Abstract
Mitoxantrone has been approved by the FDA for the treatment of multiple sclerosis (MS). However, the mechanisms by which mitoxantrone modulates MS are largely unknown. Activated astrocytes produce nitric oxide (NO), TNF-α, and IL-1β, molecules which can be toxic to central nervous system (CNS) cells including oligodendrocytes, thus potentially contributing to the pathology associated with MS. MCP-1 is a chemokine believed to modulate the migration of monocytes to inflammatory lesions present in the CNS of MS patients. IL-12 and IL-23 have been demonstrated to play critical roles in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), an animal model of MS, by contributing to the development of CD4+ T cell lineages termed Th1 and Th17, respectively. The current study demonstrates that mitoxantrone inhibits lipopolysachharide (LPS) induction of NO, TNF-α, IL-1β, and MCP-1 production by primary astrocytes. Mitoxantrone also inhibited IL-12 and IL-23 production by these cells. Furthermore, mitoxantrone suppressed the expression of C-reactive protein (CRP). Finally, we demonstrate that mitoxantrone suppressed LPS induction of NF-κB DNA-binding activity, suggesting a novel mechanism by which mitoxantrone suppresses the expression of proinflammatory molecules. Collectively, these studies demonstrate that mitoxantrone represses astrocyte production of potentially cytotoxic molecules, as well as molecules capable of altering T-cell phenotype. These in vitro studies suggest mechanisms by which mitoxantrone may modulate inflammatory diseases including MS.
Keywords: Mitoxantrone, Astrocyte, Nitric oxide, cytokine, chemokine, T cell
1. Introduction
Multiple sclerosis (MS) is characterized by inflammation of the central nervous system (CNS) and demyelination. MS is believed to be an autoimmune disorder mediated by autoreactive T cells specific for myelin antigens (Martin and McFarland, 1995). In addition to T cells, chronically activated astrocytes are believed to contribute to the pathology associated with MS (De Keyser et al., 2003; Holley et al., 2003; Zeinstra et al., 2003).
Astrocytes are resident CNS cells that protect other neuronal cells through the production of trophic factors and through the uptake of neurotoxic molecules including glutamate. In response to pathogens and pro-inflammatory cytokines, astrocytes may become activated resulting in the production of nitric oxide (NO), the cytokines including TNF-α, IL-1β, IL-6, and the chemokine MCP-1 (Dong and Benveniste, 2001; Farina et al., 2007). These astrocyte products play an important role in clearing pathogens (Dong and Benveniste, 2001; Farina et al., 2007). However, when chronically activated, as is believed to occur in MS, these same molecules can be toxic to host neural cells including myelin producing oligodendrocytes and neurons (Raine, 1997; Trapp et al., 1998).
Autoreactive CD4+ T cells are believed to modulate the development of EAE. Classically, CD4+ T cells have been separated into two subtypes termed Th1 cells which are believed to contribute to the development of EAE and Th2 cells which protect against disease (Sospedra and Martin, 2005). The cytokine IL-12 is believed to play an essential role in differentiation of Th1 cells. Recently, we and others have demonstrated that a novel subset of CD4+ T cells termed Th17 cells, which are distinct from Th1 cells, play a critical role in the development of EAE, and that IL-23 plays a critical role in the development of Th17 cells (Langrish et al., 2005; Petermann and Korn; Solt et al.; Xu et al., 2009).
Mitoxantrone is an anti-neoplastic agent which has been used since the 1970s for the treatment of leukemia and other forms of cancer. Mitoxantrone is a DNA topoisomerase II inhibitor (Koeller and Eble, 1988). As such, mitoxantrone is believed to suppress leukemia by blocking DNA synthesis and inhibiting cell cycle progression (Fox and Smith, 1990; Nathanson, 1984; Osheroff et al., 1994). More recently, mitoxantrone has been used in the treatment of MS. However, the mechanisms by which mitoxantrone alters the pathology associated with MS are largely unknown. The current studies were designed to determine the effect of mitoxantrone on astrocyte function, as these cells are capable of regulating MS pathology.
2. Results
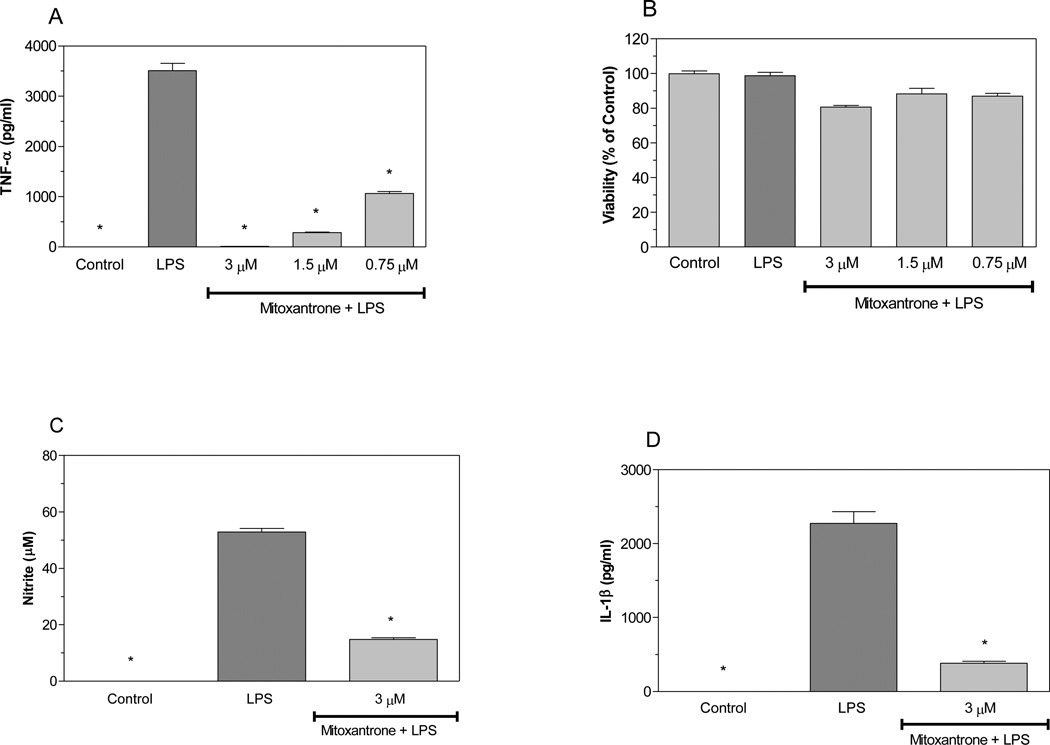
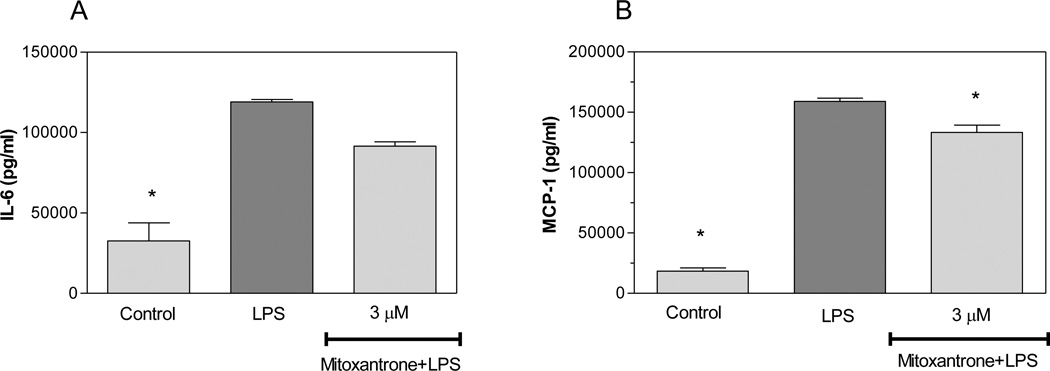
Activated astrocytes are believed to contribute to the pathology of MS by producing cytotoxic molecules including TNF-α, IL-1β, and NO. We evaluated the effects of mitoxantrone on production of these pro-inflammatory molecules by astrocytes. LPS increased the production of TNF-α by primary astrocytes as determined by ELISA (Pharmingen, San Diego, CA). Interestingly, mitoxantrone inhibited LPS induction of TNF-α production by astrocytes in a dose-dependent manner (Figure 1A). Mitoxantrone was not toxic to astrocytes at the concentrations used in these studies as determined by MTT assays (Figure 1 B). Mitoxantrone also inhibited LPS induction of NO (Figure 1C) and IL-1β (Figure 1D). Collectively, these studies suggest that mitoxantrone inhibits the production of molecules potentially toxic to CNS cells including those which are compromised in MS including oligodendrocytes and neurons. IL-6 plays a role in a variety of immune responses including a role in the differentiation of Th17 cells known to contribute to EAE and MS. MCP-1 is chemotactic to monocytic cells and believed to regulate the migration of peripheral monocytic cells into the CNS as well as CNS microglia migration to sites of inflammatory lesions in EAE mice. The current studies indicate that mitoxantrone slightly suppressed LPS induction of IL-6 expression by astrocytes, but that this suppression did not reach statistical significance (Figure 2A). Mitoxantrone did however suppress LPS induction of MCP-1 expression by these cells (Figure 2B), suggesting that mitoxantrone may act in part by inhibiting migration of monocytic cells to sites of inflammation.
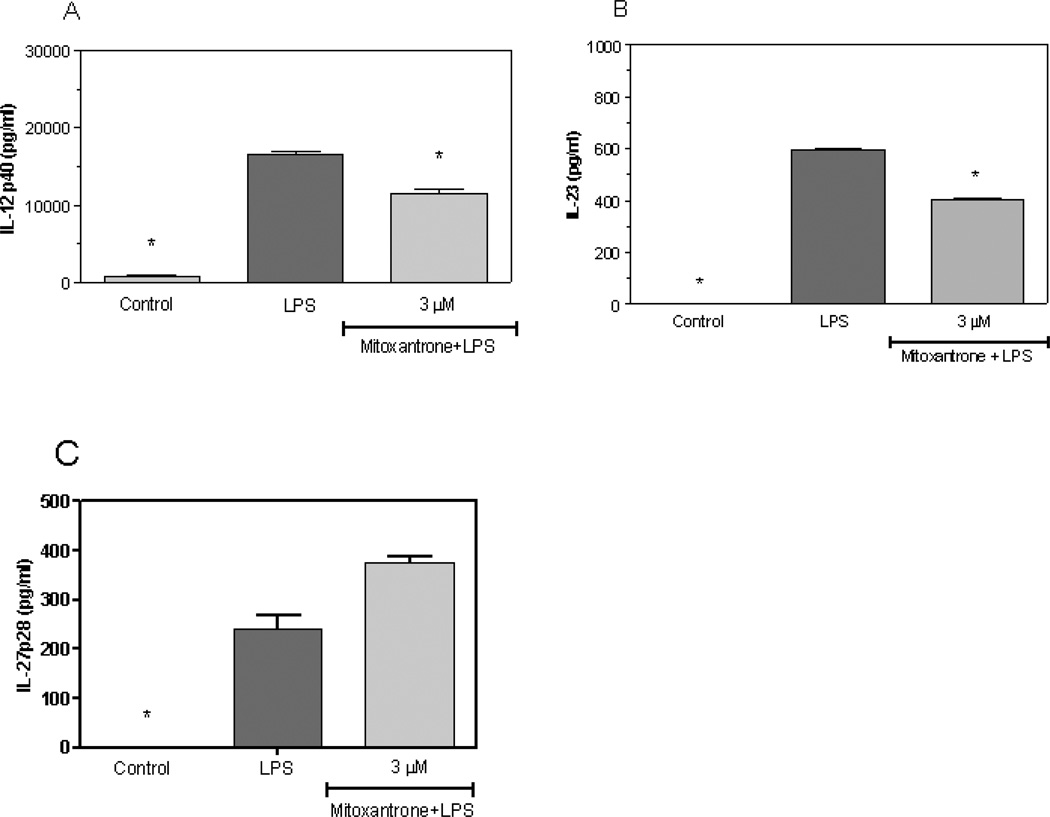
1.1.1 IL-12 and IL-23 are critical to the development of Th1 and Th17 cells, respectively, which stimulate the development of EAE. Our current studies demonstrate that LPS induces IL-12 (Figure 3A) and IL-23 (Figure 3B) expression by primary astrocytes, and mitoxantrone blocks this induction. However, mitoxantrone did not alter the expression of a third member of the IL-12 family of proteins, IL-27 (Figure 3C), suggesting a specificity of action by mitoxantrone. These studies suggest that mitoxantrone may block the development of EAE and MS, at least in part, by suppressing IL-12 and IL-23 production by astrocytes resulting in decreased production of encephalitogenic Th1 and Th17 cells.
Figure 1.
Mitoxantrone inhibits LPS induction of pro-inflammatory molecules by primary astrocytes. Cells were pre-treated for 1h with the indicated concentrations of mitoxantrone. LPS (2 µg/ml) was added as indicated, and 24h later the concentration of TNF-α in the culture medium was determined by ELISA (A). Cell viability was determined by MTT assay (B). The concentration of nitrite in the culture was determined by Greiss reaction (C). The concentration of IL-1β (D) in the culture supernatant was determined by ELISA. Values represent the mean +/− s.e.m. for triplicate cultures. *p<0.001 vs. LPS treated cultures. Data were analyzed by ANOVA followed by a Bonferroni test to determine the significance of difference. The experiment was performed three times independently.
Figure 2.
Mitoxantrone inhibits LPS induction of MCP-1 by primary astrocytes. Cells were pre-treated for 1h with the indicated concentration of mitoxantrone. LPS (2 µg/ml) was added as indicated, and 24h later the concentration of IL-6 (A) and MCP-1 (B) in the culture medium was determined by ELISA. Values represent the mean +/− s.e.m. for triplicate cultures. *p<0.001 vs. LPS treated cultures. Data were analyzed by ANOVA followed by a Bonferroni test to determine the significance of difference. The experiment was performed three times independently.
Figure 3.
Mitoxantrone inhibits LPS induction of IL-12 and Il-23 by primary astrocytes. Cells were pre-treated for 1h with the indicated concentration of mitoxantrone. LPS (2 µg/ml) was added as indicated, and 24h later the concentration of IL-12 p40 (A), IL-23 (B), and IL-27 p28 in the culture medium was determined by ELISA. Values represent the mean +/− s.e.m. for triplicate cultures. *p<0.001 vs. LPS treated cultures. Data were analyzed by ANOVA followed by a Bonferroni test to determine the significance of difference. The experiment was performed three times independently.
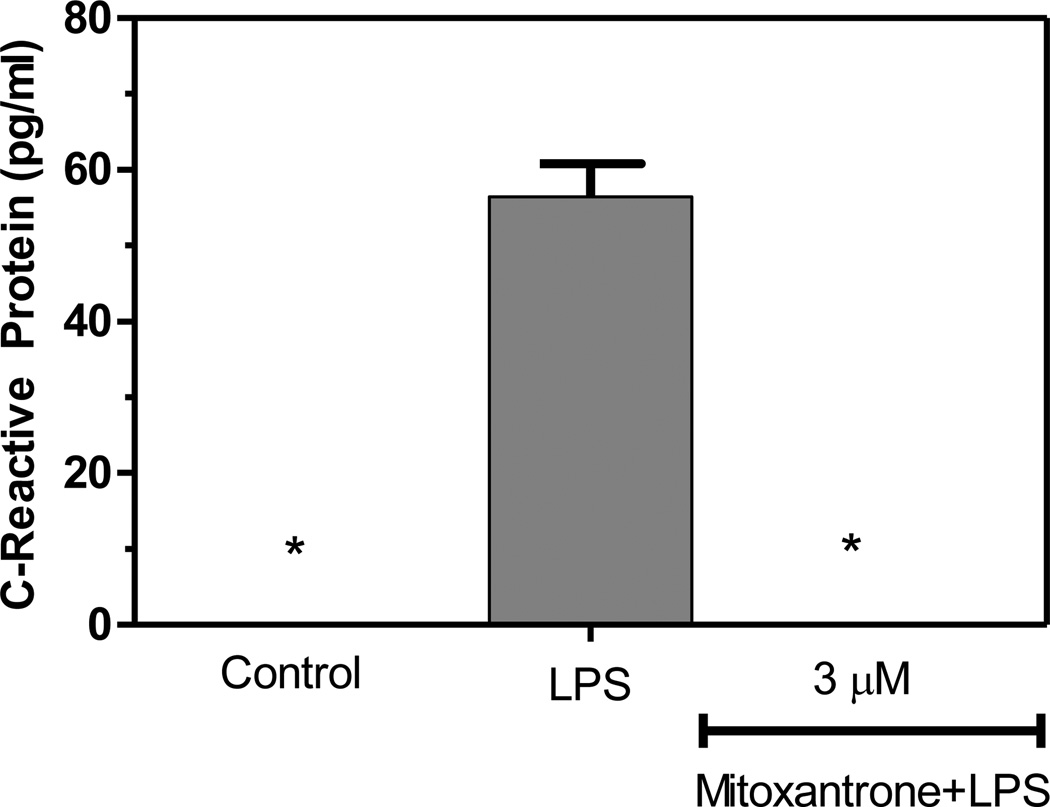
CRP is a generalized marker for inflammation, and elevated serum levels of CRP have been extensively studied in relation to cardiopathology. However, elevated levels of CRP have more recently been linked to CNS pathologies characterized by CNS inflammation including Alzheimer’s disease (AD) and Parkinson’s disease (PD). We demonstrate for the first time that LPS induces the expression of CRP by astrocytes, and that mitoxantrone blocks this induction (Figure 4).
Figure 4.
Mitoxantrone inhibits LPS induction of C-reactive protein by primary astrocytes. Cells were pre-treated for 1h with the indicated concentration of mitoxantrone. LPS (2 µg/ml) was added as indicated, and 24h later the concentration of CRP in the culture medium was determined by ELISA. Values represent the mean +/− s.e.m. for triplicate cultures. *p<0.001 vs. LPS treated cultures. Data were analyzed by ANOVA followed by a Bonferroni test to determine the significance of difference. The experiment was performed three times independently.
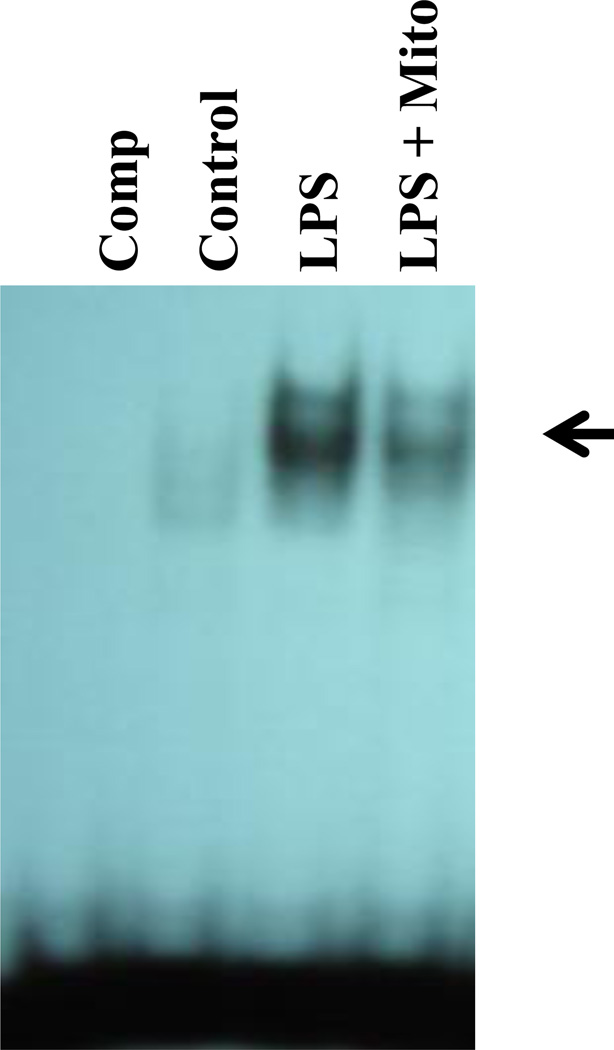
The transcription factor NF-κB is critical to the activation of a variety of genes encoding proinflammatory molecules including NO, as well as cytokines and chemokines. We demonstrate that mitoxantrone inhibits LPS induction of NF-κB DNA-binding activity in primary astrocytes (Figure 5). This suggests a novel mechanism by which mitoxantrone blocks the production of proinflammatory molecules by astrocytes.
Figure 5.
Mitoxantrone inhibits NF-κB DNA-binding activity. Nuclear extracts were prepared from untreated primary astrocytes (Control), astrocytes treated with 2 µg/ml LPS (LPS) or astrocytes pre-treated for 1h with 3 µM mitoxantrone followed by an additional treatment for 4h with 2 µg/ml LPS (LPS + Mito). An excess of cold competitor was added to some EMSA assays to demonstrate the specificity of binding (Comp). Specific NF-κB bands are indicated by the arrow. The experiment was performed three times independently.
3. Discussion
Mitoxantrone inhibits the proliferation of lymphocytes including T cells and B cells, and alters cytokine production by these cells (Neuhaus et al., 2004). This agent also suppresses the development of EAE (Levine and Saltzman, 1986; Lublin et al., 1987; Ridge et al., 1985) and is currently used in the treatment of MS. However, relatively little is known concerning the mechanisms by which mitoxantrone suppresses EAE and MS, and the effect of mitoxantrone on resident cells of the CNS including astrocytes has not been investigated. Our current studies indicate that mitoxantrone suppressing LPS induction of NO, TNF-α, and IL-1β by primary astrocytes. The chronic presence of these molecules contributes to oligodendrocyte death and associated demyelination and axonal transection common to EAE and MS. Our studies also demonstrate that mitoxantrone suppresses the expression of the chemokine MCP-1 which is believed to modulate the migration of monocytic cells to sites of inflammation in EAE and MS. Furthermore, our studies indicate that mitoxantrone suppresses astrocyte expression of CRP. Since CRP expression has been linked to AD and PD (Holmes et al., 2009; Song et al., 2011), this suggests that CRP may also play a role in modulating MS. Thus, our studies collectively suggest that mitoxantrone acts in part in suppressing MS by suppressing astrocyte production of these proinflammatory molecules.
The role of Th1 cells in modulating EAE is well established. It is now clear that Th17 cells also contribute to the development of EAE (Petermann and Korn; Solt et al.). This is based on early seminal studies which indicate that IL-6 in combination with TGF-β stimulates the differentiation of Th17 cells, and IL-23 is required for the maintenance and stability of differentiated Th17 cells (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006). Previously, we demonstrated that primary astrocytes are capable of producing IL-23 (Xu and Drew, 2007; Xu et al., 2007). Our current studies indicate that mitoxantrone suppresses IL-23 production by astrocytes. This suggests that mitoxantrone suppression of IL-23 expression by astrocytes may block development of Th17 cells resulting in suppression of EAE and MS. Collectively, our studies suggest that mitoxantrone may suppress EAE and MS, at least in part, by altering astrocyte function.
4. Experimental Procedure
4.1 Preparation of primary mouse astrocyte cultures
Primary astrocyte cultures were obtained through a modification of the McCarthy and deVellis protocol (McCarthy and de Vellis, 1980). Briefly, cerebral cortices from 1–2 day-old C57BL/6 mice were excised, meninges removed, and cortices minced into small pieces. Cells were separated by trypsinization followed by trituration of cortical tissue. Cells were plated into tissue culture flasks and allowed to grow to confluence (approximately 10 days) in DMEM media containing 10% FBS, 1.4mM glutamine, and OPI media supplement (Sigma, St. Louis, MO). Flasks were shaken overnight (200 rpm at 37°C) in a temperature controlled shaker to loosen microglia and oligodendrocytes from the more adherent astrocytes. The mitotic inhibitor L-leucine methyl ester (LME) (0.1 mM) was added to the cultures to eliminate any residual microglia. Using this procedure, astrocyte cultures of greater than 95% purity were obtained as determined by immunohistochemistry with antibodies prepared against GFAP for astrocytes and the lectin, Griffonia simplicifolia (GSA) to measure contaminating microglia. Astrocytes were seeded into 96-well plates. The following day, cultures were treated for 1 h with mitoxantrone, followed by treatment for 24 h with lipopolysaccharide (LPS), as indicated in the figure legends.
4.2 Analysis of astrocyte cell viability, NO and ELISA
Following treatment, astrocyte cell viability was determined by MTT analysis, nitrite levels produced by astrocytes was determined by Greiss assay, and cytokine, chemokine, and CRP production was determined by ELISA assays as we have described previously (Xu and Drew, 2007).
4.3 Electrophoretic Gel Mobility Shift Assay
Electrophoretic gel mobility shift assays (EMSA) were performed as we have described previously (Barger et al., 2000).
Highlights.
Mitoxantrone is used in the treatment of multiple sclerosis (MS).
Chronically activated astrocytes are believed to contribute to MS pathology.
Mitoxantrone blocks astrocyte production of molecules which contribute to MS.
Mitoxantrone effects on astrocytes may alter production of T cells that trigger MS.
Acknowledgements
This work was supported by grants NS047546 and RR16460 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barger SW, Chavis JA, Drew PD. Dehydroepiandrosterone inhibits microglial nitric oxide production in a stimulus-specific manner. J Neurosci Res. 2000;62:503–509. doi: 10.1002/1097-4547(20001115)62:4<503::AID-JNR4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Zeinstra E, Frohman E. Are astrocytes central players in the pathophysiology of multiple sclerosis? Arch Neurol. 2003;60:132–136. doi: 10.1001/archneur.60.1.132. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Fox ME, Smith PJ. Long-term inhibition of DNA synthesis and the persistence of trapped topoisomerase II complexes in determining the toxicity of the antitumor DNA intercalators mAMSA and mitoxantrone. Cancer Res. 1990;50:5813–5818. [PubMed] [Google Scholar]
- Holley JE, et al. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathol Appl Neurobiol. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller J, Eble M. Mitoxantrone: a novel anthracycline derivative. Clin Pharm. 1988;7:574–581. [PubMed] [Google Scholar]
- Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Saltzman A. Regional suppression, therapy after onset and prevention of relapses in experimental allergic encephalomyelitis by mitoxantrone. J Neuroimmunol. 1986;13:175–181. doi: 10.1016/0165-5728(86)90063-9. [DOI] [PubMed] [Google Scholar]
- Lublin FD, et al. Suppression of acute and relapsing experimental allergic encephalomyelitis with mitoxantrone. Clin Immunol Immunopathol. 1987;45:122–128. doi: 10.1016/0090-1229(87)90118-8. [DOI] [PubMed] [Google Scholar]
- Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Martin R, McFarland HF. Immunological aspects of experimental allergic encephalomyelitis and multiple sclerosis. Crit Rev Clin Lab Sci. 1995;32:121–182. doi: 10.3109/10408369509084683. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson L. Mitoxantrone. Cancer Treat Rev. 1984;11:289–293. doi: 10.1016/0305-7372(84)90025-2. [DOI] [PubMed] [Google Scholar]
- Neuhaus O, Kieseier BC, Hartung HP. Mechanisms of mitoxantrone in multiple sclerosis--what is known? J Neurol Sci. 2004;223:25–27. doi: 10.1016/j.jns.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Osheroff N, Corbett AH, Robinson MJ. Mechanism of action of topoisomerase II-targeted antineoplastic drugs. Adv Pharmacol. 1994;29B:105–126. doi: 10.1016/s1054-3589(08)61134-5. [DOI] [PubMed] [Google Scholar]
- Petermann F, Korn T. Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett. 585:3747–3757. doi: 10.1016/j.febslet.2011.03.064. [DOI] [PubMed] [Google Scholar]
- Raine CS. The Norton Lecture: a review of the oligodendrocyte in the multiple sclerosis lesion. J Neuroimmunol. 1997;77:135–152. doi: 10.1016/s0165-5728(97)00073-8. [DOI] [PubMed] [Google Scholar]
- Ridge SC, et al. Suppression of experimental allergic encephalomyelitis by mitoxantrone. Clin Immunol Immunopathol. 1985;35:35–42. doi: 10.1016/0090-1229(85)90075-3. [DOI] [PubMed] [Google Scholar]
- Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IU, et al. Association between high-sensitivity C-reactive protein and risk of early idiopathic Parkinson's disease. Neurol Sci. 2011;32:31–34. doi: 10.1007/s10072-010-0335-0. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Trapp BD, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-alpha agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, et al. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeinstra E, Wilczak N, De Keyser J. Reactive astrocytes in chronic active lesions of multiple sclerosis express co-stimulatory molecules B7-1 and B7-2. J Neuroimmunol. 2003;135:166–171. doi: 10.1016/s0165-5728(02)00462-9. [DOI] [PubMed] [Google Scholar]