Abstract
In only three steps and in 21–67% overall yields from the natural trioxane artemisinin, a series of 21 new trioxane C-10 thioacetals was prepared. Upon receiving a single oral dose of only 6 mg/kg of the monomeric trioxane 12c combined with 18 mg/kg of mefloquine hydrochloride, Plasmodium berghei-infected mice survived on average 29.8 days after infection. Two of the four mice in this group had no parasites detectable in their blood on day 30 after infection and they behaved normally and appeared healthy. One of the mice had 11% blood parasitemia on day 30, and one mouse in this group died on day 29. Of high medicinal importance, the efficacy of this ACT chemotherapy is much better than (almost double) the efficacy under the same conditions using as a positive control the popular trioxane drug artemether plus mefloquine hydrochloride (average survival time of only 16.5 days).
INTRODUCTION
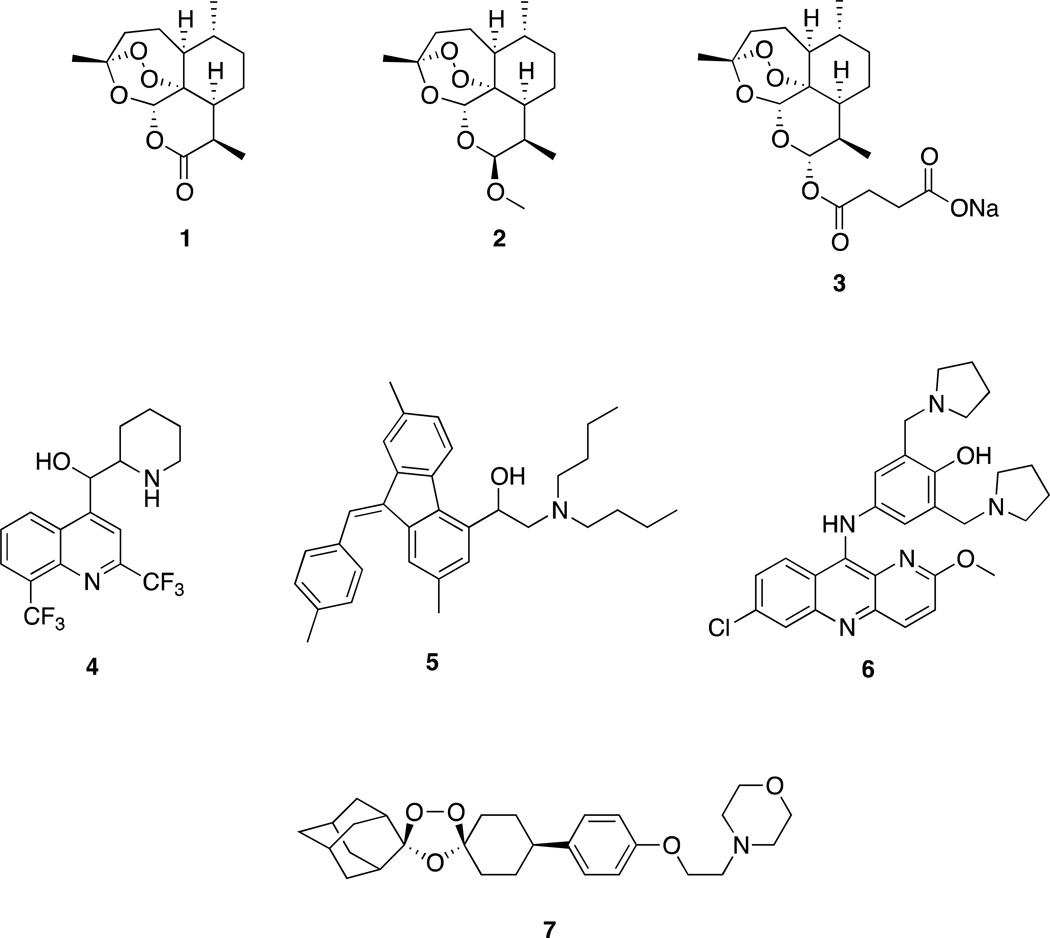
Malaria kills approximately one million people, mostly children, each year.1,2,3,4 No vaccine is yet a fully effective prophylactic against malaria.5,6 Widespread resistance of Plasmodium falciparum malaria parasites to chloroquine,7,8 until recently the most popular malaria chemotherapeutic agent, catapulted rapidly-acting artemisinin (1) trioxanes like artemether (2) and sodium artesunate (3) into use. Typically the trioxane is combined with slower-acting nitrogen-aromatics like mefloquine (4), lumefantrine (5), and pyronaridine (6, Figure 1). Indeed, the World Health Organization (WHO) urges worldwide use of such Artemisinin Combination Therapy (ACT) as the best chemotherapy for malaria patients.9 Much research has been focused on increasing the efficacy of artemisinin-derived antimalarial drugs through synthetic chemistry.10,11,12,13
Figure 1.
One of the most promising synthetic (not derived from artemisinin) peroxides is trioxolane OZ439 (7) which is now in advanced human clinical trials.14 Some semi-synthetic artemisinin derivatives also are in clinical trials.15 Antimalarial drugs now on the market include the following: a fixed combination of 2 and 5;16 a fixed combination of 3 and 4;17,18 and a fixed combination of 3 and 6.19,20 Each of these three combinations requires patients to take pills daily for several days.16,17,18,19,20,21 Adherence to such a schedule of ACT multiple dosing often is not followed faithfully, leading to ineffective chemotherapy and to increased likelihood of resistance developing.22,23,24 To overcome such non-adherence to a repeated dosing regimen, curing malaria patients with a single oral dose of drug is a very important goal.25,26,27,28,29 Toward that goal, we have reported that malaria-infected mice are cured by a single, oral, 6 mg/kg dose of several new artemisinin derivatives combined with 18 mg/kg of mefloquine hydrochloride.30,31
Some thioacetal glycosides32 and thiosugar hemithioacetals33 have potent biological activities, and a thioacetal has been developed as thiol protecting group.34 Here we disclose a new series of artemisinin-derived monomeric C-10 thioacetal carbonates, thiocarbonates, nitrogen-heterocycles, and a dimeric trioxane C-10 thioacetal amide. A trioxane monomer carbonate35 as well as a dimer carbonate have been reported,36 as well as some C-10 thioacetal derivatives of artemisinin.37,38,39,40,41
RESULTS AND DISCUSSION
Chemistry
Thioacetals are known to be more hydrolytically stable than the corresponding acetals.42,43 Since some trioxane acetals are efficacious antimalarial drugs, like 2 and 3, trioxane thioacetals were expected to be of potential value as new antimalarials. Thus, our interest was to synthesize easily and to test some trioxane thioacetals for antimalarial activity in Plasmodium berghei-infected mice.
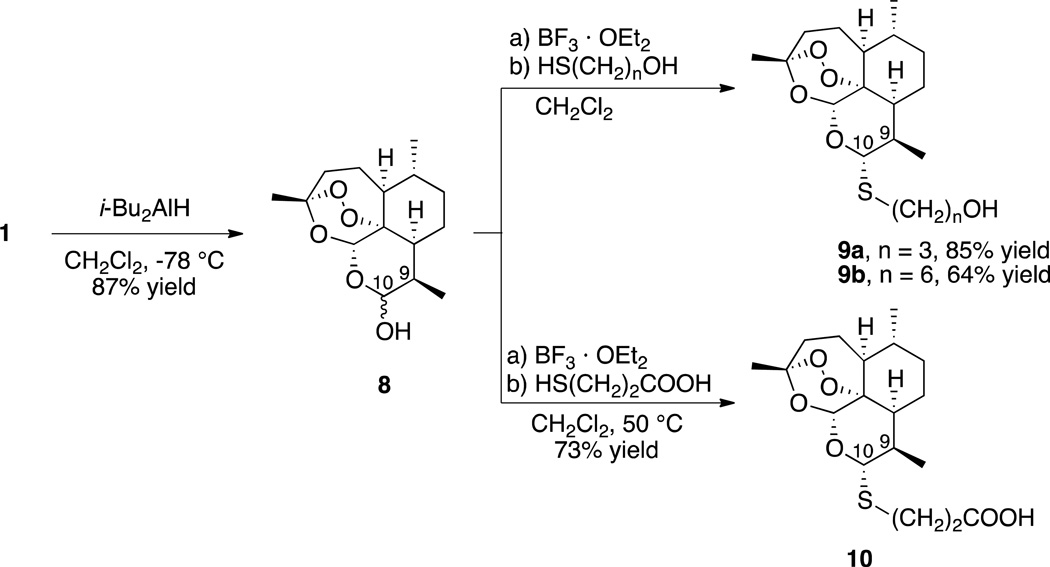
We prepared a series of C-10α thioacetals in the yields shown in Scheme 1 in three facile steps from 1. The first step is reduction of 1 into dihydroartemisinin (8)44 without destroying the crucial trioxane peroxide pharmacophore.45 Introduction of the Lewis acid boron trifluoride diethyl etherate to 8 generates an intermediate oxocarbenium ion. A variety of nucleophiles are known to react with oxocarbenium ions and, as predicted, the reaction with thiols proceeds smoothly to produce the desired C-10 thioacetals. The thiols used are commercially available bifunctional mercaptoalcohols and a mercaptocarboxylic acid, which leaves a synthetic handle, the hydroxyl or carboxylic acid group, for further manipulation and SAR studies.
Scheme 1.
The stereochemistry at the C-10 position in thioacetals 9 and 10 was determined to establish the diastereoselectivity of the thioacetalization reaction. Since the reactive C-10 site of the intermediate oxocarbenium ion is planar, the nucleophile could attack from either face of the molecule. Consequently, each synthesized thioacetal could be a mixture of both C-10α and C-10β diastereomers. Determination of the stereochemistry of the major diastereomer formed was achieved by comparing 1H NMR coupling constants between the C-10 proton and the adjacent C-9 proton in the crude thioacetals (4–9:1 ratio of diastereomers). After column chromatography purification, the major diastereomers 9a, 9b, and 10 were isolated in the yields shown. In each case the major diastereomer formed was the C-10α thioacetal based on its C-10 to C-9 coupling constant of 9–11 Hz. These values were characteristic of C-10α thioacetals in previous reports.37,38,39,40,41 Several C-10α and C-10β phenylthioacetals have been shown to be antimalarially efficacious in mice, with the C-10α thioacetals being more potent than the C-10β thioacetals.37,38,39,40,41
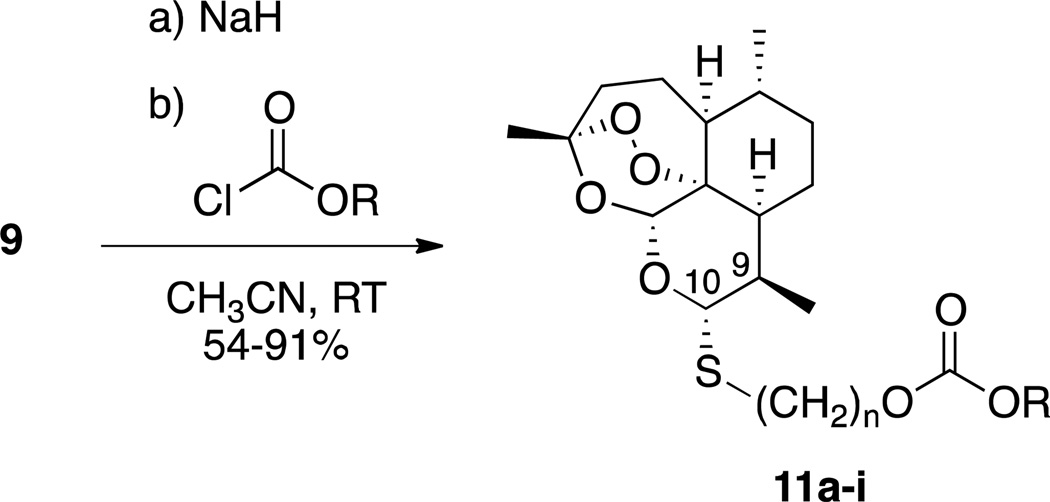
Carbonates are more stable than esters,46,47 and the carbonate functional group has been used effectively before as a prodrug, subsequent hydrolysis of which releases the parent alcohol.46,47 The steps in Scheme 2 are high-yielding and provide access to a small library of thioacetal carbonates. Thioacetal carbonates 11 were synthesized from the parent alcohol 9 by reaction with base and a chloroformate (Scheme 2). Besides aliphatic and aromatic carbonates 11a–11h, sulfone carbonate 11i was of interest because of a report demonstrating the usefulness of this particular functionality in a prodrug.48
Scheme 2.
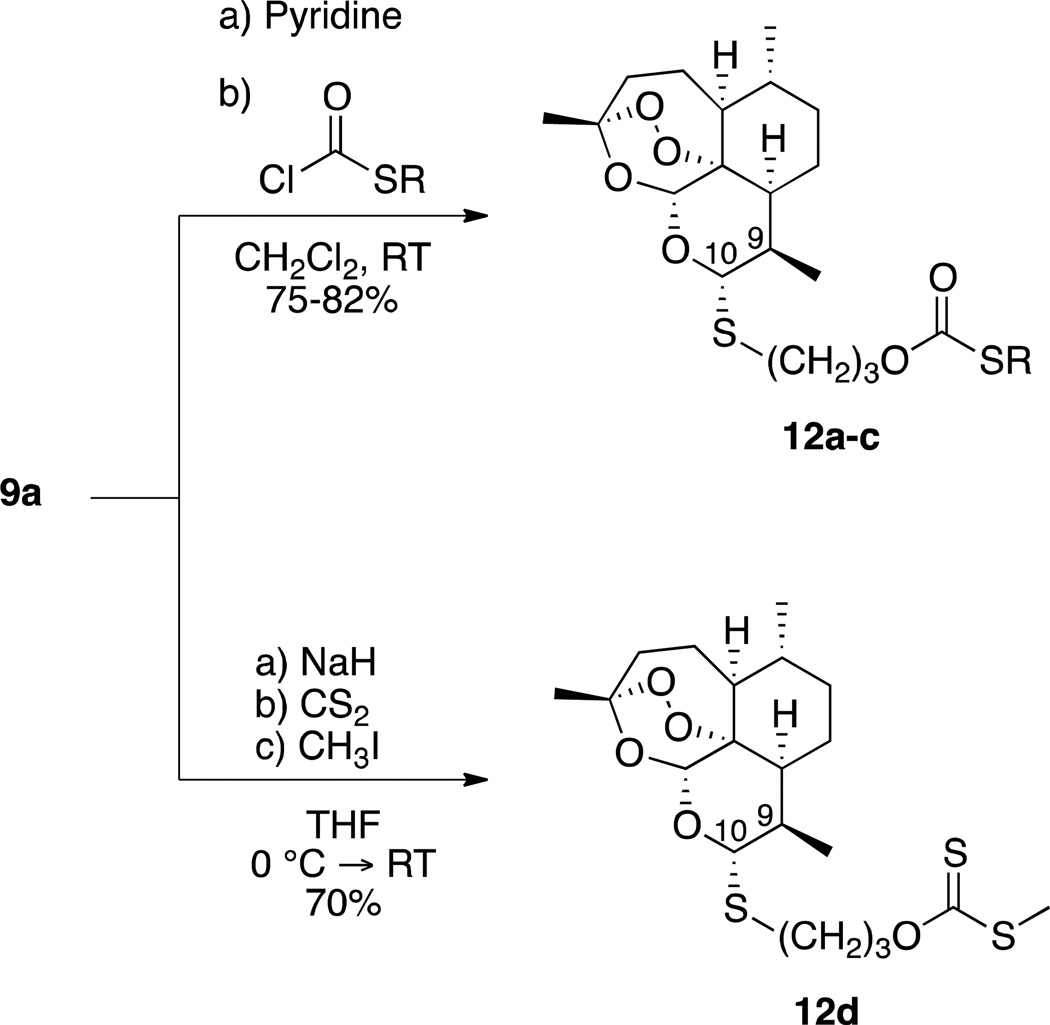
With the intention to test some compounds that were similar in structure to the thioacetal carbonates, we synthesized the series C-10α thioacetal thiocarbonates 12a–c as well as C-10α thioacetal xanthate ester 12d from diastereomerically pure thioacetal alcohol 9a. Preparation of 12a–c involved similar chemistry to that for the thioacetal carbonates, although change of solvent as well as base was necessary to optimize reaction conditions. In the case of 12d, 9a was treated with base, carbon disulfide, and methyl iodide in tetrahydrofuran (Scheme 3).
Scheme 3.
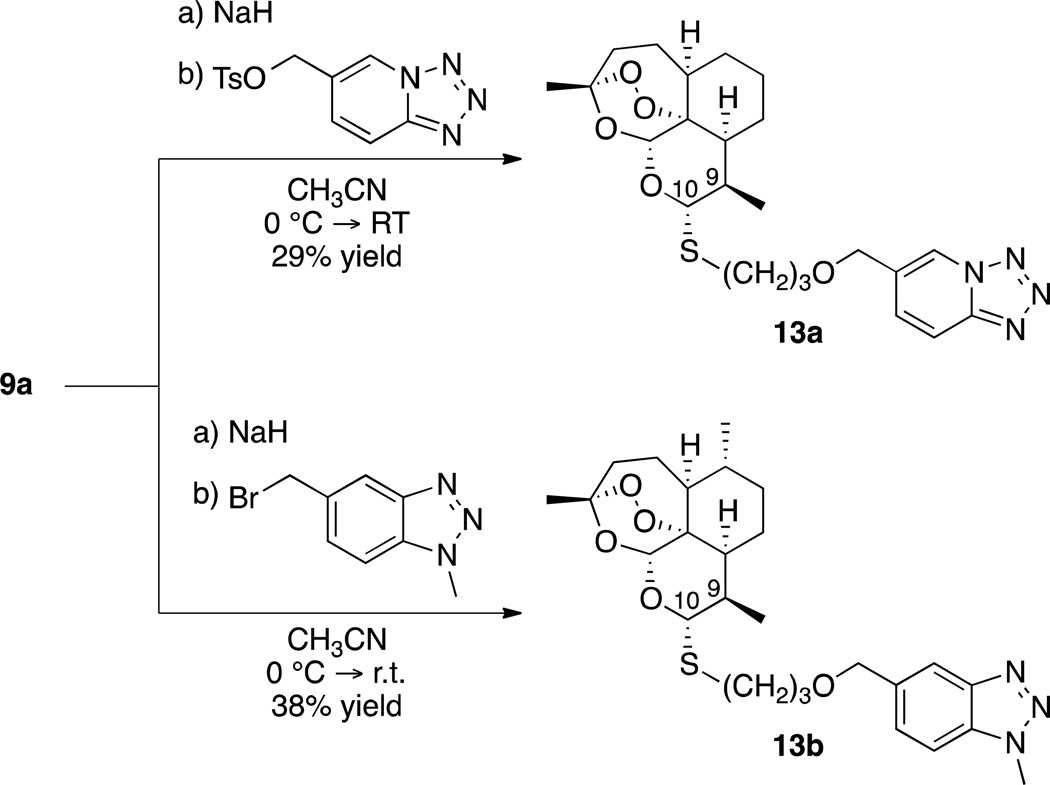
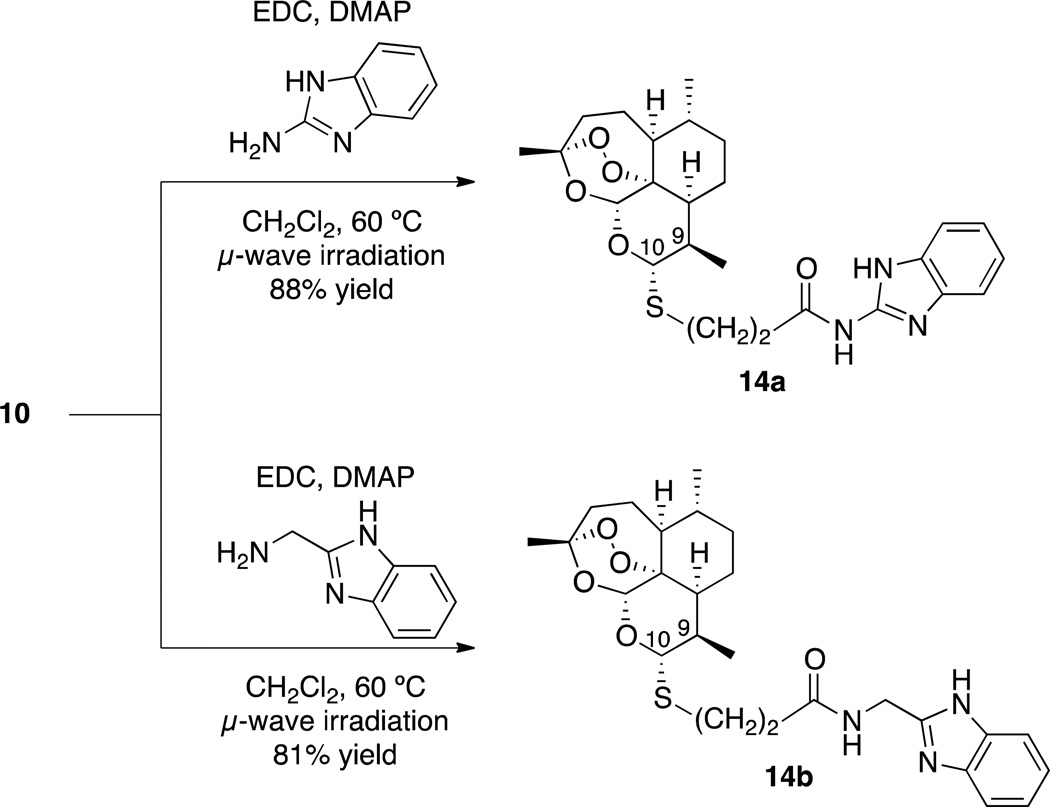
Since the development of chloroquine in the 1940s, nitrogen-containing heterocycles have been a cornerstone of antimalarial drug research.49,50,51 These moieties possess unique acid/base properties and mimic many important biological molecules. With these characteristics in mind, we prepared nitrogen-heterocycle-containing C-10 thioacetal ethers 13 (Scheme 4) and benzimidazole-containing C-10 thioacetal amides 14 (Scheme 5).
Scheme 4.
Scheme 5.
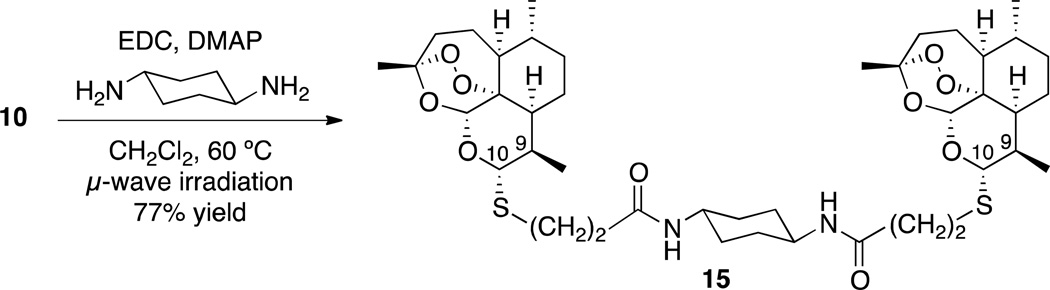
Making dimers of drug molecules is a common practice in medicinal chemistry; for every one molecule of drug, two pharmacophore moieties reach the active site within the body.52,53 Therefore, dimer drug compounds are sometimes considerably more efficacious than their monomer counterparts.52,53 Due to recent success with amide-containing monomeric trioxanes as antimalarials,30,54 we synthesized bis-amide bisthioacetal dimer 15 (Scheme 6).
Scheme 6.
Based on our previous in vivo results, we have observed some trends between log P values and antimalarial efficacy.30,31 Typically, monomeric trioxanes are most efficacious when the log P value is between 4 and 6. Dimeric trioxanes have displayed optimal results when the log P value is between 7 and 9.
Although literature suggested that our new C-10 thioacetals 9–15 would be much more stable to acid than the analogous acetals,42,43 we subjected lead trioxane 12c to a series of stability tests since it was the most efficacious of our antimalarial thioacetals. First, hydrolytic stability was tested based on a published protocol that simulated stomach acidic conditions, which is important for a drug that is administered orally.55 Trioxane 12c was dissolved a solution of aqueous hydrochloric acid and acetonitrile (pH = 2) and heated in a temperature-controlled oil bath set to 37 °C. This solution was allowed to stir for 24 h and was periodically analyzed by TLC. Additionally, the solution was analyzed by 1H NMR after completion of the 24 h. Since we knew that the product formed through hydrolysis of the C-10 thioacetal functionality would be 8, we compared the 1H NMR spectrum of 12c after 24 h to that of 8 and to that of parent alcohol 9a. Less than 2% hydrolysis of 12c had occurred. Similarly, 9a and 9b were stable to hydrolysis under the same conditions. Full experimental details of the hydrolysis study are included in the supporting information.
Because ACT is used predominantly in tropical areas where malaria is endemic, it was important to determine the thermal stability of lead trioxane 12c at 60 °C, neat, for 7 days; TLC as well as 1H NMR showed no decomposition.
Biology
To each thioacetal trioxane (0.64 mg), 100 µL of 7:3 Tween 80:ethanol with mefloquine hydrochloride (1.92 mg) was added. This mixture was then diluted with 965 µof water for oral administration to 5-week old C57BL/6J male mice (from the Jackson Laboratory) weighing about 20 g that were infected with P. berghei ANKA strain (2 × 107 parasitized erythrocytes). Each of four mice in a group was treated orally 24 h post infection with a single dose of 200 µL of diluted compound solution, corresponding to a dose of 6 mg/kg trioxane combined with 18 mg/kg of mefloquine hydrochloride. Determining blood parasitemia levels as well as monitoring the duration of animal survival compared to survival time of animals receiving no drug are both widely accepted as measures of a drug’s antimalarial efficacy. An average of 8.8% blood parasitemia was observed in the control (no drug) group on day 3 post infection. Infected animals receiving no drug died on an average of 8 days post infection. The antimalarial efficacy results of our C-10 thioacetals as well as controls are summarized in Table 4, which includes the parasitemia levels for mice on day 3 post infection.
Table 4.
In vivo antimalarial efficacy using a single oral dose of 6 mg/kg trioxane and 18 mg/kg mefloquine hydrochloride in P. berghei-infected mice.
| Trioxane | Average survival (days) after infection |
% Suppression of parasitemia (on day 3 post infection) |
|---|---|---|
| 11a | 12.8 (13, 13, 13, 12) | >99.9% |
| 11b | 18.5 (30, 16, 15, 13) | >99.9% |
| 11c | 17.8 (28, 17, 13, 13) | >99.9% |
| 11d | 19.3 (29, 17, 17, 14) | >99.9% |
| 11e | 18.8 (30, 17, 15, 13) | 99.9% |
| 11f | 21.8 (30, 30, 15, 12) | >99.9% |
| 11g | 16.5 (28, 14, 12, 12) | >99.9% |
| 11h | 25.3 (30, 29, 27, 15) | >99.9% |
| 11i | 14.5 (17, 15, 13, 13) | 99.9% |
| 12a | 24.5 (30, 30, 21, 17) | >99.9% |
| 12b | 19.3 (30, 17, 15, 15) | >99.9% |
| 12c | 29.8 (30, 30, 30, 29) | 99.9% |
| 12d | 22.0 (30, 28, 17, 13) | 99.9% |
| 13a | 23.0 (30, 29, 17, 16) | >99.9% |
| 13b | 22.3 (30, 29, 17, 13) | >99.9% |
| 14a | 26.3 (29, 28, 27, 21) | >99.9% |
| 14b | 19.5 (29, 20, 15, 14) | >99.9% |
| 15 | 19.8 (30, 17, 17, 15) | 99.9% |
| Controls | ||
| Infected (no drug) | 8.0 (10, 8, 7, 7) | 0% |
| Artemether + mefloquine-HCl | 16.5 (28, 13, 13, 12) | >99.9% |
| Mefloquine-HCl only | 14.0 (17, 13, 13, 13) | >99.9% |
Based on these data and as expected for artemisinin-derived trioxanes,10,11,12,13 all of our new C-10α thioacetals acted rapidly to suppress parasitemia, with almost complete suppression of parasitemia as determined on day 3 post infection. However, not all of the parasites were killed after 3 days which leads to a difference, sometimes substantial, in efficacy for each individual analog over the full 30 day experiment.
Also based on these data, it is clear that each of the four C-10 thioacetals 11h (25.3 days), 12a (24.5 days), 12c (29.8 days), and 14a (26.3 days) in combination with mefloquine hydrochloride prolonged the mouse average survival time by more than one week (>23.5 days) compared to the survival time of the artemether plus mefloquine hydrochloride control (16.5 days). Mefloquine hydrochloride alone at 18 mg/kg gave an average survival time of only 14 days.
Most impressively, trioxane 12c produced a partial cure with an average survival time of 29.8 days. Two of the four mice in this group showed no signs of parasitemia in their blood on day 30 post infection and behaved normally. One mouse in this group had 11% parasitemia on day 30, and one mouse died on day 29.
CONCLUSIONS
In conclusion, several artemisinin-derived C-10α thioacetals were found to be more efficacious as antimalarials than artemether. Four of the new thioacetals (11h, 12a, 12c, and 14a) combined with mefloquine hydrochloride prolonged the life of P. berghei-infected mice by at least one week longer than the artemether plus mefloquine hydrochloride control. Remarkably, when administered only once as a single, oral dose of 6 mg/kg plus 18 mg/kg of mefloquine hydrochloride, trioxane 12c was highly efficacious with an average survival time of 29.8 days, almost double the average survival time (16.5 days) achieved by the popular antimalarial drug artemether plus mefloquine positive control using the same protocol.
EXPERIMENTAL SECTION
The purity of compounds 11h, 12a, 12c, and 14a was determined to be >98% by HPLC. HPLC data were acquired using a Varian ProStar 210 two-pump system with a Sedex Model 75 Evaporative Light Scattering Detector (ELSD). A Varian 250 × 4.6mm × ¼” Microsorb-MV 100-5 Si column was used. All other instrumentation details are included in the Supporting Information.
Thioacetal Alcohol 9a
An oven-dried, 5 dram vial, equipped with a magnetic stir bar, under argon was charged with 844 (250 mg, 0.88 mmol, 1.0 equiv) and anhydrous dichloromethane (10 mL). 3-Mercaptopropanol (89 mg, 0.97 mmol, 1.1 equiv) was added and allowed to stir for 10 min at 50 °C, under argon. Boron trifluoride diethyl etherate (0.125 mL, 0.88 mmol, 1.0 equiv) was added dropwise and the reaction was allowed to stir under argon at 50 °C for 30 min. After 30 min, the reaction was quenched with water (5 mL) and extracted with dichloromethane (3 × 10 mL). The organic layers were combined, dried over MgSO4, and concentrated on a rotary evaporator at room temperature. The 1H NMR of the crude reaction mixture indicated a mixture of 10-α and 10-β diastereomers in a ratio of 9:1 (α:β). The crude amorphous solid was purified via column chromatography (5–10% ethyl acetate in hexanes) to afford 9a as a white solid (268 mg, 85% yield). Mp = 108.6–110.0 °C; [α]D23.3 +31.49 (c. 0.58, CHCl3); IR (thin film) ν 3445, 2926, 2871, 2363, 1716, 1586, 1446, 1378, 1279, 1195, 1126, 1035, 928, 900, 878 cm−1; 1H NMR (400 MHz, CDCl3) δ 5.28 (s, 1H), 4.52 (d, J = 10.7 Hz, 1H), 3.87 – 3.76 (m, 2H), 2.94 (ddd, J = 13.5, 7.7, 5.8 Hz, 1H), 2.80 (s, 1H), 2.70 (ddd, J = 13.3, 7.1, 5.9 Hz, 1H), 2.59 (ddd, J = 11.1, 7.3, 4.3 Hz, 1H), 2.33 (ddd, J = 14.6, 13.4, 4.0 Hz, 1H), 1.98 (ddd, J = 14.6, 4.8, 2.8 Hz, 1H), 1.92 – 1.63 (m, 4H), 1.57 (dt, J = 13.5, 4.3 Hz, 1H), 1.50 – 1.15 (m, 7H), 1.10 – 0.95 (m, 1H), 0.91 (dd, J = 12.2, 6.7 Hz, 7H); 13C NMR (100 MHz, CDCl3) δ 104.7, 92.6, 80.8, 80.7, 59.8, 51.9, 46.1, 37.6, 36.4, 34.2, 32.2, 31.9, 25.9, 24.9, 24.1, 21.5, 20.4, 15.2; HRMS (FAB) m/z calcd for C18H30O5S [M + H]+ 359.1892, found 359.1888.
Thioacetal Alcohol 9b
A 2-dram vial, equipped with magnetic stir bar and argon inlet adaptor, was charged with 8 (0.18 mmol, 50 mg) in anhydrous dichloromethane (2 mL). 6-Mercaptohexanol (0.19 mmol, 26 mg) was added in one portion neat directly to the stirring solution. Boron trifluoride diethyl etherate (0.18 mmol, 25 mg) was added dropwise via a needle and plastic syringe and the reaction was stirred for 20 min. The reaction was quenched with water (2 mL) and extracted with dichloromethane (3 × 2 mL). The organic layers were pooled, dried with magnesium sulfate, vacuum filtered and concentrated via rotary evaporation at room temperature. The crude residue was purified by flash column chromatography on silica, eluting with a gradient mobile phase (5–10% ethyl acetate in hexane) to yield 9b as a clear amorphous solid (45 mg, 64%). [α]D24.0 +14.6 (c. 0.65, CHCl3); IR (thin film) ν 3458, 2927, 2871, 1455, 1377, 1128, 1037, 928, 879, 829, 666 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.28 (s, 1H), 4.51 (d, J = 10.7 Hz, 1H), 3.63 (br t, J = 6.6 Hz, 2 H), 2.78 (ddd, J = 12.5, 8.3, 6.3 Hz, 1H), 2.72 – 2.51 (m, 2H), 2.36 (ddd, J = 14.6, 13.3, 4.0 Hz, 1H), 2.01 (ddd, J = 14.5, 4.9, 2.9 Hz, 1H), 1.90 – 1.83 (m, 1H), 1.77 – 1.15 (m, including a singlet at 1.41, 19 H), 1.07 – 0.99 (m, 1H), 0.95 (d, J = 6.0 Hz, 3H), 0.92 (d, J = 6.0 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ104.4, 92.4, 80.7, 80.6, 63.0, 52.0, 46.2, 37.5, 36.4, 34.2, 32.7, 31.9, 29.9, 28.8, 28.3, 26.1, 25.4, 24.9, 21.4, 20.4, 15.2; HRMS (FAB) m/z calcd for C21H36O5S (M+H)+ 401.2362, found 401.2355.
Thioacetal Carboxylic Acid 10
An oven-dried, 2 dram vial, equipped with a magnetic stir bar, under argon was charged with 8 (100 mg, 0.35 mmol, 1.0 equiv) and anhydrous dichloromethane (4 mL). 3-Mercaptopropionic acid (40 mg, 0.39 mmol, 1.1 equiv) was added and allowed to stir for 10 min at 50 °C, under argon. Boron trifluoride diethyl etherate (49.6 µL, 0.35 mmol, 1.0 equiv) was added dropwise and the reaction was allowed to stir under argon at 0 °C for 30 min. After 30 min, the reaction was quenched with water (5 mL), extracted with dichloromethane (3 × 10 mL). The organic layers were combined, dried over MgSO4, filtered, and concentrated on a rotary evaporator at room temperature. The 1H NMR of the crude reaction mixture indicated a mixture of 10-α and 10-β diastereomers in a ratio of 6:1 (α:β). The crude amorphous solid was purified via column chromatography (5–10% ethyl acetate in hexanes) to afford 10 as an amorphous solid (96 mg, 73% yield). [α]D22.6 +26.08 (c 1.1, CHCl3); IR (thin film) ν 2926, 2872, 1707, 1449, 1378, 1268, 1230, 1195, 1128, 1086, 1069, 1036, 959, 928, 900, 879 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.29 (s, 1H), 4.57 (d, J = 10.8 Hz, 1H), 3.16 – 2.94 (m, 1H), 2.95 – 2.75 (m, 3H), 2.73 – 2.55 (m, 1H), 2.36 (ddd, J = 14.5, 13.2, 4.0 Hz, 1H), 2.01 (ddd, J = 14.5, 4.9, 2.9 Hz, 1H), 1.95 – 1.80 (m, 1H), 1.80 – 1.54 (m, 3H), 1.54 – 1.15 (m, 7H), 1.13 – 0.98 (m, 1H), 0.94 (dd, J = 9.2, 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3) δ 176.8, 104.7, 92.3, 81.1, 80.6, 51.9, 46.1, 37.5, 36.4, 35.7, 34.2, 31.4, 25.9, 24.9, 23.6, 21.4, 20.4, 15.1; HRMS (FAB) m/z calcd for C18H28O6S [M + H]+ 373.1685, found 373.1669.
Thioacetal Carbonate 11h
An oven-dried 2-dram vial, equipped with magnetic stir bar and argon gas inlet needle, was charged with 9b (17.7 mg, 0.044 mmol, 1 equiv) in dry CH3CN (1 mL). Sodium hydride (2 mg, 0.088 mmol, 2 equiv) was added as a solid in one portion and the reaction was stirred at room temperature for 30 min. Propargyl chloroformate (21 mg, 0.176 mmol, 4 equiv) was added dropwise via syringe. The reaction was stirred for 24 h at room temperature, and then more sodium hydride (2 mg, 0.088 mmol, 2 equiv) and propargyl chloroformate (21 mg, 0.176 mmol, 2 equiv) were added. After 12 h, the reaction was quenched with water (2 mL) and extracted with CH2Cl2 (3 × 2 mL). The organic layers were pooled, dried with MgSO4 (ca. 1 g), vacuum filtered, and concentrated via rotary evaporation at room temperature. The crude residue was purified by flash chromatography on silica gel to yield 11h as a clear oil (13.4 mg, 64%). [α]D22.7 +11.8 (c. 0.59, CHCl3); IR (thin film) ν 3279, 2973 – 2851, 1751, 1377, 1279, 1259, 1229, 1128, 1051, 1037, 1017, 928, 880, 678 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.27 (s, 1H), 4.71 (d, J = 2.4 Hz, 2H), 4.51 (d, J = 10.7 Hz, 1H), 4.16 (t, J = 6.6 Hz, 2H), 2.77 (ddd, J = 12.5, 8.2, 6.3 Hz, 1H), 2.68 – 2.59 (m, 2H), 2.52 (t, J = 2.4 Hz, 1H), 2.36 (ddd, J = 14.6, 13.3, 4.0 Hz, 1H), 2.00 (ddd, J = 14.4, 4.9, 2.9 Hz, 1H), 1.92 – 1.81 (m, 1H), 1.75 – 1.52 (m, 8H), 1.50 – 1.19 (m, 10H), 1.10 – 0.98 (m, 1H), 0.96 – 0.90 (m, 7H); 13C NMR (75 MHz, CDCl3) δ 154.8, 104.4, 92.4, 80.7, 80.6, 75.7, 75.7, 68.8, 55.2, 52.0, 46.2, 37.5, 36.4, 34.2, 31.9, 29.9, 28.7, 28.6, 28.3, 26.1, 25.4, 24.9, 21.5, 20.4, 15.2; HRMS (FAB) m/z calcd for C25H39O7S (M+H)+ 483.2417, found 483.2408.
Thioacetal Thiocarbonate 12a
An oven-dried, 2 dram vial, equipped with a magnetic stir bar, under argon was charged with 9a (10 mg, 0.028 mmol, 1.0 equiv) and dichloromethane (1.0 mL). Pyridine (3 mg, 0.42 mmol, 1.5 equiv) was added and the mixture was allowed to stir for 15 min. After 15 min S-methyl chlorothioformate (5 mg, 0.042 mmol, 1.5 equiv) was added and the reaction was allowed to stir under argon for 24 hr. After 24 hr the reaction was quenched with water (5 mL) and extracted with dichloromethane (3 × 10 mL). The organic layers were combined, dried over MgSO4, and concentrated on a rotary evaporator at room temperature. The crude amorphous solid was purified via column chromatography (10% ethyl acetate in hexanes) to afford 12a as a white amorphous solid (9.8 mg, 81% yield). [α]D22.1 −2.40 (c. 0.55, CHCl3); IR (thin film) ν 2925, 2871, 1709, 1452, 1377, 1149, 1033, 926, 877, 826 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.27 (s, 1H), 4.52 (d, J = 10.8 Hz, 1H), 4.35 (td, J = 6.3, 1.0 Hz, 2H), 2.87 (dt, J = 13.6, 6.9 Hz, 1H), 2.76 – 2.53 (m, 2H), 2.33 (s, 4H), 2.14 – 1.96 (m, 3H), 1.87 (ddt, J = 13.5, 6.7, 3.3 Hz, 1H), 1.77 – 1.65 (m, 2H), 1.64 – 1.21 (m, 8H), 1.11 – 0.98 (m, 1H), 0.93 (dd, J = 9.8, 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3) δ 171.7, 104.4, 92.4, 80.8, 80.5, 66.4, 51.9, 46.2, 37.5, 36.4, 34.2, 31.8, 29.3, 26.1, 24.9, 21.4, 20.4, 15.2, 13.6; HRMS (FAB) m/z calcd for C20H32O6S2 [M + Na]+ 455.1538, found 455.1532.
Thioacetal Thiocarbonate 12c
An oven-dried, 2 dram vial, equipped with a magnetic stir bar, under argon was charged with 9a (10 mg, 0.028 mmol, 1.0 equiv) and dichloromethane (1.0 mL). Pyridine (3 mg, 0.042 mmol, 1.5 equiv) was added and the mixture was allowed to stir for 15 min. After 15 min S-t-butyl chlorothioformate (6 mg, 0.042 mmol, 1.5 equiv) was added and the reaction was allowed to stir under argon for 24 hr. After 24 hr the reaction was quenched with water (5 mL) and extracted with dichloromethane (3 × 5 mL). The organic layers were combined, dried over MgSO4, and concentrated on a rotary evaporator at room temperature. The crude amorphous solid was purified via column chromatography (10% ethyl acetate in hexanes) to afford 12c as a white solid (10.9 mg, 82% yield). Mp = 102.4–103.9 °C; [α]D22.1 +2.40 (c. 0.29, CHCl3); IR (thin film) ν 2961, 2922, 2872, 1706, 1455, 1377, 1125, 1036, 927, 879, 828 cm−1; 1H NMR (300 MHz, CDCl3) δ 5.27 (s, 1H), 4.52 (d, J = 10.8 Hz, 1H), 4.30 (td, J = 6.4, 0.9 Hz, 2H), 2.92 – 2.78 (m, 1H), 2.76 – 2.52 (m, 2H), 2.43 – 2.28 (m, 1H), 2.11 – 1.95 (m, 3H), 1.94 – 1.81 (m, 1H), 1.79 – 1.66 (m, 2H), 1.64 – 1.37 (m, 15H), 1.37 – 1.20 (m, 2H), 1.12 – 0.98 (m, 1H), 0.93 (dd, J = 9.9, 6.6 Hz, 6H); 13C NMR (75 MHz, CDCl3) δ 170.2, 104.4, 92.4, 80.8, 80.5, 65.3, 51.9, 47.2, 46.2, 37.5, 36.4, 34.2, 31.8, 30.3, 29.3, 26.1, 25.0, 24.9, 21.4, 20.4, 15.2; HRMS (FAB) m/z calcd for C23H38O6S2 [M + H]+ 475.2188, found 475.2174.
Thioacetal Benzimidazole Amide 14a
To a 2.5 mL microwave vial was added 10 (15 mg, 0.040 mmol), EDC (8.5 mg, 0.044 mmol), DMAP (5.4 mg, 0.044 mmol), and commercially available 2-aminobenzimidazole (5.86 mg, 0.044 mmol) and dissolved in CH2Cl2 (1 mL) under an Ar blanket. The solution was heated to 60 °C for 1.5 hours via microwave irradiation, at which point it was quenched with saturated NaHCO3 (2 mL) and extracted with CH2Cl2 (3 × 1.5 mL). The combined organic layers were dried with MgSO4 and concentrated in vacuo. The crude oil was purified by column chromatography (0–4% MeOH/CH2Cl2) to provide 14a as a colorless, amorphous solid (88% yield, 17.3 mg, 0.035 mmol). [α]D23.9 = −69.6 (c = 1.550, CHCl3); FTIR (thin film) ν 3341, 2926, 2872, 1686, 1633, 1578, 1456, 1271, 1127, 1085; 1H NMR (300 MHz, CDCl3) δ 11.27 (s, 1H), 7.52 (s, 3H), 7.25 – 7.16 (m, 2H), 5.19 (s, 1H), 4.51 (d, J = 10.8 Hz, 1H), 3.33 – 3.15 (m, 2H), 3.10 – 2.87 (m, 2H), 2.62 (dqd, J = 11.0, 7.2, 4.0 Hz, 1H), 2.33 (td, J = 13.3, 3.9 Hz, 1H), 2.04 – 1.91 (m, 1H), 1.90 – 1.76 (m, 1H), 1.68 – 1.49 (m, 3H), 1.48 – 1.36 (m, 1H), 1.35 (s, 3H), 1.29 – 1.13 (m, 3H), 1.11 – 0.95 (m, 1H), 0.95 – 0.92 (d, J = 6.0 Hz, 3H), 0.86 (d, J = 7.1 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 13C NMR (75 MHz, CDCl3) δ 172.8, 172.4, 147.6, 122.2, 104.5, 92.2, 92.1, 89.6, 80.4, 80.3, 51.6, 45.8, 37.39, 37.3, 36.22, 34.0, 31.1, 25.8, 24.6, 24.0, 21.0, 20.12, 18.4, 14.8, 6.8.; HRMS (ESI) calc’d for C25H34N3O5S (M+H)+ 488.2219, found 488.2217
Supplementary Material
Table 1.
C-10 Thioacetal Carbonates 11
| Compound | n | R | log P a | Yield |
|---|---|---|---|---|
| 11a | 3 | Me | 4.6 | 91% |
| 11b | 3 | n-Bu | 5.9 | 81% |
| 11c | 3 | t-Bu | 5.5 | 85% |
| 11d | 3 | PhF-4 | 6.4 | 77% |
| 11e | 3 | CH2C≡CH | 4.9 | 54% |
| 11f | 6 | Me | 5.9 | 82% |
| 11g | 6 | Et | 6.3 | 88% |
| 11h | 6 | CH2C≡CH | 6.1 | 64% |
| 11i | 6 | O(CH2)2S(O)2Ph | 6.6 | 63%b |
Log P values were calculated using ChemOffice® Ultra 11.0
This reaction was run in dichloromethane with pyridine as the base
Table 2.
C-10 Thioacetal Thiocarbonates 12
| Compound | X | R | log P a | Yield |
|---|---|---|---|---|
| 12a | O | Me | 5.2 | 81% |
| 12b | O | n-Pr | 6.0 | 77% |
| 12c | O | t-Bu | 6.1 | 82% |
| 12d | S | Me | 5.6 | 70% |
Log P values were calculated using ChemOffice® Ultra 11.0
Table 3.
Calculated log P values for C-10 thioacetals 13–15
| Compound | log Pa |
|---|---|
| 13a | 4.7 |
| 13b | 5.3 |
| 14a | 4.8 |
| 14b | 4.5 |
| 15 | 7.4 |
Log P values were calculated using MarvinSketch and a calculator plug-in by ChemAxon Kft
ACKNOWLEDGEMENTS
We thank the NIH (AI 34885), the Johns Hopkins Malaria Research Institute, and the Bloomberg Family Foundation for financial support. We also thank Bryan T. Mott for help in obtaining some HRMS data.
ABBREVIATIONS USED
- ACT
artemisinin combination therapy
- WHO
world health organization
- DHA
dihydroartemisinin
- EDC
N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride
- DMAP
4-dimethylaminopyridine
Footnotes
ASSOCIATED CONTENT
Supporting Information. Additional experimental details, analytical data, and copies of 1H and 13C NMR spectra for all reported compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.World Malaria Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Delves M, Plouffe D, Scheurer C, Meister S, Wittlin S, Winzeler EA, Sinden RE, Leroy D. The activities of current antimalarial drugs on the life cycle stages of Plasmodium: a comparative study with human and rodent parasites. PLos Med. 2012;9 doi: 10.1371/journal.pmed.1001169. e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJL, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 4.Gulland A. Death toll from Malaria is double the WHO estimate, study finds. Brit. Med. J. 2012;344:895. doi: 10.1136/bmj.e895. [DOI] [PubMed] [Google Scholar]
- 5.Thera MA, Plowe CV. Vaccines for malaria: how close are we? Annu. Rev. Med. 2012;63:345–357. doi: 10.1146/annurev-med-022411-192402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nussenzweig V, Good MF, Hill AVS. Mixed results for a malaria vaccine. Nature Med. 2011;17:1560–1561. doi: 10.1038/nm1211-1560. [DOI] [PubMed] [Google Scholar]
- 7.Olliaro PL, Boland PB. Clinical Public Health Implications of Antimalarial Drug Resistance. In: Rosenthal PJ, editor. Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Totowa, NJ: Humana Press; 2001. pp. 65–84. [Google Scholar]
- 8.Burgess SJ, Selzer A, Kelly JX, Smilkstein MJ, Riscoe MK, Peyton DH. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J. Med. Chem. 2006;49:5623–5625. doi: 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidelines for Treatment of Malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 10.Slack RD, Jacobine AM, Posner GH. Antimalarial peroxides: advances in drug discovery and design. Med. Chem. Commun. 2012;3:281–297. [Google Scholar]
- 11.Tilley L, Charman SA, Vennerstrom JL. RSC Drug Discovery Series. Vol. 14. Royal Society of Chemistry; 2012. Semisynthetic artemisinin and synthetic peroxide antimalarials; pp. 33–64. (Neglected Diseases and Drug Discovery) [Google Scholar]
- 12.Wells TNC. Natural products as starting points for future anti-malarial therapies: going back to our roots? Malaria Journal. 2011;10:1–12. doi: 10.1186/1475-2875-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat. Rev. Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, Chiu FCK, Chollet J, Craft JC, Creek DJ, Dong Y, Matile H, Maurer M, Morizzi J, Nguyen T, Papastogiannidis P, Scheurer C, Shackleford DM, Sriraghavan K, Stingelin L, Tang Y, Urwyler H, Wang X, White KL, Wittlin S, Zhou L, Vennerstrom JL. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci USA. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill PM. The therapeutic potential of semi-synthetic artemisinin and synthetic endoperoxide antimalarial agents. Expert Opin. Investig. Drugs. 2005;14:1117–1128. doi: 10.1517/13543784.14.9.1117. [DOI] [PubMed] [Google Scholar]
- 16.Yavo W, Faye B, Kuete T, Djohan V, Oga SA, Kassi RR, Diatta M, Ama MV, Tine R, Ndiaye J-L, Evi J-B, Same-Ekobo A, Faye O, Koné M. Multicentric assessment of the efficacy and tolerability of dihydroartemisinin-piperaquine compared to artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in sub-Saharan Africa. Malaria Journal. 2011;10:198–205. doi: 10.1186/1475-2875-10-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macedo de Oliveira A, Chavez J, Ponce de Leon G, Durand S, Arrospide N, Roberts J, Cabezas C, Marquiño W. Efficacy and effectiveness of mefloquine and artesunate combination therapy for uncomplicated Plasmodium falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2011;85:573–578. doi: 10.4269/ajtmh.2011.11-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Niambele MB, Sissoko M, Dicko A, Djimde A, Doumbo OK. A randomized trial of artesunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am. J. Trop. Med. Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 19.Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, Pénali LK, Valecha N, Tien NT, Abdulla S, Borghini-Fuhrer I, Duparc S, Shin C-K, Fleckenstein L. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. N. Engl. J. Med. 2012;366:1298–1309. doi: 10.1056/NEJMoa1007125. [DOI] [PubMed] [Google Scholar]
- 20.Kurth F, Bélard S, Basra A, Ramharter M. Pyronaridine-artesunate combination therapy for the treatment of malaria. Curr. Opin. Infect. Dis. 2011;24:564–569. doi: 10.1097/QCO.0b013e32834cabdb. [DOI] [PubMed] [Google Scholar]
- 21.Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, Maiga H, Diambele MB, Sissoko M, Dicko A, Djimde A, Doumbo OK. A randomized trial of aresunate-mefloquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Am. J. Trop. Med. Hyg. 2008;79:655–661. [PubMed] [Google Scholar]
- 22.Cohen JL, Yavuz E, Morris A, Arkedis J, Sabot O. Do malaria patients adhere to over-the-counter artemisinin combination therapy for malaria? evidence from an intervention study in Uganda. Malaria Journal. 2012;11:83–93. doi: 10.1186/1475-2875-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawford H, Zurovac D, O’Reilly L, Hoibak S, Cowley A, Munga S, Vulule J, Juma E, Snow RW, Allan R. Adherence to prescribed artemisinin-based combination therapy in Garissa and Bunyala districts, Kenya. Malaria Journal. 2011;10:281–288. doi: 10.1186/1475-2875-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Brien C, Philipp HP, Passi N, Fidock DA. Recent clinical and molecular insights into emerging artemisinin resistance in Plasmodium falicaparum. Curr. Opin. Infect. Dis. 2011;24:570–577. doi: 10.1097/QCO.0b013e32834cd3ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrer P, Tripathi AK, Clark MA, Hand CC, Rienhoff HY, Jr, Sullivan DJ. Antimalarial iron chelator, FBS0701, shows asexual and gametocyte Plasmodium falciparum activity and single oral dose cure in murine malaria model. PLos One. 2012;7:e37171. doi: 10.1371/journal.pone.0037171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tun T, Tint HS, Lin K, Kyaw TT, Myint MK, Khaing W, Tun ZW. Efficacy of oral single dose therapy with artemisinin-naphthoquine phosphate in uncomplicated falciparum malaria. Acta Tropica. 2009;111:275–278. doi: 10.1016/j.actatropica.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Younis Y, Douelle F, Feng T-S, González Cabrera D, Le Manach C, Nchinda AT, Duffy S, White KL, Shackleford DM, Morizzi J, Mannila J, Katneni K, Bhamidipati R, Zabiulla KM, Joseph JT, Bashyam S, Waterson D, Witty MJ, Hardick D, Wittlin S, Avery V, Charman SA, Chibale K. 3,5-Diaryl-2-aminopyridines as a novel class of orally active antimalarials demonstrating single dose cure in mice and clinical candidate potential. J. Med. Chem. 2012;55:3479–3487. doi: 10.1021/jm3001373. [DOI] [PubMed] [Google Scholar]
- 28.Benjamin J, Morre B, Lee ST, Senn M, Griffin S, Lautu D, Salman S, Siba P, Mueller I, Davis TME. Artemisinin-naphthoquine combination therapy for uncomplicated pediatric malaria: a tolerability, safety, and preliminary efficacy study. Antimicrob. Agents Chemother. 2012;56:2465–2471. doi: 10.1128/AAC.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batty KT, Salman S, Moore BR, Benjamin J, Lee ST, Page-Sharp M, Pitus N, Ilett KF, Mueller I, Hombhanje FW, Siba P, Davis TME. Artemisinin-napthoquine combination therapy for uncomplicated pediatric malaria: a pharmacokinetic study. Antimicrob. Agents Chemother. 2012;56:2472–2484. doi: 10.1128/AAC.06250-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slack RD, Mott BM, Woodard LE, Tripathi A, Sullivan D, Nenortas E, Girdwood SCT, Shapiro TA, Posner GH. Malaria-infected mice are completely cured by one 6 mg/kg oral dose of new monomeric trioxane sulfide combined with mefloquine. J. Med. Chem. 2012;55:291–296. doi: 10.1021/jm201214d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moon DK, Tripathi A, Sullivan D, Siegler MA, Parkin S, Posner GH. A single, low, oral dose of a 5-carbon-linked trioxane dimer orthoester plus mefloquine cures malaria-infected mice. Bioorg. Med. Chem. Lett. 2011;21:2773–2775. doi: 10.1016/j.bmcl.2010.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Álvares I, Groult H, Casas J, Barreda-Manso MA, Yanguas-Casás N, Nieto-Sampedro M, Romero-Ramírez L, Fernández-Mayoralas A. Synthesis of antimitotic thioglycosides: in vitro and in vivo evaluation of their anticancer activity. J. Med. Chem. 2011;54:6949–6955. doi: 10.1021/jm200961q. [DOI] [PubMed] [Google Scholar]
- 33.Fanton J, Camps F, Castillo JA, Guérard-Hélaine C, Lemaire M, Charmantray F, Hecquet L. Enzymatic and organocatalyzed asymmetric aldolization reactions for the synthesis of thiosugar scaffolds. Eur. J. Org. Chem. 2012:203–210. [Google Scholar]
- 34.Hibino H, Nishiuchi Y. 4-Methoxybenzyloxymethyl group, a racemization-resistant protecting group for cysteine in Fmoc solid phase peptide synthesis. Org. Lett. 2012;14:1926–1929. doi: 10.1021/ol300592w. [DOI] [PubMed] [Google Scholar]
- 35.Posner GH, Cumming JN, Woo S-H, Ploypradith P, Xie S, Shapiro TA. Orally active antimalarial 3-substituted trioxanes: new synthetic methodology and biological evaluation. J. Med. Chem. 1998;41:940–951. doi: 10.1021/jm970686e. [DOI] [PubMed] [Google Scholar]
- 36.Chadwick J, Mercer AE, Park BK, Cosstick R, O’Neill PM. Synthesis and biological evaluation of extraordinarily potent C-10 carba artemisinin dimers against P. falciparum malaria parasites and HL-60 cancer cells. Bioorg. Med. Chem. 2009;17:1325–1338. doi: 10.1016/j.bmc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Venugopalan B, Karnik PJ, Bapat CP, Chatterjee DK, Iver N, Lepcha D. Antimalarial activity of new ether and thioethers of dihydroartemisinin. Eur. J. Med. Chem. 1995;30:697–706. doi: 10.1021/jm00011a012. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Oh S. A simple synthesis of C-10 substituted deoxoartemisinin and 9-epi-deoxoartemisinin with various organozinc reagents. Tetrahedron Lett. 2002;43:2891–2894. [Google Scholar]
- 39.Oh S, Jeong IH, Shin W-S, Lee S. Growth inhibition activity of thioacetal artemisinin derivatives against human umbilical vein endothelial cells. Bioorg. Med. Chem. Lett. 2003;13:3665–3668. doi: 10.1016/j.bmcl.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Oh S, Park G-M, Kim T-S, Ryu J-S, Choi H-K. Antimalarial activity of thiophenyl- and benzenesulfonyl-artemisinin. Korean. J. Parasitol. 2005;43:123–126. doi: 10.3347/kjp.2005.43.3.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh S, Jeong IH, Ahn CM, Shin W-S, Lee S. Synthesis and antiangiogenic activity of thioacetal artemisinin derivatives. Bioorg. Med. Chem. 2004;12:3783–3790. doi: 10.1016/j.bmc.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 42.Satchell DPN, Satchell RS. Mechanisms of hydrolysis of thioacetals. Chem. Soc. Rev. 1990;19:55–81. [Google Scholar]
- 43.Wuts PGM, Greene TW. Greene’s Protective Groups in Organic Synthesis. Fourth Ed. Hoboken, NJ: John Wiley & Sons, Inc.; 2007. pp. 501–503. [Google Scholar]
- 44.Ma J, Katz E, Kyle DE, Ziffer H. Syntheses and antimalarial activities of 10-substituted deoxoartemisinins. J. Med. Chem. 2000;43:4228–4232. doi: 10.1021/jm000195l. [DOI] [PubMed] [Google Scholar]
- 45.Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 46.Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discovery. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 47.Ueda Y, Wong H, Matiskella JD, Mikkilineni AB, Farina V, Fairchild C, Rose WC, Mamber SW, Long BH, Kerns EH, Casazza AM, Vyas DM. Synthesis and antitumor evaluation of 2′-oxycarbonylpaclitaxels (paclitaxel-2′-carbonates) Bioorg. Med. Chem. Lett. 1994;4:1861–1864. [Google Scholar]
- 48.Nicolaou KC, Riemer C, Kerr MA, Rideout D, Wrasidlo W. Design, synthesis and biological activity of protaxols. Nature. 1993;364:464–466. doi: 10.1038/364464a0. [DOI] [PubMed] [Google Scholar]
- 49.Skinner-Adams TS, Davis TM, Manning LS, Johnston WA. The efficacy of benzimidazole drugs against Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 1997;91:580–594. doi: 10.1016/s0035-9203(97)90035-3. [DOI] [PubMed] [Google Scholar]
- 50.Berger O, Kaniti A, Tran van BaC, Vial H, Ward SA, Biagini GA, Bray PG, O’Neill PM. Synthesis and antimalarial activities of a diverse set of triazole-containing furamidine analogues. ChemMedChem. 2011;6:2094–2108. doi: 10.1002/cmdc.201100265. [DOI] [PubMed] [Google Scholar]
- 51.Saify ZS, Azim MK, Ahmad W, Nisa M, Goldberg DE, Hussain SA, Akhtar S, Akram A, Arayne A, Oksman A, Khan IA. New benzimidazole derivatives as antiplasmodial agents and plasmepsin inhibitors: synthesis and analysis of structure–activity relationships. Bioorg. Med. Chem. Lett. 2012;22:1282–1286. doi: 10.1016/j.bmcl.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 52.Taudon N, Margout D, Wein S, Calas M, Vial HJ, Bressole FMM. Quantitative analysis of a bis-thiazolium antimalarial compound, SAR97276, in mouse plasma and red blood cell samples, using liquid chromatography mass spectrometry. J. Pharm. Biomed. Anal. 2008;46:148–156. doi: 10.1016/j.jpba.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 53.Ban K, Duffy S, Khakham Y, Avery VM, Hughes A, Montagnat O, Katneni K, Ryan E, Baell JB. 3-Alkyl-1,2,4-triazine dimers with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2010;20:6024–6029. doi: 10.1016/j.bmcl.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 54.Woodard LE, Chang W, Chen X, Liu JO, Shapiro TA, Posner GH. Malaria-infected mice live until at least day 30 after a new monomeric trioxane combined with mefloquine are administered together in a single low oral dose. J. Med. Chem. 2009;52:7458–7462. doi: 10.1021/jm9005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung M, Lee S. Stability of acetal and non acetal-type analogs of artemisinin in simulated stomach acid. Bioorg. Med. Chem. Lett. 1998;8:1003–1006. doi: 10.1016/s0960-894x(98)00160-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.