calf thymus. Biochem Biophys Res Commun. 1982 Jan 15;104(1):266–271. doi: 10.1016/0006-291x(82)91969-6. [DOI] [PubMed] [Google Scholar]
- Iwata T., Incefy G. S., Good R. A. Interaction between thymopoietin and facteur thymique serique in the rosette inhibition assay. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1419–1424. doi: 10.1016/0006-291x(79)91138-0. [DOI] [PubMed] [Google Scholar]
- Kenny C., Moschera J. A., Stein S. Purification of human fibroblast interferon produced in the absence of serum by Cibacron Blue F3GA-Agarose and high-performance liquid chromatography. Methods Enzymol. 1981;78(Pt A):435–447. doi: 10.1016/0076-6879(81)78154-0. [DOI] [PubMed] [Google Scholar]
- Low T. L., Goldstein A. L. The chemistry and biology of thymosin. II. Amino acid sequence analysis of thymosin alpha1 and polypeptide beta1. J Biol Chem. 1979 Feb 10;254(3):987–995. [PubMed] [Google Scholar]
- Low T. L., Hu S. K., Goldstein A. L. Complete amino acid sequence of bovine thymosin beta 4: a thymic hormone that induces terminal deoxynucleotidyl transferase activity in thymocyte populations. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1162–1166. doi: 10.1073/pnas.78.2.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low T. L., Thurman G. B., McAdoo M., McClure J., Rossio J. L., Naylor P. H., Goldstein A. L. The chemistry and biology of thymosin. I. Isolation, characterization, and biological activities of thymosin alpha1 and polypeptide beta1 from calf thymus. J Biol Chem. 1979 Feb 10;254(3):981–986. [PubMed] [Google Scholar]
- Tarr G. E. Improved manual sequencing methods. Methods Enzymol. 1977;47:335–357. doi: 10.1016/0076-6879(77)47036-8. [DOI] [PubMed] [Google Scholar]
- Wara D. W., Barrett D. J., Ammann A. J., Cowan M. J. In vitro and in vivo enhancement of mixed lymphocyte culture reactivity by thymosin in patients with primary immunodeficiency disease. Ann N Y Acad Sci. 1979;332:128–134. doi: 10.1111/j.1749-6632.1979.tb47106.x. [DOI] [PubMed] [Google Scholar]
- Wara D. W., Goldstein A. L., Doyle N. E., Ammann A. J. Thymosin activity in patients with cellular immunodeficiency. N Engl J Med. 1975 Jan 9;292(2):70–74. doi: 10.1056/NEJM197501092920204. [DOI] [PubMed] [Google Scholar]
- Weigele M., DeBernardo S., Leimgruber W. Fluorometric assay of secondary amino acids. Biochem Biophys Res Commun. 1973 Jan 23;50(2):352–356. doi: 10.1016/0006-291x(73)90847-4. [DOI] [PubMed] [Google Scholar]



