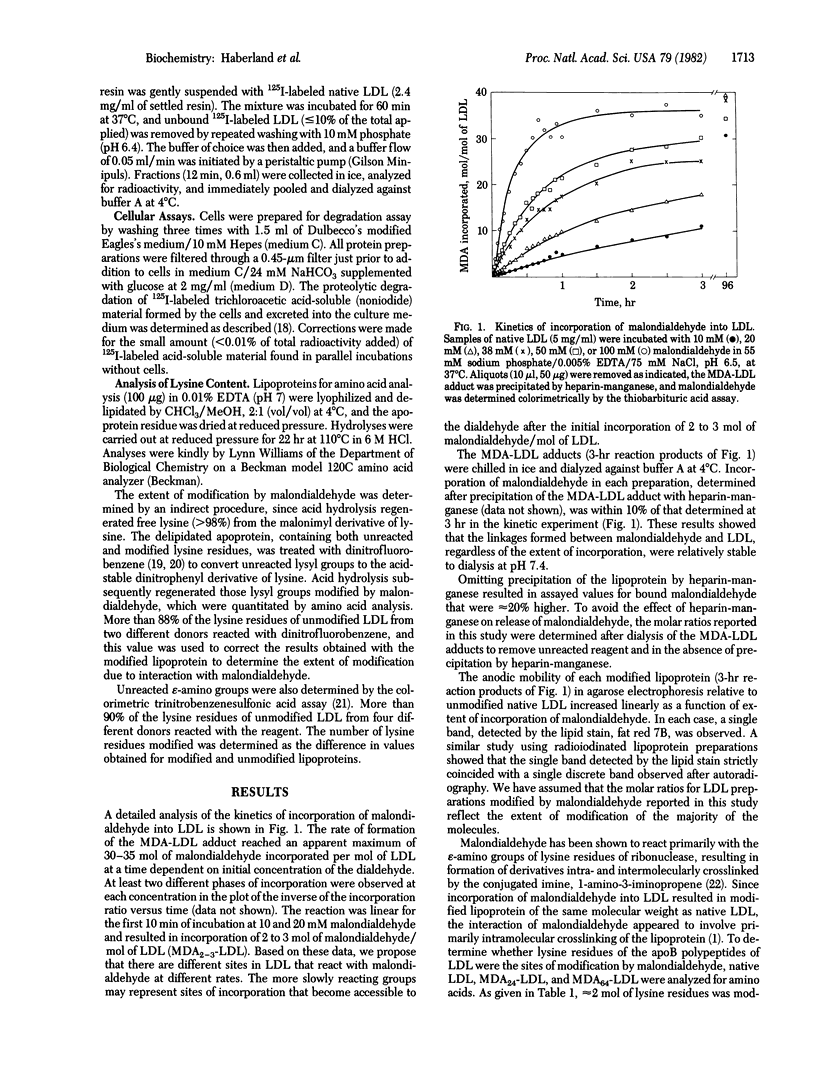
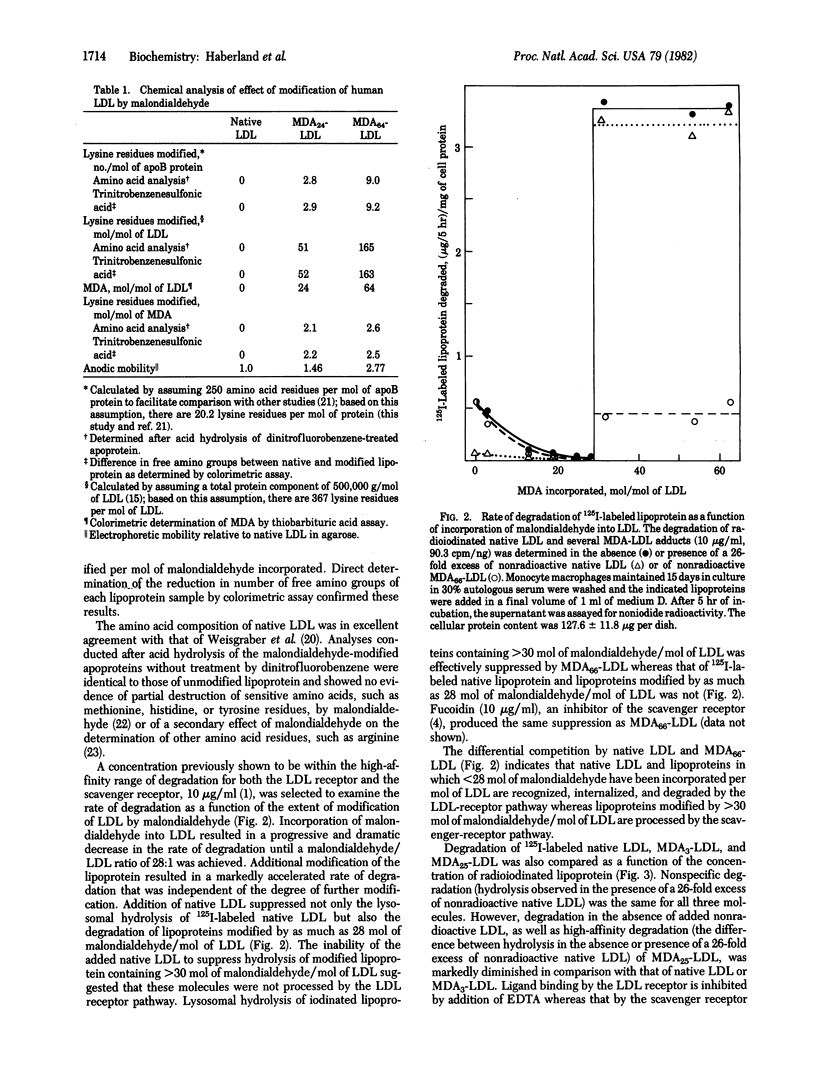
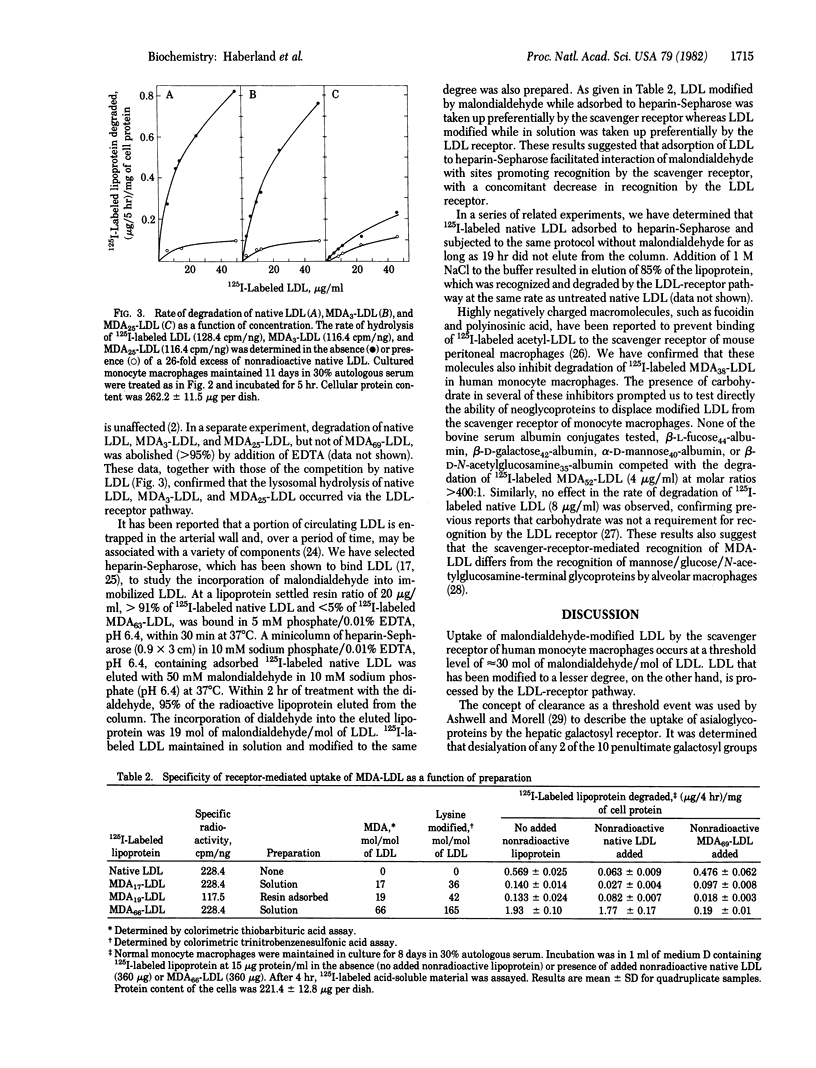
Abstract
Blood-borne human monocytes and macrophages derived from human monocytes in vitro express an active low density lipoprotein (LDL) receptor and an active receptor for negatively charged proteins, the scavenger receptor. When less than 15% of the lysine residues of human LDL were modified by malondialdehyde while the lipoprotein was in solution, recognition and uptake of the modified lipoprotein occurred via the LDL receptor. Further modification resulted in threshold recognition and uptake by the scavenger receptor with concomitant loss of recognition by the LDL receptor. The rate of degradation via the LDL receptor pathway was inversely related to the degree of modification whereas that mediated by the scavenger receptor was independent of the extent of incorporation of malondialdehyde once threshold recognition was achieved. In contrast to the interaction of LDL with malondialdehyde in solution, modification of less than 15% of the lysine residues of LDL adsorbed to heparin-Sepharose resulted in recognition and uptake by the scavenger receptor. The scavenger receptor-mediated uptake of malondialdehyde-modified LDL may be dependent on formation of recognition sites involving specific modified lysine residues or changes in the conformation of LDL induced by neutralization of specific lysine residues of the apoB polypeptides or both.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Basu S. K., Falck J. R., Ho Y. K., Goldstein J. L. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13(1):67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Ho Y. K., Goldstein J. L. The cholesteryl ester cycle in macrophage foam cells. Continual hydrolysis and re-esterification of cytoplasmic cholesteryl esters. J Biol Chem. 1980 Oct 10;255(19):9344–9352. [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Popják G. Mechanism of induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human leukocytes. J Biol Chem. 1977 Jan 25;252(2):644–651. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Wakabayashi T., Samuelsson B. Isolation and structure of two prostaglandin endoperoxides that cause platelet aggregation. Proc Natl Acad Sci U S A. 1974 Feb;71(2):345–349. doi: 10.1073/pnas.71.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Falardeau P. Resolution of prostaglandin endoperoxide synthase and thromboxane synthase of human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3691–3695. doi: 10.1073/pnas.74.9.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. P. Selective chemical modification of arginyl residues. Biochemistry. 1966 Nov;5(11):3454–3459. doi: 10.1021/bi00875a011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L. Interaction of plasma lipoproteins containing apolipoproteins B and E with heparin and cell surface receptors. Biochim Biophys Acta. 1979 Oct 26;575(1):81–91. doi: 10.1016/0005-2760(79)90133-4. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mitchell C. D., King W. C., Applegate K. R., Forte T., Glomset J. A., Norum K. R., Gjone E. Characterization of apolipoprotein E-rich high density lipoproteins in familial lecithin:cholesterol acyltransferase deficiency. J Lipid Res. 1980 Jul;21(5):625–634. [PubMed] [Google Scholar]
- Santos M. T., Valles J., Aznar J., Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980 Oct;33(10):973–976. doi: 10.1136/jcp.33.10.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter I., Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. The metabolism of native and malondialdehyde-altered low density lipoproteins by human monocyte-macrophages. J Lipid Res. 1981 Jan;22(1):63–71. [PubMed] [Google Scholar]
- Shireman R. B., Fisher W. R. The absence of a role for the carbohydrate moiety in the binding of apolipoprotein B to the low density lipoprotein receptor. Biochim Biophys Acta. 1979 Mar 29;572(3):537–540. doi: 10.1016/0005-2760(79)90161-9. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C. M., Silver M. J. Malondialdehyde formation as an indicator of prostaglandin production by human platelets. J Lab Clin Med. 1976 Jul;88(1):167–172. [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Molecular weight and hydrodynamic properties of apolipoprotein B in guanidine hydrochloride and sodium dodecyl sulfate solutions. J Biol Chem. 1979 Mar 10;254(5):1639–1643. [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Smith A. L. Lipid peroxidation by human blood phagocytes. J Clin Invest. 1974 Sep;54(3):638–645. doi: 10.1172/JCI107801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Hamer C. J., Morell A. G., Scheinberg I. H., Hickman J., Ashwell G. Physical and chemical studies on ceruloplasmin. IX. The role of galactosyl residues in the clearance of ceruloplasmin from the circulation. J Biol Chem. 1970 Sep 10;245(17):4397–4402. [PubMed] [Google Scholar]
- WOFSY L., SINGER S. J. Effects of the amidination reaction on antibody activity and on the physical properties of some proteins. Biochemistry. 1963 Jan-Feb;2:104–116. doi: 10.1021/bi00901a019. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]



