Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is associated with increased production of reactive oxygen species and a reduction in antioxidant defenses leading to oxidative stress. Glutathione S-transferases (GSTs) modulate oxidative stress. The present cross-sectional study was aimed at investigating the association between the GSTP1 gene polymorphism and T2DM and to clarify their effect on the glycemic control parameters.
Material and methods
From the Egyptian population, we enrolled 112 T2DM patients and 188 healthy controls matched for age, sex and origin. Serum lipid profile, blood-glucose level, glycated hemoglobin (HbA1c) and body mass index (BMI) were measured. DNA was extracted from the blood samples. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was used to measure GSTP1 Ile105Val gene polymorphism of study participants.
Results
The frequency of the Val allele in exon 5 of the GSTP1 gene in patients with T2DM was higher than that observed in healthy controls (15.2% vs. 9.6%); the difference was considered statistically significant when compared to Ile allele carriers (p = 0.03). The presence of the GSTP1 heterozygous mutant allele Ile/Val was more common in subjects with T2DM than in the control group (30.4% and 19.2%, respectively; p = 0.02). Variation in the GSTP1 gene was associated with BMI (p = 0.02) and not associated with glycemic control parameters (fasting serum glucose and HbA1c) or smoking-related risk of T2DM.
Conclusions
GSTP1 gene polymorphism may play a significant role in increasing the susceptibility to and risk of T2DM and obesity regardless of smoking status and had no apparent effect on HbA1c in patients with diabetes mellitus.
Keywords: GSTP1, type 2 diabetes mellitus, glycated hemoglobin, body mass index, polymerase chain reaction-restriction fragment length polymorphism
Introduction
Type 2 diabetes mellitus (T2DM) represents a significant global health problem. It is estimated that six people die every minute from the disease worldwide, a figure that will soon make the disease one of the world's most prevalent causes of preventable mortality [1].
Reactive oxygen species (ROS) production induced by chronic hyperglycemia is implicated as a potential molecular mechanism behind diabetic vascular complications. The ROS activates protein kinase C (PKC) and increases the production of advanced glycation end products (AGEs), leading to superoxide generation [2], which triggers atherosclerosis [3]. Pancreatic β-cells express low levels of anti-oxidant enzymes and become sensitive to cytotoxic stress that leads to higher risk of oxidative damage [4, 5].
Glutathione S-transferases (GSTs) belong to a group of multigene and multifunctional detoxification enzymes, which defend cells against a wide variety of toxic insults from chemicals, metabolites, and oxidative stress [6]. An important condition affecting GST expression is oxidative stress, usually observed in diabetes.
The gene expressing GST enzymes is polymorphic and therefore it is possible that individual variations in metabolic activities of each enzyme may regulate the clearance of toxic DNA intermediates and may be partially responsible for individual host susceptibility to oxidative stress damage of β-cells [7]. The GSTs comprise a class of enzymes that detoxify tobacco-related carcinogens by conjugating glutathione to facilitate their removal [8].
Improved glycemic control in people with diabetes reduces the risk of long-term complications. The Diabetes Control and Complications Trial [9] and the United Kingdom Prospective Diabetes Study [10] provided evidence for the benefits of tight and sustained glycemic control among type 1 and 2 diabetic patients. Chronic hyperglycemia confers increased risk for long-term diabetes-associated complications and repeated hemoglobin A1c (HbA1c) measures are a widely used marker for glycemic control in diabetes treatment and follow-up [11].
A GSTP1 variant with A to G transition in exon 5 at codon 105 leads to Ile105Val amino acid substitution, which reduces the ability to conjugate reactive electrophiles with glutathione and may therefore sensitize cells to free radical-mediated damage. The Val105 variant has been associated with susceptibility to smoking-related cancer [12] and cardiovascular disease [13, 14].
Studies have so far reported contradictory results regarding any association between GSTP1 gene polymorphism and T2DM [15, 16]. There has been no previous report of its effect on the glycemic control parameters. Thus, we conducted a case-control study in the Egyptian population with age- and sex-matched T2DM to evaluate whether the GSTP1 variants modulate the risk of T2DM patients and to clarify their effect on the glycemic control parameters.
Material and methods
Subjects
A total of 112 T2DM patients and a control group of 188 healthy subjects were examined in this study. Diagnosis of T2DM was based on the American Diabetes Association definition of diabetes [17]. Healthy subjects did not meet the criteria for the diagnosis of diabetes mellitus.
Laboratory measurements
Venous blood samples were drawn after an overnight fast. HbA1c was measured with an ADVIA 1800 chemistry analyzer (Siemens Healthcare Diagnostics, USA). Fasting serum glucose, serum total cholesterol, HDL cholesterol and triglycerides (TG) were measured by automated enzymatic methods on an Hitachi-912 analyzer; LDL cholesterol was calculated according to the Friedewald formula [18]. Hypertension was defined as systolic blood pressure (BP) ≥ 140 mm Hg, diastolic BP ≥ 90 mm Hg and/or a history of hypertension [19]. Current smoker was defined as a subject who continued to smoke cigarettes regularly.
Study subjects
All the procedures were carried out according to the principles of the Declaration of Helsinki. The study was approved by the Suez Canal University Research Ethics Committee and all the patients and controls provided written informed consent. Table I summarizes the clinical features of patients and controls.
Table I.
General characteristics of the study population
| Variable | Control (n = 188) | T2DM (n = 112) | Value of p |
|---|---|---|---|
| Age [years] | 48.54 ±8.07 | 48.67 ±7.3 | 0.89 |
| Sex (M/F) | 101/87 | 60/52 | 0.98 |
| FBGL level [mg/dl] | 96.16 ±8.8 | 208.5 ±95.1 | 0.0001* |
| HbA1c | 5 ±0.74 | 9.67 ±1.89 | 0.0001* |
| BMI [kg/m2] | 28.23 ±3.94 | 29.42 ±4.08 | 0.013* |
| Total cholesterol [mg/dl] | 210.33 ±85.05 | 217.69 ±46.13 | 0.39 |
| TG [mg/dl] | 151.52 ±77.09 | 168.56 ±89.45 | 0.82 |
| HDL-C [mg/dl] | 46.91 ±11.19 | 45.16 ±10.78 | 0.18 |
| VLDL-C [mg/dl] | 30.3 ±15.42 | 33.7 ±17.89 | 0.08 |
| LDL-C [mg/dl] | 133.11 ±85.62 | 138.82 ±47.45 | 0.52 |
| Current smoking (+/–) | 35/153 | 28/84 | 0.89 |
| Systolic BP [mm Hg] | 120.29 ±15.52 | 142.99 ±25.34 | 0.0001* |
| Diastolic BP [mm Hg] | 75.13 ±8.03 | 83.21 ±12.23 | 0.0001* |
FBGL – fasting blood glucose level [mg/dl]. Comparisons were performed by Student t-test and χ2 test; data are mean ± SD.
Significant differences between groups (p < 0.05)
Determination of genotype at the GSTP1 locus
Genomic DNA was obtained from peripheral blood samples collected in EDTA tubes. The DNA was extracted using DNA purification kit cat #1120 (Promega, USA). The assay for the polymorphisms in GSTP1-105 was performed as described previously [20]. Each PCR reaction mixture (40 µl) contained 200 ng each of primers P105F (5’-ACC CCA GGG CTC TAT GGG AA-3’) and P105R (5’-TGA GGG CAC AAG AAG CCC CT-3’), 100 ng of genomic DNA, 1.5 mM MgCl2, 100 mM of each dNTP and 1 U Taq polymerase (Promega, USA). Initial denaturation was carried out at 95°C for 5 min. The reaction involved 30 cycles of incubation at 94°C (30 s), 55°C (30 s), and 72°C (30 s). A final polymerization step of 72°C for 5 min was carried out to complete the elongation processes. After the confirmation of an amplified fragment of the expected size (176 bp) on an agarose gel, the PCR products were digested with 5 U of restriction enzyme Alw261 (Fermentas, UK) in a total volume of 25 µl. DNA fragments were submitted to electrophoresis through a 3.5% agarose gel and stained with ethidium bromide (10 mg/ml).
Statistical analysis
All data was entered, independently verified and analyzed using SPSS Statistics 17.0. Chi-squared statistics were performed to assess differences between case and control populations in relation to non-continuous variables. Student's t-test was used to test differences based on continuous variables.
Results
A total of 300 subjects were enrolled in this study (112 T2DM patients and 188 gender- and age-matched controls). The basic demographic data, including body mass index (BMI), age, gender and clinical characteristics of the study populations are summarized in Table I. No significant difference was found between patients and controls in age (45.67 ±7.3 vs. 48.54 ±8.07) or gender (male: female ratio = 60: 52 vs. 101: 87) respectively. In diabetics the established risk factors (hypertension, BMI, HbA1c and fasting blood glucose level) for diabetes were higher than in control subjects. On the other hand, no significant difference in the lipid profiles was observed among the studied groups.
GSTP1 alleles in the two groups
There was no deviation in the distribution of GSTP1 exon 5 polymorphism genotypes from Hardy-Weinberg equilibrium in the groups studied. The results of exon 5 variants of GSTP1 evaluated by comparing the T2DM group with controls are listed in Table II.
Table II.
Frequencies and genotype distribution of GSTP1 gene A/G polymorphism in control and type II diabetic patients
| GSTP1 gene | Control, n (%), n = 188 | T2DM, n (%), n = 112 | χ2 | Value of p | OR | 95% CI |
|---|---|---|---|---|---|---|
| A allele (Ile) | 340 (90.4) | 190 (84.8) | 4.27 | 0.03* | 1.69 | 1.024-2.78 |
| G allele (Val) | 36 (9.6) | 34 (15.2) | ||||
| AA (Ile/Ile) | 152 (80.8) | 78 (69.6) | ||||
| AG (Ile/Val) | 36 (19.2) | 34 (30.4) | 4.92 | 0.02* | 1.84 | 1.07-3.17 |
| GG (Val/Val) | 0 (0) | 0 (0) |
χ2 – Chi square test, CI – confidence interval, OR – odds ratio.
G vs. A
AG vs. AA. Value of p < 0.05 is statistically significant
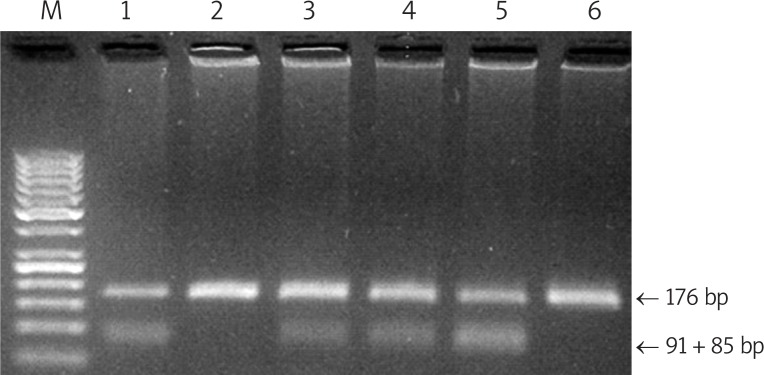
The G allele (Val) was more prevalent among patients (15.2%) than among control subjects (9.6%) (p = 0.03). We found that 30.4% of the diabetic patients and 19.2% of the control subjects were heterozygous for the G allele (Ile/Val) (p = 0.02) and G allele homozygosity (Val/Val) could not be detected in any of the control subjects or the patients. Compared with the Ile/Ile genotype, subjects with the Ile/Val genotype had an elevated risk of type 2 diabetes mellitus (OR = 1.84, 95% CI = 1.07-3.17). The risk was increased with the G allele frequency (p = 0.03) (Figure 1).
Figure 1.
Genotyping of GSTP1 Ile105Val by PCR-RFLP on 3.5% agarose gel, which distinguishes between the GSTP1 genotypes: A/A (Ile/Ile) – lines 2 and 6 (176 bp); A/G (Ile/Val) – lines 1 and 3-5 (176, 91 and 85 bp)
Clinical and functional characteristics in relation to GSTP1 genotypes
The correlation between different genotypes of exon 5 of the GSTP1 gene with clinical and functional parameters is presented in Table III. We found no statistically significant interactions between GSTP1 genotypes and the age of onset of diabetes (p = 0.27). There was no significant influence of these genotypes on lipid profile, smoking or blood pressure. On the other hand, there was a significant influence of these genotypes on BMI (p = 0.02).
Table III.
The relationship between GSTP1 genotypes with different clinical parameters in diabetic patients
| Variables | Carriers of AA (n = 78) | Carriers of AG (n = 34) | Value of p |
|---|---|---|---|
| Onset age [years] | 5.17 ±2.05 | 5.62 ±1.84 | 0.27 |
| Sex (M/F) | 33/45 | 12/22 | 0.49 |
| FBGL | 207.38 ±23.93 | 211.08 ±99.06 | 0.85 |
| HbA1c [%] | 9.7 ±1.96 | 9.59 ±1.77 | 0.78 |
| BMI | 28.83 ±4.21 | 30.76 ±3.47 | 0.02* |
| Total cholesterol [mg/dl] | 214.03 ±45.77 | 226.12 ±46.5 | 0.2 |
| TG [mg/dl] | 174.13 ±92.02 | 155 ±79 ±83.18 | 0.32 |
| HDL-C [mg/dl] | 45.56 ±11.65 | 44.24 ±8.52 | 0.55 |
| VLDL-C [mg/dl] | 34.83 ±18.4 | 31.16 ±16.64 | 0.32 |
| LDL-C [mg/dl] | 133.64 ±47.05 | 150.72 ±46.88 | 0.08 |
| Current smoking (+/–) | 20/58 | 8/26 | 0.81 |
| Systolic BP | 144.74 ±23.93 | 138.97 ±28.28 | 0.27 |
| Diastolic BP | 82.82 ±11.83 | 84.12 ±13.23 | 0.6 |
Comparisons were performed by Student-t test and χ2 test; data are mean ± SD.
Significant differences between groups (p < 0.05)
The association of polymorphism in GSTP1 with glycemic control parameters of diabetes mellitus
Diabetic control is expressed by glycated hemoglobin (HbA1c) and fasting serum glucose level. No effect of polymorphism in the GSTP1 gene on glycemic control parameters was found. Glycated hemoglobin was 9.59 ±1.77 in Ile/Val genotype of the GSTP1 gene and 9.7 ±1.96 in Ile/Ile genotype of the GSTP1 gene, with p = 0.78.
Discussion
Oxidative stress has been considered to be a common pathogenic factor in diabetes and its complications [21]. The family of GST genes plays an important role in protecting cells from oxidative stress. GSTP1 catalyses the detoxification of products arising from DNA oxidation [22]. A defect in detoxifying reactive oxygen species, which is genetically determined, may influence the development and severity of diabetes mellitus [16].
There are many studies dealing with GSTP1 polymorphism in various diseases, but only a few studies have addressed the role of GSTP1 gene polymorphism in diabetes. Therefore, the current study was designed to investigate the role of GST-P1 (Ile105Val) gene polymorphism in T2DM patients and healthy controls and whether this variant modulates the glycemic control in Egyptian patients with T2DM.
Our results demonstrate that the frequency of the G allele was higher in the diabetics compared to controls (OR = 1.69, 95% CI = 1.024-2.78, p = 0.03). Additionally, significant differences in the frequencies of the Ile/Val genotype between patients and the control group were observed (30.4% vs. 19.2% respectively). We therefore suggest that the G allele (Val) of GSTP1 Ile105Val plays an important role in predisposition to T2DM.
There have been controversial results regarding the association between GSTP1 Ile105Val gene polymorphism and diabetes development. We are in agreement with Ramprasath et al. [15] and Bid et al. [23], who demonstrated that the GSTP1 G allele (Val) and its variant genotype (Il/Val) play a vital role in the development of diabetes mellitus. In contrast, Yalin et al. [16] and Oniki et al. [24] suggested that GSTP1 Ile105Val polymorphism may not play a significant role in the etiopathogeneses of DM in the Turkish population and Japanese population respectively. These inconsistent data could be explained by ethnic differences in the selected study groups [25].
To investigate the association between GSTP1 Ile105Val gene polymorphism and glycemic control in relation to T2DM we evaluated HbA1c and serum glucose levels in different genotypes. No association between the gene polymorphism and glycemic control parameters in T2DM patients was detected. To our knowledge, this study is the first attempt to evaluate the effect of the GSTP1 genotypes with regard to glycemic control parameters in T2DM.
The epidemic of T2DM observed in recent years is a clear indication of the importance of environmental factors in diabetes onset, in particular obesity and physical inactivity [23]. Obesity, mainly when fat is distributed predominantly at the abdominal level, is the main risk factor for T2DM. Our study is probably the first study to show that GSTP1 heterozygosity (Ile/Val) is significantly associated with BMI.
Dyslipidemia observed in T2DM is one of the major factors contributing to vascular risk [26]. In the current study, we further investigated the effect of the genotypes on the lipid profile. There was no association between the genotypes and lipid profile in diabetic patients. This data are consistent with the previous reports which demonstrated that there was no association between GSTP1 genotypes and blood lipids in T2DM patients [15, 23].
GSTP1 detoxifies cigarette smoke-derived toxins and endogenously derived reactive oxygen species. Therefore, it is conceivable that genetic polymorphism in GSTP1 may have an effect on smoking-related risk in T2DM. Concerning the relation to smoking status, previous report showed that active smoking is associated with an increased risk of T2DM [27]. We found that GSTP1 polymorphism was not significantly linked with smoking in T2DM patients. These data are in accordance with Oniki et al. [24].
In conclusion, this is the first study to determine the association of type 2 diabetes with GSTP1 Ile105Val gene polymorphism in the Egyptian population and its effect on glycemic control parameters. These results show that GSTP1 Ile/Val genotype may play a significant role in the etiopathogeneses of T2DM, and the GSTP1 gene may be a useful marker in the prediction of T2DM susceptibility of the Egyptian population regardless of smoking status. Our findings suggest that the GSTP1 gene polymorphism had no apparent effect on glycemic control in type 2 diabetes patients; meanwhile, there was a significant correlation with BMI in diabetics. Although some of our data were statistically significant, we acknowledge that the findings presented here are preliminary because of the small number of subjects and that the study requires confirmation in a separate, larger cohort. Additionally, a wide epidemiological study is needed to test the possible association between genotypic and phenotypic effects of other GST gene polymorphisms in diabetic patients.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2000;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:685–94. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- 3.Creager MA, Lüscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences and medical therapy: part I. Circulation. 2003;108:1527–32. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 4.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–42. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 5.Robertson RP, Harmon J, Tran PO, Tanaka Y, Takahashi H. Glucose toxicity in beta-cells: type 2 diabetes, good radicals gone bad, and the glutathione connection. Diabetes. 2003;52:581–7. doi: 10.2337/diabetes.52.3.581. [DOI] [PubMed] [Google Scholar]
- 6.Chasseaud LF. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang G, Zhang L, Li Q. Genetic polymorphisms of GSTT1, GSTM1, and NQO1 genes and diabetes mellitus risk in Chinese population. Biochem Biophys Res Commun. 2006;341:310–3. doi: 10.1016/j.bbrc.2005.12.195. [DOI] [PubMed] [Google Scholar]
- 8.Mao GE, Morris G, Lu QY, et al. Glutathione S-transferase P1 Ile105Val polymorphism, cigarette smoking and prostate cancer. Cancer Detect Prev. 2004;28:368–74. doi: 10.1016/j.cdp.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 10.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 11.Hertel JK, Johansson S, Ræder H, et al. Evaluation of four novel genetic variants affecting hemoglobin A1c levels in a population-based type 2 diabetes cohort (the HUNT2 study) BMC Med Genet. 2011;12:20. doi: 10.1186/1471-2350-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DP, Neuberg D, de Vivo I, et al. Smoking and the risk of lung cancer: susceptibility with GSTP1 polymorphisms. Epidemiology. 2003;14:545–51. doi: 10.1097/01.ede.0000073120.46981.24. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, El Sohemy A, Cornelis MC, Kim HA, Kim SY, Bae SC. Glutathione S-transferase M1, T1, and P1 gene polymorphisms and carotid atherosclerosis in Korean patients with rheumatoid arthritis. Rheumatol Int. 2004;24:157–63. doi: 10.1007/s00296-003-0347-7. [DOI] [PubMed] [Google Scholar]
- 14.Palmer CN, Young V, Ho M, Doney A, Belch JJ. Association of common variation in glutathione S-transferase genes with premature development of cardiovascular disease in patients with systemic sclerosis. Arthritis Rheum. 2003;48:854–5. doi: 10.1002/art.10955. [DOI] [PubMed] [Google Scholar]
- 15.Ramprasath T, Senthil Murugan P, Prabakaran AD, Gomathi P, Rathinavel A, Selvam GS. Potential risk modifications of GSTT1, GSTM1 and GSTP1 (glutathione-S-transferases) variants and their association to CAD in patients with type-2 diabetes. Biochem Biophys Res Commun. 2011;407:49–53. doi: 10.1016/j.bbrc.2011.02.097. [DOI] [PubMed] [Google Scholar]
- 16.Yalin S, Hatungil R, Tamer L, et al. Glutathione S-transferase gene polymorphisms in Turkish patients with diabetes mellitus. Cell Biochem Funct. 2007;25:509–13. doi: 10.1002/cbf.1339. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2007;30:42–7. [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 20.Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR. Identification of genetic polymorphisms at the glutathione S-transferase pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis. 1997;18:641–4. doi: 10.1093/carcin/18.4.641. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann R, Schleicher ED. Molecular mechanisms of diabetic nephropathy. Clin Chim Acta. 2000;297:135–44. doi: 10.1016/s0009-8981(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 22.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 23.Bid HK, Konwar R, Saxena M, Chaudhari P, Agrawal CG, Banerjee M. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in north Indian population. J Postgrad Med. 2010;56:176–81. doi: 10.4103/0022-3859.68633. [DOI] [PubMed] [Google Scholar]
- 24.Oniki K, Umemoto Y, Nagata R, et al. Glutathione S-transferase A1 polymorphism as a risk factor for smoking-related type 2 diabetes among Japanese. Toxicol Lett. 2008;178:143–5. doi: 10.1016/j.toxlet.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Alshagga MA, Mohamed N, Suhid AN, Ibrahim IA, Zakaria SZS. Frequencies of glutathione s-transferase (GSTM1, GSTM3 AND GSTT1) polymorphisms in a Malaysian population. Arch Med Sci. 2011;7:572–8. doi: 10.5114/aoms.2011.24123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United kingdom prospective diabetes study (UKPDS: 23) BMJ. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]