Abstract
Background
Mesocorticolimbic neurocircuitry and impulsivity have both been implicated in pathological gambling (PG) and in reward processing. However, the neural underpinnings of specific phases of reward and loss processing in PG and their relationships to impulsivity remain only partially understood. The present functional magnetic resonance imaging study examined brain activity associated with different phases of reward and loss processing in PG. Given an inverse relationship between ventral striatal recruitment during anticipation of monetary rewards and impulsivity in alcohol dependence, the current study explored whether a similar association might also be present in PG.
Methods
Fourteen adults with PG and 14 control comparison (CC) participants performed the Monetary Incentive Delay Task (MIDT) to identify brain activation changes associated with reward/loss prospect, reward/loss anticipation and reward/loss notification. Impulsivity was assessed separately using the Barratt Impulsiveness Scale.
Results
Relative to the CC group, the PG group exhibited significantly reduced activity in the ventromedial prefrontal cortex, insula and ventral striatum during several phases, including the prospect and anticipation phases of both gain and losses. Activity in the ventral striatum correlated inversely with levels of impulsivity in PG participants, consistent with prior findings in alcohol dependence.
Conclusions
Relatively decreased activity in cortico-striatal neurocircuitry during multiple phases of reward processing suggests consistent alterations in neurocircuitry underlying incentive valuation and loss prediction. Together with findings in alcohol dependence, these results suggest that impulsive tendencies in addictions may be reflected in diminished ventral striatal activations to reward anticipation and may represent targets for treatment development in addictions.
Keywords: fMRI, vmPFC, ventral striatum, insula, incentive, gambling
Introduction
Pathological gambling (PG) shares clinical features with substance addictions, and both demonstrate similar alterations in motivational and reward neurocircuitry (1–7). Both PG and substance-dependent individuals show differences in neural responses to drug and monetary reward cues (2,6,8,9). Relatively little is known, however, about the neural correlates of specific temporal phases of reward and loss processing in PG. In non-addicted adults, investigations into the neural underpinnings of reward processing have identified distinct anticipation and outcome phases, with reward anticipation linked to activation of the ventral striatum (VS) and reward notification or outcome linked to activation of the ventromedial prefrontal cortex (vmPFC) (10–14). Neural responses during anticipation are significant as they are temporally ordered to influence decision-making processes and behavior (15). Different patterns of cortico-striatal activations during anticipatory phases of reward processing are observed in substance-dependent patients relative to healthy adults. For example, persons with alcohol dependence show relatively diminished VS activation during reward anticipation (8). Furthermore, this activation correlates inversely with self-reported impulsivity (8), the tendency to act quickly, without planning or regard for negative consequences ((16,17), which has been linked to propensities to develop addictions and to addiction treatment outcomes (8,18–20).
Neuroimaging studies indicate diminished activation of cortico-striatal circuitry in PG. Diminished vmPFC activation has been reported in PG during cognitive control (21), gambling cue presentation (2), simulated gambling (3), and amongst those with co-occurring substance abuse/dependence, during risk/reward decision-making (4). Relatively diminished VS activation has also been observed in PG during simulated gambling (3) and in response to gambling related cues (6). Amongst individuals with Parkinson’s disease (PD) and impulse control disorders (including PG) as compared to persons with PD alone, diminished VS activation occurs during risk-taking, with differences in perfusion also observed (22). Reduced insula activity has been reported in PG individuals viewing gambling cues (6). In non-addicted individuals, insula activation is implicated in loss prediction and financial risk-taking (23–25). Alterations in reward and loss processing circuitry appear particularly relevant to PG as they may generate misrepresented valuations of rewards or punishments and promote risky choices and continued gambling (26,27). For example, some neurophysiological data suggest hypersensitivity to reward following losses in problem gamblers (28). To date, however, PG studies examining monetary incentives have included paradigms that do not fully disambiguate specific variables such as probability, response preparation, certainty, guessing and choice – all of which may differentially contribute to reward processes.
No functional magnetic resonance (fMRI) study in PG has examined the neural correlates during different phases of reward and loss processing, thus limiting understanding of temporal fluctuations attributable to aspects of incentive processing in PG. A widely-used fMRI task for investigating monetary reward processing is the monetary incentive delay task (MIDT), which can parse anticipatory and outcome phases (8,11,12,15,29,30). This task has recently been modified to model two distinct anticipatory phases relating to prospect (A1) and anticipation of notification (A2) of reward/loss (18). This MIDT structure effectively separates anticipatory processes from choice, and further parses neural activity associated with motor preparation/demands. In this way, the modified MIDT provides an ordered framework to examine the neurobiological substrates underlying specific aspects of reward and loss processing in PG. In accordance with evidence for ventral striatal and vmPFC recruitment during reward anticipation and outcomes, respectively, and diminished activation of these regions in PG during simulated gambling, we hypothesized that the PG group would demonstrate relatively diminished VS activation during the A1 and A2 phases and relatively diminished activation of vmPFC during the outcome phase of the MIDT. Given insular contributions to financial risk-taking and loss prediction (23), we hypothesized relatively reduced insula activity during loss processing in PG. Given similar neurobiological contributions to substance and non-substance addictions (1–7) and findings in alcohol dependence (8), we hypothesized that VS activity during the anticipatory phase would inversely correlate with self-reported impulsivity in the PG group.
Methods
Participants
Participants were 14 individuals who met criteria for PG and 14 control comparison (CC) participants (demographic and self-reported measures are displayed in Table 1). Sample characteristics are more fully described in the Supplement. All participants except one CC individual completed the Barratt Impulsivity Scale (BIS-11; (31)). The BIS-11 is a valid and reliable measure of impulsivity that factors into motor, attention, and non-planning subscales (31). Urine toxicology at the time of scanning verified that all individuals were free of illicit substances. All participants provided written informed consent. The study was approved by the Yale Human Investigations Committee.
Table 1.
Characteristics of PG and CC participants.
| PG | CC | Test Statistics | |
|---|---|---|---|
| n | 14 | 14 | |
| Male/Female | 10/4 | 10/4 | |
| Current Smoker* | 6 | 2 | χ2 = 4.76, df = 1, p<0.05 |
| Age (SD) | 35.8 (11.7) | 37.1 (11.3) | ns |
| IQ – Shipley (SD) | 102.8 (12.4) | 106.5 (13.2) | ns |
| SOGS (SD)*** | 12.6 (3.5) | 0.3 (0.6) | F(1,26) =169.28, p<0.001 |
| BIS-11 Total Score (SD)* | 68.07 (12.26) | 59.13 (12.08) | F(1,25) =3.64, p= 0.1 |
| Attention Subscale (SD)* | 16.36 (4.47) | 13.92 (3.88) | F(1,25) =2.27, p> 0.1 |
| Motor Subscale (SD)* | 25.14 (4.54) | 22.52 (4.24) | F(1,25) =2.41, p> 0.1 |
| Non-planning subscale (SD)* | 26.57 (5.45) | 22.69 (5.19) | F(1,25) =3.58, p= 0.1 |
Monetary Incentive Delay Task
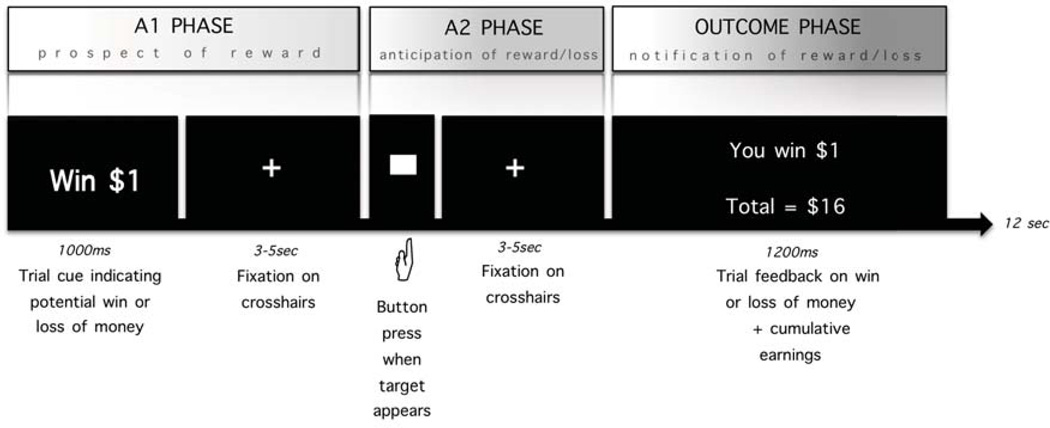
All participants completed the MIDT (Figure 1). The task and experimental methods are described elsewhere (18) and in the Supplement.
Figure 1.
Monetary Incentive Delay Task (MIDT) adapted from Knutson et al., 2001, described in Andrews et al., 2011. Participants first view an incentive cue signaling the potential to win or lose money and then fixate on a ‘+’ (A1 phase). Then, in the A2 phase, a target appears. Participants win (or avoid losing) money by pressing a button before the target disappears. Participants then wait for feedback notifying whether they’ve won or lost the trial (A2). In the outcome phase participants receive feedback on whether they have won or lost the trial and their cumulative earnings. Task difficulty (length of target presentation) is based on reaction times collected during a pre-scan practice session, such that participants win on ~66% of trials.
fMRI Acquisition and Analysis
Images were obtained using a Siemens TIM Trio 3T MRI system. Image acquisition and analysis methods are detailed further in the Supplement. Functional images were preprocessed using SPM5 (Welcome Functional Imaging Laboratory, London UK), normalized to the Montreal Neurological Institute template and smoothed with a 6mm kernel FWHM. First-level modeling was conducted using robust regression (32) to reduce the influence of strong outliers (33). Motion parameters and high-pass filter parameters were included as additional regressors of no interest. Neuroelf analysis package (www.neuroelf.net) was used for second-level random effects analysis. Correction for multiple comparisons was conducted using Monte-Carlo simulation (e.g., AlphaSim), using a combined voxel-wise and cluster thresholds to result in a family-wise error rate of 5%. To examine the effects of task on brain activation, we contrasted: 1) anticipation of monetary gain versus anticipation of no monetary outcome for the A1 and A2 phases (A1Win and A2Win, respectively); 2) anticipation of monetary loss versus anticipation of no monetary outcome for the A1 and A2 phases (A1Loss and A2Loss, respectively); 3) ‘Win’ vs. ‘Neutral’ outcome trials (OCWin); and 4) ‘Loss’ vs. ‘Neutral’ outcome trials (OCLoss). To examine between-group differences, we compared activity in PG and CC groups during A1Win, A2Win, OCWin, A1Loss, A2Loss and OCLoss in a series of t-tests.
Given the small volume of the VS, together with evidence implicating this area in reward processing, the MIDT, and the pathophysiology of PG, the VS was selected as an a priori region of interest (ROI). This ROI was defined and localized based on reward-processing findings of Breiter and colleagues (34). Activity from a spherical ROI of 3mm radius (123 structural voxels 1×1×1mm) was extracted for each individual to examine the mean BOLD percent signal change from baseline.
Then, activity during the anticipatory phases was examined between experimental groups using a one-way Analysis of Variance (ANOVA) in SPSS, version 17.0. The relationship between impulsivity and activity in the VS ROI during win and loss anticipation (win cues > neutral cues; loss cues > neutral cues) during A1 and A2 was examined using Pearson correlations.
Results
In-Scanner Behavior
Multiple one-way ANOVAs examining behavioral responses in-scanner showed no significant between-groups differences in earnings, reaction times or hit rates on the different incentive conditions (all p>0.05; see Supplement).
Group Differences: A1Win
Between-group contrasts of neural activity during the A1Win phase revealed significantly decreased activity in PG relative to CC in the mPFC extending through the vmPFC and anterior cingulate into the left VS (Table 2; Figure 2a) and another cluster in the left inferior frontal gyrus. Conversely, activity in the medial precuneus was relatively increased in the PG group relative to the CC.
Table 2.
Group differences during MIDT Trials
| MNI Coordinates | ||||||||
|---|---|---|---|---|---|---|---|---|
| MIDT Phase |
Structure | BA | Left/ Right |
x | y | z | k | F/t-value |
| A1 Winning | mPFC /vmPFC/AC/ventral striatum | 9/10/24 | L | −9 | 45 | 24 | 348 | −4.92 |
| Inferior Frontal Gyrus | 45 | L | −51 | 27 | −6 | 110 | −3.70 | |
| Precuneus | 7 | L | 3 | −54 | 54 | 47 | 3.22 | |
| A1 Losing | mPFC /ACC | 9 | L | −12 | 45 | 21 | 142 | −4.48 |
| Inferior Frontal Gyrus/Insula | 45/47 | L | −51 | 30 | −6 | 107 | −4.05 | |
| Ventral | 10/11/2 | L | −18 | 18 | −6 | 103 | −3.84 | |
| Striatum/vmPFC | 5 | |||||||
| ROI: Ventral striatum | - | R | 10 | 12 | −11 | 123 | F(1, 26)= 4.91 | |
| A2 Winning | vmPFC/ ventral striatum | 11 | R/L | 3 | 24 | −15 | 99 | −4.10 |
| ROI: Ventral striatum | - | R | 10 | 12 | −11 | 123 | F(1, 26)= 4.72 | |
| A2 Losing | ||||||||
| ROI: Ventral striatum | - | L | −10 | 12 | −11 | 123 | F(1, 26)= 4.57 | |
| Winning Outcome | mPFC/ACC/vmPFC | 9/32/24 /10 | L/R | 9 | 33 | −3 | 238 | −4.03 |
| Posterior Cingulate/Hippocampus | 30 | L | −18 | −51 | 9 | 119 | −3.83 | |
| Losing Outcome | Superior Temporal Gyrus/ Insula | 22 | R | 48 | −9 | −9 | 116 | −4.65 |
| Middle Occipital Gyrus/Posterior Cingulate/Cuneus | 18 | R | 24 | −81 | −9 | 806 | −4.39 | |
| Superior Parietal Lobule | 7 | R | 27 | −57 | 54 | 230 | −4.29 | |
| Precentral Gyrus | 6 | L | −60 | 0 | 39 | 110 | −4.24 | |
| Middle/Superior Temporal Gyrus/Insula | 37 | L | −42 | −60 | 3 | 367 | −4.16 | |
| Superior Frontal Gyrus | 8 | L | −18 | 18 | 45 | 353 | −3.49 | |
| Superior Frontal Gyrus | 8 | L | −6 | 48 | 48 | 148 | −3.39 | |
| Middle Frontal Gyrus | 10 | R | 21 | 60 | 12 | 120 | −3.19 | |
| ROI: Ventral striatum | - | L | −10 | 12 | −11 | 123 | F(1, 26)= 4.35 | |
BA = Brodman’s Area
ROI = Region of Interest
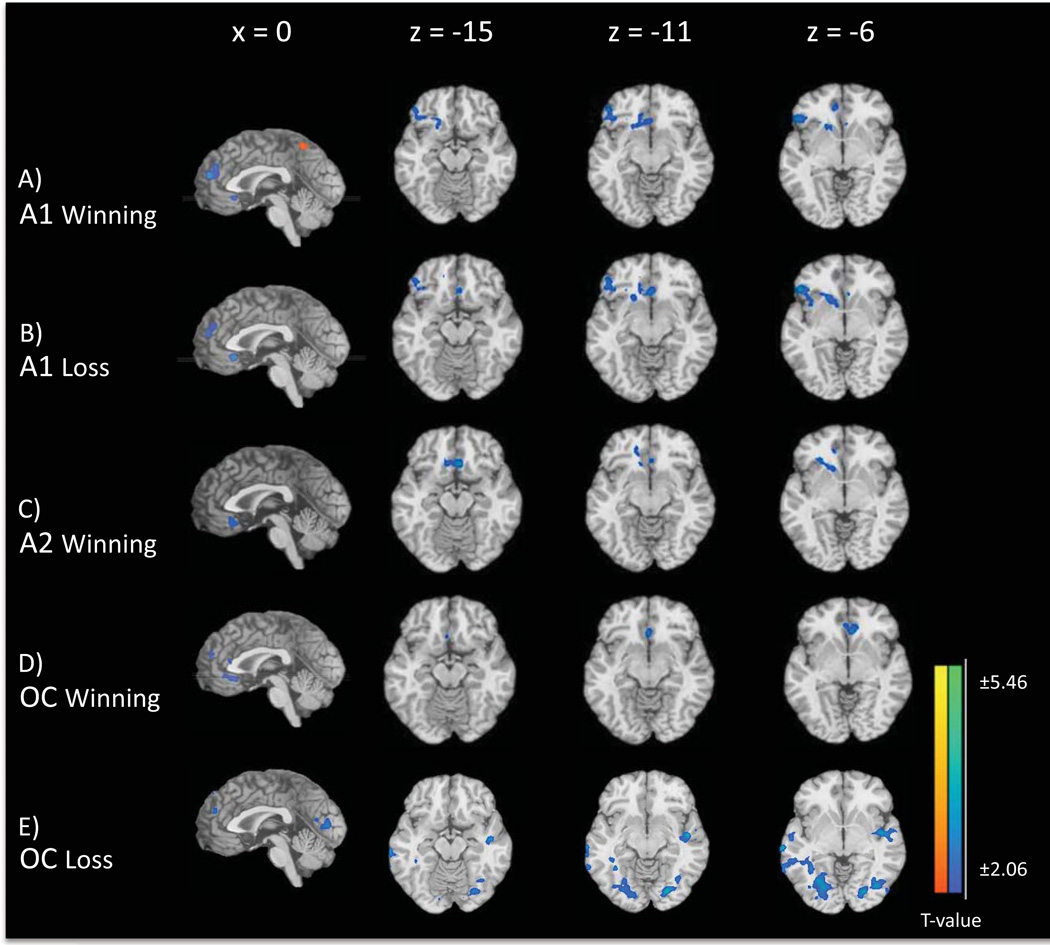
Figure 2. Group Differences on the MIDT in Ventral Fronto-striatal Areas: PGvsCC.
Brain activation maps demonstrate differences in the PG group contrasted with the CC group during the:
a) A1 winning phase, associated with the prospect of monetary wins. Maps depict significant differences in the vmPFC, mPFC and precuneus (x = 0), ventral and lateral PFC (z = −15, −11, −6) and ventral striatum (z = −11, −6);
b) A1 losing phase, associated with the prospect of monetary losses. Maps depict significant differences in the vmPFC and mPFC (x = 0), ventral and lateral PFC (z = −15, −11, −6), ventral striatum (z = −11, −6) and left insula (z= −6).
c) A2 winning phase, associated with the anticipation of winning money. Maps depict significant differences in the vmPFC (x = 0; z = −15, −11, −6) and ventral striatum (z = −11, −6).
d) OC winning phase, associated with the receipt of a monetary reward. Maps depict significant differences in the mPFC and vmPFC (x = 0), vmPFC (z = −15, −11, −6), and ventral striatum (z = −11, −6).
e) OC losing phase, associated with the receipt of a monetary loss. Maps depict significant differences in the middle PFC and the middle occipital gyrus (x = 0, z=−15,−11), superior temporal gyrus (z = −15, −11, −6), middle temporal gyrus (z = −11, −6) and insula (z = −11, −6).
All contrast maps are thresholded at an uncorrected level of p < 0.05 two-tailed and FWE-corrected at p < 0.05 with a cluster threshold of 91. Blue color demonstrates areas where PG subjects show relatively less activation and red color indicates areas where PG subjects show relatively greater activation. For axial slices, the right side of the brain is on the right.
Abbreviations: vmPFC = ventromedial prefrontal cortex; mPFC = medial prefrontal cortex.
A1Loss
Similar between-group differences were observed during the A1Loss phase, associated with the prospect of losing money. Compared to the CC group, the PG group exhibited decreased activity in the left mPFC extending ventrally into the anterior cingulate as well as in the left inferior frontal gyrus, extending to the insula (Table 2). This pattern was also apparent in the left VS, extending to the vmPFC (Figure 2b).
A2Win
During A2Win, between-group differences involved the left vmPFC extending to the VS (Table 2; Figure 2c). This difference involved relatively decreased activity in the PG group.
A2Loss
No significant between-group differences were observed during A2Loss.
OCWin
During OCWin, decreased activity in the PG group was observed in the right vmPFC extending dorsally through the anterior cingulate and medially to the mPFC (Figure 2d). Another between-group difference involved the left posterior cingulate extending ventrally to the hippocampal gyrus (Table 2).
OCLoss
OCLoss was characterized by decreased activation in the PG group in multiple regions (Table 2, Figure 2, Figure S2 in the Supplement). These included the right superior temporal gyrus extending into the insula, right occipital gyrus extending bilaterally into the lingual gyrus, cuneus and posterior cingulate, right superior parietal lobule, and left precentral gyrus, and, within a large cluster, in the left middle temporal gyrus extending into superior temporal and insular areas. Greater decreases in activity in the PG group were observed in the left superior and middle frontal gyri and bilateral mPFC.
ROI Analyses
Multiple one-way ANOVAs examining between-group differences in right VS activity revealed a significant difference during A1Loss [F(1, 26)= 4.91, p < 0.05], A2W [F(1, 26)= 4.72, p < 0.05], and A2L [F(1, 26)= 5.12, p < 0.05]. Between-group differences were observed in the left VS during A2L [F(1, 26)= 4.57, p < 0.05] and OCLoss [F(1, 26)= 4.35, p < 0.05]. Inspection of ANOVAs revealed for all differences relatively decreased activation in the PG group.
Correlations Between Impulsivity and Reward Anticipation
BIS-11 Total and subscale scores are listed in Table 1. To test our hypotheses regarding VS activity and impulsivity, based on prior findings in alcohol dependence, Pearson correlations were calculated between ROI activity during the anticipatory phases and total and subscale scores on the BIS-11. During A2Win in the PG group, left VS activation correlated inversely with BIS-11 Motor subscale scores (r = −.55, p < 0.05) and right VS activation correlated inversely with BIS-11 Total (r = −.63, p < 0.05) and Attention subscale (r = −.76, p < 0.01) scores during the A2Loss phase. There were no other significant correlations between the VS and BIS-11 scores in any other anticipatory phase for either the PG or CC groups (see Table S2 and Figure S1 in the Supplement).
Discussion
Consistent with our hypotheses, PG as compared to CC participants showed diminished VS activation during reward anticipation, diminished vmPFC activation during reward outcome and diminished insula activation during loss outcome. However, these patterns extended to wins and losses, were less phase-specific than hypothesized, and involved additional brain regions. As hypothesized, VS activity during the A2Win phase inversely correlated with impulsivity measures in the PG group. The biological and clinical implications are discussed below with respect to the relevant brain areas.
Between-Group Differences in vmPFC Activation
PG participants demonstrated relatively decreased activity in overlapping vmPFC areas during the initial (prospect) anticipatory phase corresponding with the impending possibility of winning (A1Win) or losing (A1Loss) money, as well as during the second anticipation phase corresponding with the possibility of winning (A2Win; Figure 2a,b,c). Similar between-group differences were observed across winning and loss trials in the A1 phase involving overlapping areas of the mPFC (including vmPFC), VS and left inferior frontal gyrus. These results provide evidence for significantly reduced recruitment of brain areas implicated in coding reward values, reward anticipation, and impulse control (11,12,29,35–37) in PG relative to CC groups. Decreased activity in PG during anticipatory phases suggests alterations in the ability to signal and integrate the short-term value of an incentive cue. These findings have significant implications, as value integration can influence choice; indeed, in healthy populations, vmPFC recruitment during affective judgment is associated with adaptive decision-making (38,39). Therefore, reduced vmPFC recruitment in PG may contribute to less adaptive money-related decision-making.
The vmPFC has been ascribed a role in integrating and updating information of executive processes from dorsolateral PFC areas with affective information from insular and cingulate regions, thereby registering stimulus contingencies that can be used to forecast future consequences (35,40). In the MIDT, increased vmPFC as well as posterior cingulate activity when an expected reward is obtained supports roles for these areas in monitoring monetary outcomes (12). In the present study, decreased activity in the vmPFC and posterior cingulate during the outcome phase of a winning trial in the PG group suggests possible deficits in PG related to tracking reinforcement contingencies. Relatively diminished vmPFC activity in the PG group accompanying anticipation and receipt of wins and losses therefore suggests diminished integration of incentive information that might be used to guide subsequent behavior. This result resonates with findings in PG of perseverative response styles, deficits in decision-making tasks dependent on vmPFC function (41–43), and diminished vmPFC activation in PG during simulated gambling (3), cognitive control (21), gambling stimuli exposure (2) and decision-making (4). Together, results suggest that reduced vmPFC activity is an important neural feature of PG across a range of cognitive processes.
Between-Group Differences in VS Activation
The vmPFC connects directly to the VS, predominantly with the nucleus accumbens, a region heavily implicated in reward processing, particularly as related to changes in affective states and goal-directed behaviors (44–46). The findings of relatively diminished VS responses in PG participants during the anticipation and outcome phases are consistent with findings of reduced VS activity in PG individuals during a simulated gambling/guessing task (3).
Reduced VS activity was observed in all anticipatory phases (A1Win, A1Loss, A2Win and A2Loss) (Figure 2a,b,c; Table 2). Anticipatory processing may involve aspects of prospect and related motivations, anticipation of working for winning or avoiding losing, motoric responses, and anticipation of potential reward/loss. In an effort to model these phases more accurately than in some prior studies, the current experimental design models both A1 and A2 anticipatory phases, with the latter period occurring following motoric response. The behavioral results show no between-group differences in response times or correct hits, suggesting that the group differences in VS activity in A2Win and A2Loss may reflect differences in anticipatory processing rather than motoric demands or performance.
Relatively reduced VS activity in PG during both winning and losing anticipatory phases suggests a hypoactive reward system in response to monetary incentives and potential difficulties in maintaining reward expectations. The VS also contributes to temporal difference learning during aversive processing, whereby deviations of expected outcomes are signaled through striatal activity (47). In the current study, reduced VS response during the losing outcome phase in the PG group may denote that this result was unexpected. Together with the decreased vmPFC activity, further lend support to the idea of a hypo-responsive fronto-striatal system as important to PG.
Between-Group Differences in Insula Activation
Relative to the CC group, PG participants demonstrated decreased anterior insula activity during the A1Loss phase (associated with the prospect of losing money) and during the loss outcome phase (OCLoss). In healthy populations, the representation of aversive value recruits the anterior insula, as does the processing of uncertainty and risky choices (48–50). This area contributes to loss prediction since activity here predicts switching from more to less risky choices during financial risk-taking (23,49). Individuals with insular damage demonstrate increased betting on a gambling task, characterized by higher wagers and failures to adjust betting behavior when probabilities of losing increase (51).
In the current study, relative diminished insula activity in the PG group during the prospect of losing money may relate to altered loss-prediction signaling in this population. In healthy individuals, heightened insular activity during loss anticipation on the MIDT can predict future loss avoidance learning, suggesting that a loss-prediction signal may represent an important marker of adaptive avoidance behavior (52). Increased insular activity, together with ventrolateral prefrontal cortical function, appears to signal changes in the context of varying rewards (53), consistent with insular contributions to integrating homeostatic signals with prior experiences and promoting adaptive choices and decision-making (24,25,54). Therefore, decreased insula activity in the PG group observed during the prospect of loss may indicate diminished anticipatory signaling of information related to predicting and monitoring losses and could result in failures to adjust betting behavior or avoid risks.
Altered interoceptive awareness through blunted insular activity, particularly during the processing of losses, may relate to clinically relevant behavioral and cognitive processes in PG, such as loss-chasing and cognitive distortions involving inflated confidence or illusions of control (55,56). The findings from the present study support a role for altered insula activity in PG populations during loss processing and suggest neural mechanisms that may underlie poor risk estimation in PG. Relative to control participants, diminished insula activity has previously been noted in PG during initial exposure to gambling cues and in an overlapping area in cocaine dependent individuals when viewing cocaine cues (6). Diminished insula activity also has clinical relevance, as activity here during a decision-making task predicts time to relapse in substance-dependent individuals (57). Altogether, the role for the insula in signaling aversive value has led to the proposal of this area as an important therapeutic target in both PG and substance dependence (54,58).
Brain Activation and Impulsivity
Consistent with findings in alcohol dependence, we observed an inverse relationship between VS activity and measures of impulsiveness in the PG group, with whole-brain analyses implicating a broader range of cortico-striatal areas. Analogous to alcohol-dependent individuals, higher BIS-11 Motor subscale scores inversely correlated with VS activity in the PG group during reward anticipation (8). However, in contrast to the alcohol-dependence study, we separately modeled prospect (A1) and anticipation of notification (A2) phases of processing and thus linked the impulsivity finding more specifically to the A2 phase of processing. Another MIDT study separately modeling A1 and A2 phases also found a negative correlation between VS activity and impulsivity during the A2 phase in individuals with a positive family history for alcoholism (18). Our results therefore lend additional support to distinct neural phases associated with the prospect and the anticipation of reward/loss and further demonstrate consistent similarities across at-risk and addicted populations in relationships between impulsivity and VS activity during reward anticipation.
The current study further observed inverse correlations during the A2Loss phase between VS activity and both the BIS-11 total scores and the BIS-11 attention subscale scores, indicating diminished VS-related responsiveness to anticipated loss in association with elevated impulsivity. Notably, all VS correlations occurred during the A2 (rather than the A1) phase, highlighting in PG a specific relationship between impulsivity and VS activity during the anticipation-of-notification (rather than prospect) phase of reward and loss processing. Evidence in non-addicted individuals suggests not only that individual sensitivity to future reward magnitude is proportionally reflected in VS activation, but that increased impulsivity is additionally inversely related to this diminished VS response (11,59). Together, data suggest that reduced VS responsiveness during reward and loss processing in PG may be reflected in elevated impulsivity and may influence decision-making and/or reward-seeking behaviors related to PG.
Whole-brain correlations related impulsivity to other cortico-striatal regions including the vmPFC and insula during anticipatory phases. Interestingly, impulsivity correlated negatively with anterior cingulate activity in both A2Win and A2Loss. As the anterior cingulate contributes to and loss-chasing during gambling (26), the finding suggests that impulsivity may influence excessive gambling through cingulate mechanisms related to reward and loss processing. Future research should further examine these relationships as impulsivity has been related to clinically relevant aspects of PG and its treatment.. In a randomized clinical trial of paroxetine, self-reported impulsivity correlated with problem gambling severity at treatment onset, and changes in impulsivity correlated with changes in problem gambling severity during treatment (61). In an open-label trial of memantine, PG differed from control participants at treatment onset but not at treatment end on a behavioral measure of motor impulsivity, the stop-signal task (62). Thus, impulsivity may represent an important treatment target for PG. Given the relationship between impulsivity and VS activation during reward and loss processing, drugs that influence ventral striatal function and have data supporting efficacy in PG (e.g., opioid antagonists like naltrexone and nalmefene and glutamatergic agents like n-acetyl cysteine (63–65)) may be exerting their influences through decreasing impulsivity and normalizing VS function. This hypothesis warrants direct examination, particularly given the broader range of cortico-striatal associations with impulsivity in PG.
Strengths, Limitations & Future Directions
Previous studies examining monetary reward processes in PG have not parsed specific phases of processing that may differentially contribute to PG and clinically relevant aspects thereof. The current fMRI study is the first in PG to investigate distinct phases of reward and loss processing relating to prospect, anticipation and notification. Moreover, relative to research in substance dependence in which brain changes may be attributable to the effects of a drug, the use of a PG population provides complementary information.
While this study incorporated both men and women, it is nonetheless limited by a sample size that does not permit examination of gender-related differences. Another drawback is the slightly greater number of smokers in the PG sample and the inclusion in the PG group of people with past psychiatric illnesses. Given the frequencies of comorbid psychiatric conditions in PG, particularly smoking (66), the current sample is representative of the general PG population. However, future studies should examine directly the influences of specific co-occurring disorders.
Although the findings were less phase-specific than originally hypothesized, the brain areas in which differences were observed represent predominant projection areas of the mesocorticolimbic dopamine system, which is consistent dopamine’s role in reward processing (67,68). While fMRI cannot relate activity changes to specific neurotransmitters, recent conjoint fMRI and Positron Emission Tomography (PET) studies identified increased dopaminergic activity in prefrontal cortical areas as individuals anticipate and receive monetary rewards (69). Therefore, our findings of diminished activity in corticostriatal-limbic areas may reflect differences in dopaminergic function, particularly as alterations in striatal dopamine functioning have been reported in PET studies of both PG and substance dependence (70–73). Reward and error prediction signaling in the VS and orbitofrontal cortex are attenuated by alterations in dopamine transmission (74); consequently, this neurotransmitter’s effect on neuronal processing may impact an individual’s ability to attribute value to cues, anticipate events and learn from negative feedback. Future direct investigation of the relationships between the neural correlates of reward and loss processing as they related to dopamine and other neurotransmitter function in PG is needed.
Understanding how the brain appraises incentive value further represents a fundamental parameter for decision-making processes. The idea that adaptive decision-making is promoted through activation of somatic and visceral states previously associated with advantageous choices has spurred research into identifying the neural substrates of affective signaling (15,75,76). For example, the vmPFC and insula have been ascribed roles in representing somatic and visceral states, particularly as they relate to negative arousal, with increased activity during negative or uncertain incentives (15,76,77). In linking the current body state with previously experienced outcomes, these brain areas may provide anticipatory signals to guide risky decision-making (24,25,54,76). However, as the MIDT does not investigate choice, future research examining choice with respect to reward processing in PG is needed. Given the correlations between VS activity and impulsivity in PG without significant between-group differences in self-reported impulsivity or task performance, future experiments could more closely examine this relationship (e.g., using larger samples and/or behavioral measures of impulsivity). To better understand interoceptive states associated with gambling, future studies should use integrative approaches, including subjective, physiological, neural and behavioral measures, in order to gauge homeostatic changes in PG. Additionally, such measures should be examined with respect to treatment outcome, and include both self-report and behavioral measures, as these may differentially relate to addictive behaviors and their treatment (20,78).
Conclusions
The current study compared neural responses in anticipation of monetary rewards and punishments using a modified MIDT that parses prospect, anticipation and outcome phases. Although the findings were less phase-specific than originally hypothesized, our findings demonstrate that during prospect and anticipation phases involving potential wins and losses, individuals with PG exhibit hypoactivity in neurocircuitry coding for the incentive value of stimuli. Specifically, this group shows a similar pattern of diminished VS and vmPFC responding during both winning and losing phases. Consistent with studies of alcohol dependence, we observed an inverse association during reward anticipation between impulsivity and VS activity in PG. These data provide evidence for similar alterations in neurocircuitry mediating anticipatory processing in both PG and substance addictions, and that impulsivity may be similarly involved in these relationships. Treatment development efforts for PG might target normalizing activity in mesocorticolimbic neurocircuitry as related to impulsive thoughts and behaviors.
Supplementary Material
Acknowledgments
We gratefully acknowledge Jennifer Bellegarde, Mike Bernabeo, Scott Bullock, Cameron DeLeone, Stephen Healy, Jessica Montoya, Naaila Panjwani, Monica Solorzano, Jocelyn Topf and Katie VanBuskirk for their help with the project. Support was provided by the following Grants: National Institutes of Health grants R01-DA019039, P20-DA027844, P50-AA012870, R01-DA020908, R01-DA020709, R01-AA016599, RL1-AA017539, K12-DA00167, the Veterans Integrated Service Network 1 Mental Illness Research, Education, and Clinical Center (MIRECC), and a Center of Excellence in Gambling Research Grant from the National Center for Responsible Gaming and its Institute for Research on Gambling Disorders. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Responsible Gaming or the Institute for Research on Gambling Disorders or any of the other funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr Potenza has received financial support or compensation for the following: consults for and is an advisor to Boehringer Ingelheim; has consulted for and has financial interests in Somaxon; has received research support from the National Institutes of Health, Veteran’s Administration, Mohegan Sun Casino, the National Center for Responsible Gaming and its affiliated Institute for Research on Gambling Disorders, and Forest Laboratories, Psyadon, Ortho-McNeil, Oy-Control/Biotie and Glaxo-SmithKline pharmaceuticals; has participated in surveys, mailings or telephone consultations related to drug addiction, impulse control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse control disorders; provides clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has given academic lectures in grand rounds, CME events and other clinical or scientific venues; and has generated books or book chapters for publishers of mental health texts. All other authors report that they have no biomedical financial interests or potential conflicts of interest with respect to the content of this manuscript.
References
- 1.Holden C. Psychiatry. Behavioral addictions debut in proposed DSM-V. Science. 2010;327:935. doi: 10.1126/science.327.5968.935. [DOI] [PubMed] [Google Scholar]
- 2.Potenza MN, Steinberg MA, Skudlarski P, Fulbright RK, Lacadie CM, Wilber MK, et al. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2003b;60:828–836. doi: 10.1001/archpsyc.60.8.828. [DOI] [PubMed] [Google Scholar]
- 3.Reuter J, Raedler T, Rose M, Hand I, Glascher J, Buchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potenza MN. Review. The neurobiology of pathological gambling and drug addiction: an overview and new findings. Philos Trans R Soc Lond B Biol Sci. 2008;363:3181–3189. doi: 10.1098/rstb.2008.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frascella J, Potenza MN, Brown LL, Childress AR. Shared brain vulnerabilities open the way for nonsubstance addictions: carving addiction at a new joint? Ann N Y Acad Sci. 1187:294–315. doi: 10.1111/j.1749-6632.2009.05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 9.Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biol Psychiatry. 2005;58:787–795. doi: 10.1016/j.biopsych.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 11.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 13.Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- 14.McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- 15.Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 17.Moeller F, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 18.Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, et al. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–683. doi: 10.1016/j.biopsych.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Potenza MN, Sofuoglu M, Carroll KM, Rounsaville BJ. Neuroscience of behavioral and pharmacological treatments for addictions. Neuron. 2011;69:695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, et al. An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry. 2003a;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. [DOI] [PubMed] [Google Scholar]
- 22.Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Mov Disord. 25:1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 25.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 26.Campbell-Meiklejohn DK, Woolrich MW, Passingham RE, Rogers RD. Knowing when to stop: the brain mechanisms of chasing losses. Biol Psychiatry. 2008;63:293–300. doi: 10.1016/j.biopsych.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MG, Holroyd CB, et al. Hypersensitivity to reward in problem gamblers. Biol Psychiatry. 2010;67:781–783. doi: 10.1016/j.biopsych.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 30.Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 31.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, et al. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 107:14811–14816. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wager TD, Keller MC, Lacey SC, Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. Neuroimage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 35.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 36.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 37.Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127:1108–1126. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- 38.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Northoff G, Grimm S, Boeker H, Schmidt C, Bermpohl F, Heinzel A, et al. Affective judgment and beneficial decision making: ventromedial prefrontal activity correlates with performance in the Iowa Gambling Task. Hum Brain Mapp. 2006;27:572–587. doi: 10.1002/hbm.20202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cavedini P, Riboldi G, Keller R, D'Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biol Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- 42.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res. 2005;23:137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: a comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101:534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 44.Carlezon WA, Jr, Wise RA. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 47.Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc Lond B Biol Sci. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 49.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 50.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–531. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 51.Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson A, Grant JE, Kim SW, Odlaug BL, Gotestam KG. Risk factors for problematic gambling: a critical literature review. J Gambl Stud. 2009;25:67–92. doi: 10.1007/s10899-008-9088-6. [DOI] [PubMed] [Google Scholar]
- 56.Sacco P, Torres LR, Cunningham-Williams RM, Woods C, Unick GJ. Differential Item Functioning of Pathological Gambling Criteria: An Examination of Gender, Race/Ethnicity, and Age. J Gambl Stud. 2011 doi: 10.1007/s10899-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 58.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45:143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanco C, Potenza MN, Kim SW, Ibanez A, Zaninelli R, Saiz-Ruiz J, et al. A pilot study of impulsivity and compulsivity in pathological gambling. Psychiatry Res. 2009;167:161–168. doi: 10.1016/j.psychres.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant JE, Chamberlain SR, Odlaug BL, Potenza MN, Kim SW. Memantine shows promise in reducing gambling severity and cognitive inflexibility in pathological gambling: a pilot study. Psychopharmacology (Berl) 2010;212:603–612. doi: 10.1007/s00213-010-1994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grant JE, Kim SW, Hollander E, Potenza MN. Predicting response to opiate antagonists and placebo in the treatment of pathological gambling. Psychopharmacology (Berl) 2008;200:521–527. doi: 10.1007/s00213-008-1235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62:652–657. doi: 10.1016/j.biopsych.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 65.Wareham JD, Potenza MN. Pathological gambling and substance use disorders. Am J Drug Alcohol Abuse. 2010;36:242–247. doi: 10.3109/00952991003721118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, et al. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 68.Robbins TW. Chemical neuromodulation of frontal-executive functions in humans and other animals. Exp Brain Res. 2000;133:130–138. doi: 10.1007/s002210000407. [DOI] [PubMed] [Google Scholar]
- 69.Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF. Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A. 2008;105:15106–15111. doi: 10.1073/pnas.0802127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Linnet J, Moller A, Peterson E, Gjedde A, Doudet D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction. 2010a;106:383–390. doi: 10.1111/j.1360-0443.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- 71.Linnet J, Peterson E, Doudet DJ, Gjedde A, Moller A. Dopamine release in ventral striatum of pathological gamblers losing money. Acta Psychiatr Scand. 2010b;122:326–333. doi: 10.1111/j.1600-0447.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- 72.Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132:1376–1385. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 74.van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease? Neuropsychopharmacology. 2009;34:2758–2766. doi: 10.1038/sj.npp.npp2009124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 76.Damasio AR. Descartes'Error. New York, NY: Grosset/Putnam, GP: Putnam's Sons; 1994. [Google Scholar]
- 77.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 78.Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88:79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.