Abstract
Gene transfer technologies enable the controlled, targeted and sustained expression of gene products at precise anatomical locations, such as the joint. In this way, they offer the potential for more-effective, less-expensive treatments of joint diseases with fewer extra-articular adverse effects. A large body of preclinical data confirms the utility of intra-articular gene therapy in animal models of rheumatoid arthritis and osteoarthritis. However, relatively few clinical trials have been conducted, only one of which has completed phase II. This article summarizes the status in 2010 of the clinical development of gene therapy for arthritis, identifies certain constraints to progress and suggests possible solutions.
Introduction
Arthritis gene therapy has been discussed for nearly 20 years,1 and a large body of impressive preclinical safety and efficacy data has been generated. However, there have been few clinical trials of this therapeutic strategy. As discussed in this article, the main impediments to progress are not so much scientific and technological as financial and socio logical. Only one phase II study has been completed2 and a gene-based therapy for arthritis is unlikely to become clinically available in the near future. Despite this slow progress, gene therapy holds promise to fulfill unmet needs in the treatment of arthritis and other joint diseases. This article takes stock of the field and examines the issues restraining the clinical development of arthritis gene therapy.
Arthritis gene therapy: the basics
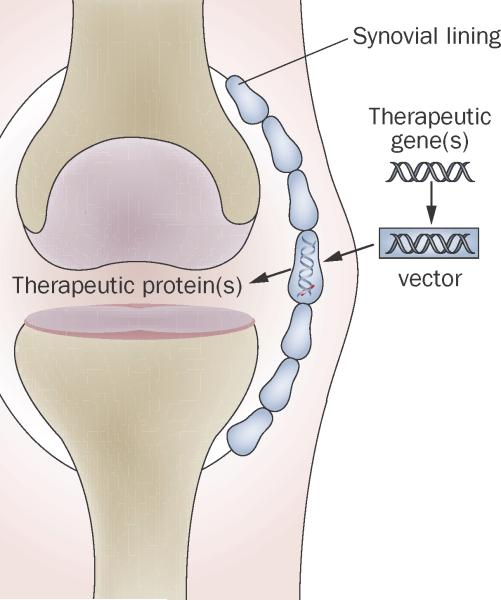
As extensively detailed in previous reviews,3,4 several strategies can be used to treat arthritis with genes (Box 1). Only local, intra-articular gene therapy, however, has entered clinical trials. As no other gene-based strat egies are likely to do so in the near future, this article focuses on gene delivery to joints. The concept of this approach is simple (Figure 1). Therapeutic complementary DNA (cDNA) is incorporated into a vector, which is used to deliver the transgene by direct intra-articular injection (in vivo delivery) or by modifying cells that are subsequently introduced into the joint (ex vivo delivery). Because the dense matrix of cartilage excludes most vectors, the major sites of genetic modification and transgene expression are usually cells within the synovium and intra-articular ligaments,5 although there is evidence that adeno-associated virus (AAV) could be small enough to transduce chondrocytes in situ.6
Figure 1.
Basic concept behind local, intra-articular gene therapy for arthritis. Complementary DNA encoding an anti-arthritic product, typically a secreted protein, is introduced into the joint. Cells within the synovium become transduced and synthesize the encoded transgene endogenously in a sustained fashion. Adapted from Future Rheumatology (2006) 1(2), 173–178 with permission of Future Medicine Ltd.
As a result of gene delivery, therapeutic gene products are synthesized locally within individual joints for an extended period of time. This capability is, at present, unique to gene transfer-based therapy. Additional advantages include a dramatic reduction in the risk of extra-articular adverse effects and a reduced frequency of dosing which, as well as being better for the patient, reduces costs, given that less of the therapeutic agent is required.
Although numerous different transgenes have been successfully used to treat animal models of arthritis,3,4 most attention has focused on cDNAs that encode secreted anti-arthritic proteins because they do not necessarily require highly efficient gene transfer to articular cells. A variety of cyto kines, cytokine antagonists, anti-inflammatory agents and immuno modulatory proteins have been used for this purpose in animal models of rheumatoid arthritis (RA). In animal models of osteoarthritis (OA), treatment has tended to focus on cDNAs encoding anti-inflammatory proteins and cartilage growth factors. An additional therapeutic strategy for RA is the use of gene transfer to ablate the synovium and achieve a genetic synovectomy. As well as using cDNAs, the products of which promote apoptosis,7 the synovectomy approach has followed the lead of certain cancer gene therapy strategies where the transgene encodes an enzyme that locally converts a prodrug to its active, cytotoxic form.8,9 Cell death under these circumstances usually has a pronounced by-stander effect that improves the efficiency of the therapy. One advantage of this approach is that transgene expression need not be prolonged.
In general, nonviral gene delivery to the synovium has not proved very effective in animal models.4 For this reason, the preponderance of research has used different types of viral vectors for in vivo or ex vivo gene delivery. Successful treatment of joint diseases in animal models has been accomplished using retroviral vectors in an ex vivo fashion, and using vectors derived from adenovirus, AAV, herpes simplex virus (HSV) and lentivirus for in vivo delivery (Table 1). Adenovirus and HSV are inflammatory and cytotoxic, respectively, and do not permit prolonged transgene expression; thus, these vectors are not suitable for the sustained delivery of anti-arthritic proteins but they do lend themselves well to synovial ablation. Recombinant retroviruses provide two major types of vector: oncoretroviruses, often referred to simply as retroviruses, which require host-cell division, and lenti-viruses, which, although also retroviruses, transduce non-dividing cells. Because of the need for host-cell division, oncoretroviruses are normally used in an ex vivo fashion. When autologous synovial or skin fibroblasts are used as vehicles, the genetically modified cells colonize the synovium after intra-articular injection and express transgenes for an extended period. The direct injection of AAV or lentivirus into joints leads to in situ synovial cell transduction and the sustained intra-articular expression of transgenes. No detailed studies have directly compared the effectiveness of different promoters of transgene expression, but the retroviral long terminal repeat, the human cytomegalo virus immediate-early promoter and the elongation factor-1 promoter have all been able to support sustained transgene expression at therapeutic levels in the joints of laboratory animals. Certain inducible promoters have also shown value, and these may prove useful in the future to permit regulated transgene expression, something that could improve both the safety and efficacy of gene therapy.10
Table 1.
Salient properties of the main viral vectors used for human gene therapy
| Parent virus | Key properties of wild-type virus | Advantages | Disadvantages | Comments |
|---|---|---|---|---|
| Adenovirus | Double-stranded DNA genome, ~ 35 Kb long Non-enveloped Over 50 serotypes ~100 nm in size Genome remains episomal in infected cells |
Straightforward production of recombinant vectors at high titers Transduces non-dividing cells Wide choice of serotypes |
Inflammatory and antigenic | Various generations with increasingly deleted genomes have been developed ‘Gutted’ vectors have no viral coding sequences and a large carrying capacity but are dificult to produce Tropism can be modified by altering coat proteins |
| HSV | Double stranded DNA genome, ~150 Kb long Enveloped ~200 nm in size Genome remains episomal in infected cells |
Transduces non-dividing cells Very efficient transduction of dividing and non-dividing cells Has a natural latency in neurons Very large carrying capacity |
Complex genome—difficult to produce recombinant virus Cytotoxic | HSV1 and HSV2 are most widely used as vectors Herpes family includes EBV, CMV and others |
| AAV | Single-stranded DNA genome, 4.8 Kb long Non-enveloped Growing number of serotypes identified ~20 nm in size |
Perceived to be safe (wild-type virus causes no known disease) Transduces non-dividing cells Thought to have low immunogenicity, but this is being re-evaluated |
Dificult to produce Carrying capacity is insuficient for certain applications Transduction eficiency sometimes low |
Wild-type virus cannot replicate without helper virus Wild-type virus integrates in a site-specific manner; recombinant virus remains as a stable, concatemeric plasmid Limitations of single-stranded genome can be overcome by the development of double-copy (self-complementary) DNA viruses |
| Oncoretrovirus | RNA genome ~8–10 Kb long Enveloped ~100 nm in size |
Straightforward production of recombinant vectors at moderate titers Pseudotyped vectors have wide host range |
Require host-cell division Risk of insertional mutagenesis |
Usually used ex vivo Two genomes per virion, reverse transcribed into DNA |
| Lentivirus | RNA genome ~8–10 Kb long Enveloped ~100 nm in size |
Straightforward production of recombinant vectors at moderate titers Pseudotyped vectors have a broad host range and are often very eficient Transduces non-dividing cells |
Risk of insertional mutagenesis, but nonintegrating vectors are being developed, and have proved effective in animal models | Two genomes per virion, reverse transcribed into DNA |
Abbreviations: AAV, adeno-associated virus; CMV, cytomegalovirus; EBV, Epstein–Barr virus; HSV, herpes simplex virus.
Progress in clinical trials
Rheumatoid arthritis
Ex vivo trials
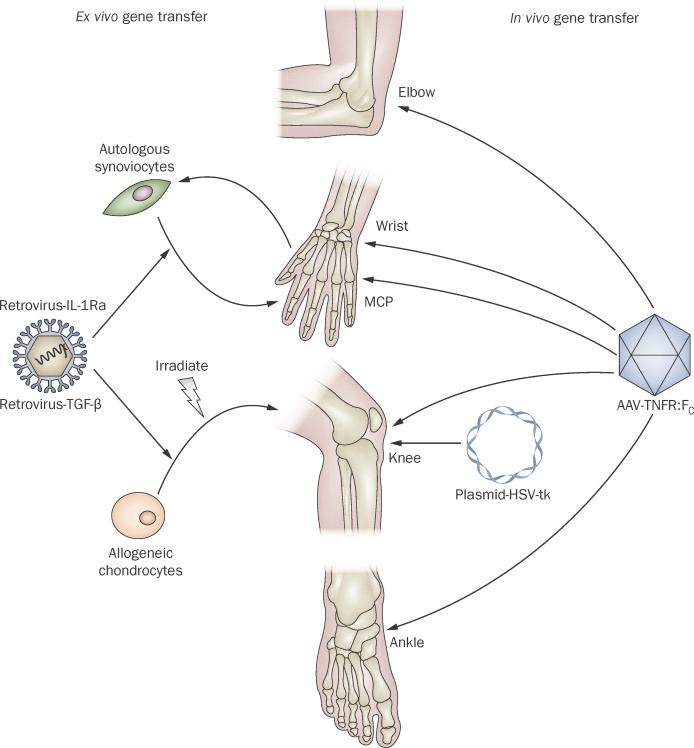
Although numerous transgenes and vectors have shown efficacy in animal models of RA,3,4 only a small number of these have advanced to be tested in clinical trials (Table 2, Figure 2). The first clinical trial of gene therapy for RA, which took place in the USA, used the interleukin-1 receptor antagonist (IL-1Ra) as the transgene and an oncoretrovirus (MFG-IRAP) as the vector.11 IL-1Ra is particularly well-suited for delivery by gene transfer because its biological clearance is so rapid that it is difficult to maintain at therapeutic concentrations in vivo using conventional delivery systems.12 As noted above, retroviral vectors are normally used in an ex vivo fashion and preclinical studies have confirmed that autologous synovial fibroblasts are suitable vehicles for this purpose.13,14
Table 2.
Clinical trials of gene therapy for rheumatoid arthritis
| Transgene | Method of delivery | Phase | Institution or sponsor (principal investigator[s]) | NIH OBA protocol number | Number of subjects | Reference |
|---|---|---|---|---|---|---|
| IL-1Ra | Retrovirus, ex vivo | I | University of Pittsburgh, USA (Evans & Robbins) | 9406-074 | 9 | Evans et al. (2005)11 |
| IL-1Ra | Retrovirus, ex vivo | I | University of Düsseldorf, Germany (Wehling) | NA | 2 | Wehling et al. (2009)15 |
| HSV-tk* | Plasmid, in vivo | I | University of Michigan, USA (Roessler) | 9802-237 | 1 | Unpublished |
| TNFR–Fc (etanercept) | AAV, in vivo | I | Targeted Genetics, USA (Mease) | 0307-588‡ | 15 | Mease et al. (2009)19 |
| TNFR–Fc (etanercept) | AAV, in vivo | I/II | Targeted Genetics, USA (Mease) | 0504-705§ | 127 | Mease et al. (2010)2 |
| NFκB decoy oligonucleotide | No vector, in vivo | I | Osaka University, Japan (Tomita) | N/A | 9 | Unpublished |
When expressed in conjunction with ganciclovir administration, HSV-tk kills synovial cells and produces a synovectomy.
This study also included one subject with ankylosing spondylitis.
This study also included subjects with psoriatic arthritis and ankylosing spondylitis.
Abbreviations: AAV, adeno-associated virus; HSV-tk, herpes simplex virus thymidine kinase; IL-1Ra, IL-1 receptor antagonist; NA, not applicable; NFκB, nuclear factor κB; OBA, Office of Biotechnology Activities; TNFR–Fc, tumor necrosis factor receptor–immunoglobulin Fc fusion protein.
Figure 2.
Local gene therapy for arthritis in clinical trials. Ex vivo gene transfer using retroviral vectors has been used to transfer IL-1Ra cDNA to the MCP joints of subjects with RA, and TGF-β1 cDNA to the knee joints of subjects with OA. In the latter trials, cells were irradiated before injection. In vivo gene transfer using AAV has been used to deliver etanercept (TNFR–Fc) cDNA to the ankle, knee, wrist, MCP and elbow joints of subjects with RA, psoriatic arthritis and ankylosing spondylitis. Plasmid DNA encoding HSV-tk has been injected into the knee joint of a patient with RA, who was subsequently administered ganciclovir. Abbreviations: AAV, adeno-associated virus; cDNA, complementary DNA; HSV-tk, herpes simplex virus thymidine kinase; IL-1Ra, interleukin-1 receptor antagonist; MCP, metacarpophalangeal; OA, osteoarthritis; RA, rheumatoid arthritis; TNFR–Fc, tumor necrosis factor receptor–immunoglobulin Fc fusion protein.
The protocol for human gene therapy that was developed for this trial was the first in the world for the treatment of a nonlethal disease to come before regulatory bodies and thus it raised considerable safety issues. Because retroviruses promiscuously insert their genetic material into the chromosomal DNA of the cells they infect, there was a small, but finite, chance of insertional mutagenesis, although this had never been observed up to that point in human clinical trials using retroviruses. To address this issue, it was agreed that the genetically modified cells would be inserted into metacarpophalangeal joints that were destined to undergo joint replacement surgery 1 week later. This protocol would provide sufficient time to determine whether the cells colonized the synovium and continued to express the transgene, while addressing, in a preliminary fashion, issues of safety and practicality. The trial was accomplished successfully, demonstrating for the first time the transfer of a gene to a human joint. Study of tissues retrieved after joint replacement provided evidence of the intra-articular expression of an active transgene product.11
A second trial using MFG-IRAP, published in 2009, was conducted in Germany.15 The only major difference from the first protocol was the period between the injection of the genetically modified cells and their removal by surgical synovectomy, which was 1 month in the German study as opposed to 1 week in the previous study. Because several participants in the previous study had reported subjective, symptomatic relief, efficacy measurements were included in the German trial, although these measurements are not well-developed for studies that target individual joints of patients with RA. Permission was granted to include six subjects, but the trial was terminated after only two had completed the protocol because of reports of leukemia, with one death, in an unrelated gene therapy trial in France for severe combined immunodeficiency disease (SCID) that used the MFG retrovirus backbone.16 Nevertheless, both of the subjects who completed the RA study reported symptomatic improvement; in one case the improvement was dramatic.15
No further clinical trials of this approach to gene therapy have been undertaken. A number of intrinsic and extrinsic factors have contributed to this circumstance. The occurrence of leukemia in subjects who received retroviral vectors has demonstrated that insertional mutagenesis resulting in aberrant expression of proto-oncogenes is not just of theoretical concern.16 This potential complication makes it much more difficult to justify the use of retroviral vectors in the treatment of nonlethal diseases. Moreover, the US FDA now requires 15-year follow-up data in human trials of gene therapy that use integrating vectors. Conducting both the US and German trials brought home to the investi gators the cumber some, expensive and inefficient nature of ex vivo gene therapy that requires expanded autologous-cell cultures. If immune barriers could be overcome, the use of cell lines as allografts would obviate this limitation. Surmounting the immune barriers to allotransplantation would thus provide a major advance for ex vivo gene delivery. Mesenchymal stem cells are thought to be immunosuppressive and thus may enable successful allo transplantation into joints, but this possibility has not been thoroughly investigated.
In vivo trials
The first in vivo arthritis gene therapy protocol involved the intra-articular injection of plasmid DNA encoding herpes simplex thymidine kinase cDNA. Expression of this enzyme renders cells susceptible to the cytotoxic effects of the prodrug ganciclovir, and this strategy has been widely used in clinical trials of cancer gene therapy.17 Soon after this arthritis trial had treated its first subject, the field of gene therapy was shaken by the death of Jesse Gelsinger in an unrelated trial for ornithine transcarbamylase (OTC)-deficiency that used an adenoviral vector for gene transfer to the liver.18 Because this was the first human death that could be reliably ascribed to gene transfer, it disrupted the entire field and the arthritis trial was unable to recruit further subjects. The data and safety monitoring board thus terminated the study.
Two further in vivo trials have been completed. These trials used AAV, a choice that resonates with the growing popularity of this vector for human gene therapy. AAV is perceived to be safe and can be de livered by direct injection. Drawbacks of this approach include the technological challenges of making large amounts of clinical-grade material, its relative inefficiency and, for some applications, its small packaging capacity (Table 1). The two in vivo studies in RA used a transgene that encoded human tumor necrosis factor receptor–immunoglobulin Fc fusion protein (TNFR:Fc), equivalent to etanercept. This vector, known as rAAV2-TNFR:Fc or tgAAC94, was injected into single, symptomatic joints in over 100 patients with RA, as well as patients with ankylosing spondylitis, and psoriatic arthritis, whose disease was not adequately controlled by standard therapy. The phase I trial was accomplished without incident,19 but the phase II study was marred by the death of a subject shortly after receiving a second intra-articular injection of the highest dose of rAAV2-TNFR:Fc.2,20 This death was widely publicized in the press and the trial was put on hold while the authorities investigated the matter. They concluded that the death was probably not the result of the gene transfer procedure and the FDA eventually allowed the trial to continue with minor modifications to the protocol, which was completed without further incident. Although there were trends towards clinical improvements among the patients in the phase II study, the effects of the gene therapy were not statistically significant.2
Whether there will be further clinical trials of rAAV2-TNFR:Fc, or indeed of any other gene therapeutic, for RA in the near future is unknown. Apart from anything else, the clinical and commercial success of biologic agents for the treatment of RA does not encourage the development of alternative approaches that have a perceived risk. Nevertheless, 25–40% of patients fail to respond to biologic therapy21 and are thus exposed to the morbidity and increased mortality that accompany RA.
A different and possibly less-intimidating strategy involves the intra-articular injection of noncoding nucleic acids (Box 1). Tomita and colleagues have injected decoy deoxyribose oligonucleotides that sequester NF-κB into the joints of subjects with RA.22 This approach may not be strictly speaking gene therapy, but it illustrates an alternative molecular therapeutic avenue. There is also interest in using RNA interference in this fashion.
Osteoarthritis
Unlike RA, no highly effective drugs are available for the treatment of OA and this disease affects a far greater number of people. Moreover, OA is also a major clinical problem in veterinary medicine. Because OA affects a limited number of weight-bearing joints and has no major extra-articular or systemic components, it is well-suited to local, intra-articular gene therapy. Two similar phase I trials for OA have been completed in Korea and the USA, and phase II trials have been initiated (Table 3). All use ex vivo gene delivery via a retrovirally transduced, established line of chondrocytes that overexpress transforming growth factor-β1 (TGF-β1). Use of an allograft cell line obviates the disadvantages associated with the use of primary cultures of autologous cells. To avoid the safety issues associated with retroviral transduction, the cells are irradiated before intra-articular injection, which renders them incapable of cell division and hence removes any possibility of these aneuploid cells causing cancer. The expectation is that the irradiated cells will survive long enough to secrete sufficient levels of TGF-β1 to trigger a sustained reparative response within the cartilage damaged by OA. Whether transient transgene expression will produce prolonged improvement in OA—a chronic condition—remains to be seen. The results of these studies have not yet been published, but the phase I data suggest that the procedure is safe.23
Table 3.
Clinical trials of gene therapy for osteoarthritis
| Transgene | Method of delivery | Phase | Institution or sponsor (principal investigator) | Status | Number of subjects |
|---|---|---|---|---|---|
| TGF-β1 | Retrovirus, ex vivo | I | Kolon Life Sciences, Korea (Ha) | Closed | 12 |
| TGF-β1 | Retrovirus, ex vivo | I | TissueGene Inc. (Mont)* | Closed | 12 |
| TGF-β1 | Retrovirus, ex vivo | IIa | Kolon Life Sciences, Korea (Ha) | Recruitment Complete | 28 |
| TGF-β1 | Retrovirus, ex vivo | II | TissueGene Inc. (Mont)‡ | Pending | 100 |
NIH Office of Biotechnology Activities protocol number: 0307-594; ClinicalTrials.gov identifier: NCT00599248.
NIH Office of Biotechnology Activities protocol number: 0912-1016.
Abbreviation: TGF-β1, transforming growth factor β1.
An additional phase I trial in OA is planned, based upon the delivery of IL-1Ra cDNA to affected knee joints. This study developed from the RA trial discussed in the previous section. Because of the problems associated with ex vivo delivery using retroviruses and expanded autologous cells, the trial will use in vivo delivery with AAV. Intra-articular delivery of IL-1Ra cDNA has demonstrated efficacy in canine,24 lapine,25 and equine26 models of OA both in terms of protecting the articular cartilage and in reducing symptoms. Progress in moving this protocol into a phase I clinical trial has stalled because of the high cost of the extensive safety testing now required by the FDA for this type of application.
Other indications
The ability to introduce genes into joints opens additional therapeutic possibilities. Evidence from a murine model of hemophilia B suggests that the hemarthrosis due to intra-articular bleeding suffered by individuals with hemophilia B can be treated by the intra-articular injection of AAV that encodes factor IX. In mice, local gene de livery was superior to systemic introduction of recombinant factor IX as a means of preventing hemophilic arthropathy.27 The transition of this technology into human clinical trials will be facilitated by the previous use of AAV to deliver factor IX to the livers of patients with hemophilia.28 Although cell-mediated immune reactions to the AAV vector curtailed transgene expression in the liver, preliminary data suggest that cell-mediated immunity to AAV might not occur following intra-articular delivery in humans.2
Diffuse, pigmented villonodular synovitis (PVNS) is a rare proliferative disorder of the synovium that is often very difficult to treat.29 Surgical synovectomy is the standard treatment for PVNS, but removing all of the hypertrophic mass is difficult and regrowth is a common problem. Genetic syno vectomy along the lines discussed earlier in this article could provide a much-needed, nonsurgical, effective approach to managing this condition. The pathway to clinical trials is shortened by the prior use in cancer gene therapy of the vectors needed for such an application.17
Mucopolysaccharidosis type VI is a genetic, lysosomal storage disease caused by a lack of N-acetyl galactosamine-4-sulfatase. All extra-articular manifestations of the disease can be treated with systemic recombinant N-acetyl galactosamine-4-sulfatase. However, insufficient amounts of this enzyme accumulate in joints after systemic delivery, and the joints remain symptomatic. Research is thus directed toward the intra-articular delivery of cDNA encoding N-acetyl galactosamine-4-sulfatase.30
Constraints on progress
There is no getting around the fact that moving arthritis gene therapy protocols into the clinic is extremely expensive, slow and vulnerable to outside events over which the investigator has no control. Clinical trials of gene therapy for RA seem to have been particularly unlucky in the latter regard. As we have seen, a trial in Germany was curtailed because of unrelated adverse events in a study in France; the synovial ablation trial was unable to proceed because of a death elsewhere in a trial of OTC deficiency. Although the rAAV2-TNFR:Fc RA trial was allowed to continue following the death of a subject, our experience suggests that the event has made it very difficult for subsequent trials using AAV in joints.
Regulatory bodies, funding agencies and certain sections of the scientific community seem extremely sensitive to the safety issues surrounding gene therapy, despite the fact that in over 1,500 trials of human gene therapy only two deaths and a relatively small number of severe adverse events have been unequivocally linked to gene transfer. The safety bar for gene therapeutics seems to be set at a far higher level than for other therapeutics, despite the ability of non-genetic medicines to do just as much harm. This level of caution on the part of the regulatory agencies means that investigators are increasingly required to undertake prolonged, complicated and very expensive preclinical investigations in order to obtain approval through the FDA's Investigational New Drug (IND) mechanism.
To give an idea of the sums of money involved, additional preclinical work required for the AAV-IL-1Ra OA study discussed above has been estimated to cost as much as $2 million. Such an enormous sum is well beyond the typical resources of academic investigators. Indeed, there are few granting opportunities that would cover such applied, non-hypothesis-driven research. Moreover, there may be a disconnect between what agencies are willing to fund and what the FDA accepts as evidence in support of an IND application. Under such conditions, the private sector almost certainly has to get involved in providing funding, but this has not proved easy. The large pharmaceutical companies do not embrace gene therapy because they see liability, long timelines, and uncertain returns on investment.31 Certain types of bio technology companies show more interest, but usually lack sufficient funding.
Despite these issues, however, the field of gene therapy as a whole is experiencing something of a resurgence. Gene therapies for diseases such as adrenal leukodystrophy, β-thalassemia, adenosine deaminase (ADA) deficiency, X-linked SCID, chronic granulomatous disease, Leber congential amaurosis, and lipoprotein lipase deficiency have all shown clinical efficacy.32 Indeed, gene therapy is increasingly used to treat ADA-SCID and X-linked SCID, and orphan drug status has been granted to AAV-based therapies for familial lipoprotein lipase deficiency and X-linked juvenile retinoschisis.
Conclusions
The emerging appreciation that gene therapy is a clinical reality could increase enthusiasm for its application to arthritis; as described in this Perspectives article, numerous preclinical studies confirm its efficacy and safety in various animal models and there is preliminary evidence of its efficacy and safety in humans. With sufficient resources and regulatory pragmatism, arthritis gene therapy stands a good chance of success.
Box 1 Potentially therapeutic nucleic acids.
Genes (complementary DNA)
The gene, or more usually its complementary DNA, encodes a therapeutic product. When the gene is expressed, the encoded RNA or protein is synthesized and exerts its therapeutic influence. The therapeutic gene or complementary DNA is delivered through a vector by direct injection (in vivo delivery) or through modified cells that are introduced into the target cell or tissue (ex vivo delivery).
Oligodeoxyribonucleotides
Oligodeoxyribonucleotides are short sequences of DNA, often 15–25 base pairs in length, which are usually used to downregulate the expression of genes whose products have important pathophysiologic roles. Two major approaches are employed: when used as double-stranded decoys, oligodeoxyribonucleotides bind transcription factors required for the expression of the target gene; when used as antisense molecules, usually in a single-stranded form, they bind to mRNA molecules transcribed from the target gene. Oligodeoxynucleotides are delivered directly by transfection.
Oligoribonucleotides (RNA)
The three basic strategies that use oligonucleotides—antisense, ribozyme and RNA interference—all rely on binding to target RNA molecules by complementary base-pairing, which results in degradation of the target. This degradation reduces the expression of genes that encode the target RNAs. Therapeutic RNA molecules can be delivered directly by transfection, or via delivery of genes encoding the therapeutic RNA.
Modified oligonucleotides
Because native RNA molecules are rapidly degraded by intracellular nucleases, there is interest in using chemically modified RNAs, where phosphodiesterase linkages are replace by something more stable. The drug fomivirsen, for example, is a synthetic, 21-base RNA oligonucleotide with phosphorothioate linkages that is used to treat cytomegalovirus infections of the eye. Morpholinos are oligonucleotides in which the bases are linked to morpholino rings instead of deoxyribose or ribose rings, and are linked through phosphorodiamidate groups; these may be single-stranded or double-stranded. Because these are chemically synthesized molecules, they are delivered only by transfection.
Acknowledgments
The authors’ work in this area has been funded by NIH grants R01 AR43623, R21 AR049606, R01 AR048566, R01 AR057422 and R01 AR051085, and by Orthogen.
Footnotes
Competing interests C. H. Evans declares associations with the following companies: TissueGene and Orthogen. S. C. Ghivizzani declares an association with the following company: Molecular Orthopedics. P. D. Robbins declares associations with the following companies: TissueGene and Molecular Orthopedics. See the article online for full details of the relationships.
Author contributions All authors contributed equally to researching data for the article, providing a substantial contribution to discussion of content, writing the article and review and/or editing of the manuscript before submission.
Contributor Information
Christopher H. Evans, Center for Advanced Orthopedic Studies, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Avenue, RN-115, Boston, MA 02215, USA.
Steven C. Ghivizzani, Gene Therapy Laboratory, Department of Orthopedics and Rehabilitation, University of Florida College of Medicine, PO Box 100137 Gainesville, FL 32610–0137, USA.
Paul D. Robbins, Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine, 523 Bridgeside Point II, 450 Technology Drive, Pittsburgh, PA 15219, USA.
References
- 1.Bandara G, et al. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–231. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- 2.Mease PJ, et al. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 study. J. Rheumatol. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- 3.Traister RS, Hirsch R. Gene therapy for arthritis. Mod. Rheumatol. 2008;18:2–14. doi: 10.1007/s10165-007-0017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghivizzani SC, et al. Perspectives on the use of gene therapy for chronic joint diseases. Curr. Gene Ther. 2008;8:273–286. doi: 10.2174/156652308785160638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouze E, et al. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol. Ther. 2007;15:1114–1120. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- 6.Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum. Gene Ther. 2003;14:393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Gao G, Clayburne G, Schumacher HR. Elimination of rheumatoid synovium in situ using a Fas ligand ‘gene scalpel’. Arthritis Res. Ther. 2005;7:R1235–R1243. doi: 10.1186/ar1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goossens PH, et al. Feasibility of adenovirus-mediated nonsurgical synovectomy in collagen-induced arthritis-affected rhesus monkeys. Hum. Gene Ther. 1999;10:1139–1149. doi: 10.1089/10430349950018139. [DOI] [PubMed] [Google Scholar]
- 9.Sant SM, et al. Molecular lysis of synovial lining cells by in vivo herpes simplex virus-thymidine kinase gene transfer. Hum. Gene Ther. 1998;9:2735–2743. doi: 10.1089/hum.1998.9.18-2735. [DOI] [PubMed] [Google Scholar]
- 10.Miagkov AV, Varley AW, Munford RS, Makarov SS. Endogenous regulation of a therapeutic transgene restores homeostasis in arthritic joints. J. Clin. Invest. 2002;109:1223–1229. doi: 10.1172/JCI14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans CH, et al. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc. Natl Acad. Sci. USA. 2005;102:8698–8703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bendele A, et al. Efficacy of sustained blood levels of interleukin-1 receptor antagonist in animal models of arthritis: comparison of efficacy in animal models with human clinical data. Arthritis Rheum. 1999;42:498–506. doi: 10.1002/1529-0131(199904)42:3<498::AID-ANR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Makarov SS, et al. Suppression of experimental arthritis by gene transfer of interleukin 1 receptor antagonist cDNA. Proc. Natl Acad. Sci. USA. 1996;93:402–406. doi: 10.1073/pnas.93.1.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandara G, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc. Natl Acad. Sci. USA. 1993;90:10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehling P, et al. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum. Gene Ther. 2009;20:97–101. doi: 10.1089/hum.2008.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- 17.Miles BJ, et al. Prostate-specific antigen response and systemic T cell activation after in situ gene therapy in prostate cancer patients failing radiotherapy. Hum. Gene Ther. 2001;12:1955–1967. doi: 10.1089/104303401753204535. [DOI] [PubMed] [Google Scholar]
- 18.Raper SE, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–58. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Mease PJ, et al. Local delivery of a recombinant adenoassociated vector containing a tumor necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann. Rheum. Dis. 2009;68:1247–1254. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- 20.Frank KM, et al. Investigation of the cause of death in a gene-therapy trial. N. Engl. J. Med. 2009;361:161–169. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]
- 21.Gibbons LJ, Hyrich KL. Biologic therapy for rheumatoid arthritis: clinical efficacy and predictors of response. BioDrugs. 2009;23:111–124. doi: 10.2165/00063030-200923020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Tomita N, Morishita R, Tomita T, Ogihara T. Potential therapeutic applications of decoy oligonucleotides. Curr. Opin. Mol. Ther. 2002;4:166–170. [PubMed] [Google Scholar]
- 23.Safety study of TissueGene-C in degenerative joint disease of the knee (TGC-03-01). ClinicalTrials.gov identifier: NCT00599248 [online], http://clinicaltrials.gov/ct2/show/ NCT00599248 (2010).
- 24.Pelletier JP, et al. In vivo suppression of early experimental osteoarthritis by interleukin-1 receptor antagonist using gene therapy. Arthritis Rheum. 1997;40:1012–1019. doi: 10.1002/art.1780400604. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Mao Z, Yu C. Suppression of early experimental osteoarthritis by gene transfer of interleukin-1 receptor antagonist and interleukin-10. J. Orthop. Res. 2004;22:742–750. doi: 10.1016/j.orthres.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9:12–20. doi: 10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, et al. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532–4541. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 29.Tyler WK, Vidal AF, Williams RJ, Healey JH. Pigmented villonodular synovitis. J. Am. Acad. Orthop. Surg. 2006;14:376–385. doi: 10.5435/00124635-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Byers S, Rothe M, Lalic J, Koldej R, Anson DS. Lentiviral-mediated correction of MPS VI cells and gene transfer to joint tissues. Mol. Genet. Metab. 2009;97:102–108. doi: 10.1016/j.ymgme.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Evans CH. Arthritis gene therapy at an inflection point. Future Rheumatol. 2008;3:207–210. [Google Scholar]
- 32.Gene therapy deserves a fresh chance. Nature. 2009;461:1173. doi: 10.1038/4611173a. [No authors listed] [DOI] [PubMed] [Google Scholar]