Abstract
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus-associated malignancy most common in East Asia and Africa. Aberrant expression of Jab1/CSN5, a negative regulator of the cell cycle inhibitor p27, is correlated with reduced p27 expression and associated with advanced tumor stage and poor prognosis in several human cancers. In this study, we examined the functional relationship between Jab1 and p27 protein expression in NPC. Immunohistochemical analysis showed an inverse association between Jab1 and p27 in NPC tissue samples, and overexpression of Jab1 correlated with poor survival in NPC patients. Mechanistically, Jab1 and p27 were found to interact directly in NPC cells, with Jab1 mediating p27 degradation in a proteasome-dependent manner. Knockdown of Jab1 resulted in a remarkable increase in p27 levels and inhibition of cell proliferation, indicating that Jab1 targets p27 for degradation, thereby controlling its stability. Jab1 depletion also enhanced the antitumor effects of cisplatin in NPC cells. Together, our findings suggest that Jab1 overexpression plays an important role in the pathogenesis of NPC through Jab1-mediated p27 degradation. Jab1 therefore represents a novel diagnostic marker and therapeutic target in patients with NPC.
Keywords: nasopharyngeal carcinoma, tumorigenesis, Epstein-Barr virus-associated malignancy, p27Kip1, Jab1/CSN5
Introduction
Nasopharyngeal carcinoma (NPC), originating from the epithelial lining of the nasopharynx, is a squamous-cell carcinoma (1). This neoplasm has remarkable ethnic and geographic distribution, with a high prevalence in southern China, Southeast Asia, northern Africa, and Alaska (2, 3). The annual incidence peaks at 50 cases per 100,000 people in the endemic regions, but is rare in the Western world (1 per 100,000) (2). Etiologic studies have indicated that Epstein-Barr virus (EBV) infection, environmental factors, and genetic susceptibility are associated with NPC (1, 4). In China more than 95% of NPCs are non-keratinizing carcinoma, while fewer than 5% are keratinizing carcinoma; thus, in our study we use CNE1 (keratinizing carcinoma) and CNE2 (non-keratinizing carcinoma) cells to represent the two mainly histologic types (5).
Radiotherapy and adjuvant cisplatin chemotherapy have become the standard treatments for NPC (6). The 5-year survival rate after treatment is approximately 70% (7). Thirty to forty percent of patients will develop distant metastasis within 4 years (8), and once metastasis occurs, the prognosis is very poor.
To develop better treatment approaches, it is important to understand the molecular basis of the development and progression of NPC. Genetic alterations have been reported in NPC including a frequent reduction of p27 expression (9, 10). Furthermore, low p27 expression levels may contribute to the aggressive behavior of NPC (11). Overexpression of cyclin E mRNA has been shown to predict poor prognosis in NPC and has been correlated with an advanced stage of NPC and a low overall survival rate (12). Recently, it has been reported that PIK3CA gene amplification is frequently observed in advanced-stage NPC, which emphasizes the association between PIK3CA gene amplification and poor prognosis (11). It has also been shown that Akt promotes cell proliferation and survival in NPC (4, 13). However, additional molecular abnormalities resulting in the deregulation of cell-cycle progression may also occur.
Jab1/CSN5 (Jab1 hereafter) as we initially identified as a c-Jun coactivator, is also known as the fifth component of the COP9 signalosome (CSN) complex (CSN5) (14, 15). Jab1 promotes cell proliferation and inactivates p27 by inducing translocation of p27 from the nucleus to the cytoplasm, which accelerates p27 degradation through the ubiquitin-dependent proteasome pathway and promotes cell-cycle progression (16). p27 is a universal cyclin-dependent kinase (Cdk) inhibitor that directly inhibits the enzymatic activity of cyclin-Cdk complexes, resulting in cell-cycle arrest at G1 (17). In addition, p27 protein levels are increased in quiescent cells and rapidly decrease after cells are stimulated with mitogens (18). Although transcriptional regulation is possible, the cellular abundance of p27 is primarily regulated at the posttranslational level by the ubiquitin-proteasome pathway (19). Jab1 overexpression is correlated with a loss of p27 and a lower rate of survival in patients with breast cancer, suggesting a role in breast cancer pathogenesis (20). This inverse association between Jab1 and p27 expression has also been observed in anaplastic large cell lymphoma (21), ovarian cancer (22), pancreatic adenocarcinomas (23, 24), and other cancer types (25–27). However, the mechanisms leading to p27 downregulation in NPC remain undefined. Because Jab1 overexpression is correlated with the loss of p27 in several cancers, and low p27 expression is associated with higher tumor grades (28), we hypothesized that Jab1 functions as a negative regulator of p27 and as such may play a role in the pathogenesis of NPC.
To test our hypothesis, we assessed Jab1 and p27 expression in a series of 45 NPC and 30 nasopharyngeal inflammation tissue specimens. We found that Jab1 overexpression was associated with absent or low expression of p27 in these samples. To further elucidate the role of Jab1 in p27 degradation in NPC, we infected NPC cell lines with an adenoviral vector overexpressing Jab1 and found that p27 levels were significantly reduced. We also detected a direct physical interaction between Jab1 and p27 in NPC cells. Furthermore, inhibition of endogenous Jab1 expression with specific short interfering RNAs (siRNAs) resulted in a substantial increase of p27 levels and inhibition of cell proliferation, indicating that Jab1 controls the stability of p27 by targeting it for degradation in NPC. Interestingly, siRNA-mediated depletion of Jab1 inhibited cell proliferation and accelerated apoptotic cell death in NPC. Moreover, Jab1 depletion enhanced the antitumor effects of cisplatin in NPC cells. This may suggest that Jab1 is a potential target for treating NPC.
Materials and Methods
Patients and tissue samples
All patients were from the Cancer Center of Sun Yat-Sen University in 2003. The study group consisted of 36 men and 9 women with NPC who underwent radiotherapy and the control group consisted of 13 men and 17 women with nasopharyngeal inflammation. Patients that had preoperative diagnosis and did not receive preoperative chemo-radiation treatment were selected for this study based on the availability of archived paraffin-embedded NPC and nasopharyngitis tissue blocks for immunohistochemical analysis. Ethical approval was obtained from the cancer center and fully informed consent from all patients before sample collection. Surgical staging of tumors had been done according to the American Joint Committee on Cancer tumor-node-metastasis system and tumor grading was based on currently used histopathologic criteria.
Reagents
Cell culture medium were from Mediatech Inc (Mannassas, VA) and fetal bovine serum (FBS) were obtained from Gibco (Grand Island, NY, USA). The antibodies used were Jab1 (Santa Cruz, CA), p27, and PARP (BD Biosciences PharMingen, San Diego, CA); caspase-3, Lamin A/C, and Myc-tag (Cell Signaling Technology, Beverly, MA); and Flag and β-actin (Sigma-Aldrich, St. Louis, MO). The Lipofectamine Plus and Oligofectamine reagents were from Invitrogen (Carlsbad, CA). NE-PER nuclear and cytoplasmic extraction reagents and Western Lightning Chemiluminescence Plus reagent were from Thermo Scientific Pierce (Rockford, IL, USA). Annexin V/PI kit was from BD Biosciences (Palo Alto, CA). Cell Proliferation Kit was from Roche (Indianapolis, IN, USA).
Human tissues and immunohistochemical analysis
A total of 75 formalin-fixed, paraffin-embedded human primary NPC and noncancerous inflamed nasopharyngeal specimens from 45 NPC and 30 nasopharyngitis patients were analyzed. The p27 and Jab1 levels in the formalin-fixed paraffin-embedded tissue sections were measured by immunohistochemical analysis, as previously described (24). Briefly, heat-induced retrieval of Jab1 and p27 antigens was performed, and the slides were incubated with the primary antibodies. An immunoreaction was detected with the LSAB+ kit from Dakocytomation. We used 3, 3'-diaminobenzidine as the chromogen and hematoxylin as the counterstain. Expression levels of Jab1 and p27 were evaluated by counting at least 500 tumor cells in representative high-power fields. Tumor cells were considered positive for Jab1 or p27 when nuclear or cytoplasmic staining was present. For each tumor, we determined a proportion score and an intensity score. The proportion score represented the estimated fraction of positively stained cells (0 = less than 5%; 1 = 5% to 35%; 2 = 35% to 65%; 3 = greater than 65%). The intensity score represented the estimated average staining intensity of the positive cells (0 = no staining; 1 = weak staining; 2= intermediate staining; 3= strong staining). The overall amount of protein present in each tumor was then expressed as the average of the proportion and intensity scores for negative and positive cells (ranges = 0 and 0.5 to 3, respectively). We defined the scores 0, 0.5 to 1.0, 1.5 to 2.0, and 2.5 to 3.0 as negative (−), weakly positive (+), and highly positive [(++), (+++)], respectively.
Cell cultures
Human NPC cell lines CNE1 (well-differentiated, from Cancer Center, Sun Yat-Sen University, China), CNE2 (poorly differentiated, from Cancer Center, Sun Yat-Sen University, China), HONE1 (poorly differentiated, the generous gift of Prof. Ronald Glaser, The Ohio State University Medical Center) and C666.1 (undifferentiated, the generous gift of Prof. Kwok-Wai, Lo, Prince of Wales Hospital, The Chinese University of Hong Kong) were cultured in RPMI medium containing 10% fetal bovine serum and penicillin-streptomycin sulfate. 293T human embryonic kidney cells were cultured in Dulbecco's modified Eagle medium (Invitrogen) with 10% fetal bovine serum and penicillin-streptomycin. HOK16B (Normal keratinocyte cells, the generous gift of Prof. Jeffrey N. Myers, MD. Anderson Cancer Center) were cultured in keratinocyte-SFM media containing 30 mg/ml bovine pituitary extract, 0.2 ng/ml epidermal growth factor (EGF), 5% fetal bovine serum, and penicillin-streptomycin sulfate. All cell lines were incubated at 37 °C in an atmosphere of 5% CO2.
Cell extracts and immunoblotting
Cells in the log-phase of growth were collected, washed twice in cold phosphate-buffered saline (PBS), and lysed as described previously (29). Nuclear fractions of the indicated cell lysates were separated using NE-PER nuclear and cytoplasmic extraction reagents according to the manufacturer's directions. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose membranes, and probed with anti-Jab1, anti-p27, anti-Myc, anti-PARP and anti-caspase-3. β-actin was used as the internal positive control for all immunoblots. Immunoreactive bands were detected using HRP-conjugated secondary antibodies with the Western Lightning Chemiluminescence Plus reagent. The protein levels were quantified using ImageJ software (National Institute of Health, Bethesda, MD, USA. http://rsb.info.nih.gov/ij). Activity of PARP and Caspase-3 were measure as percentage of cleavage bands intensity to the total bands and calculated as follows: % PARP or Caspase-3 = 100% × Tc/Tt, where Tc is the intensity value of the cleavage bands, and Tt is the intensity value of the total bands.
Adenoviral vectors and gene transduction
A recombinant adenoviral vector expressing a doxycycline-regulated (Tet-Off) form of human Jab1 (Ad-Myc-Jab1) was constructed, as previously described (24). NPC cells were transduced for 48 hours with a regulatory virus (adeno-X Tet-Off, Clontech, Palo Alto, CA) and Ad-Myc-Jab1 at a multiplicity of infection of 50 in a growth medium containing 10% FBS in the presence or absence of 1 μg/mL doxycycline, a tetracycline analogue.
Proteasome inhibition assays
NPC cells were treated with the proteasome inhibitors LLnL (35 μM) or MG132 (30 μM), or LLM (25 μM), and DMSO as a control. After 16 hours of treatment, the cells were harvested, whole-cell lysates were prepared, and immunoblotting was done with antibodies against p27. β-actin antibodies were used as a loading control. NPC cells were transduced with Ad-Myc-Jab1 as described above. Thirty-two hours after infection, proteasome inhibitors (LLnL or MG132 or LLM), or DMSO (control) were added to the medium for 16 hours. Forty-eight hours after infection, whole-cell lysates were prepared, and proteins were analyzed by immunoblotting.
SiRNA, DNA transfection and co-immunoprecipitation assays
For the siRNA analysis, Jab1 siRNA and control siRNA oligonucleotides were cloned into an RNAi vector (BD Biosciences PharMingen) according to the manufacturer's instructions and as described by Kouvaraki (24). The Myc-Jab1 and Flag-p27, GFP-Jab1 and Cherry-p27 plasmids were transfected into either 293T or CNE1 cells using the Lipofectamine Plus reagent, and cells were either lysed for co-immunoprecipitation assays or examined with a fluorescence microscope.
Co-immunoprecipitation assays were done with the whole cell lysates from NPC cells or DNA transfected 293T cells, as described earlier (24). Briefly, cell lysates were incubated in RIPA buffer for 4 hours at 4 °C with either anti-Myc or anti-Jab1 antibodies or non-immune mouse serum as a control. Proteins were separated by 12% SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by immunoblotting.
Cell proliferation assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to evaluate cell viability, as described previously (29). Briefly, 48 hours after transfection, NPC cells were seeded in 96-well plates (500 cells/well for growth curves or 2000 cells/well for cisplatin treatment) in 100 μl of RPMI-1640 medium with 8% fetal bovine serum. After the indicated incubation period, the MTT labeling reagent (final concentration 0.5 mg/ml) was added, and the spectrophotometric absorbance of the samples was read using a microplate (ELISA) reader at 570 nm. The inhibition ratios (%) of cell survival or colony formation were calculated as the ratio of the indicated treatment group to the control group as follows: % inhibition ratio = 100% × Nt/Nc, where Nt is the OD value (colony number) of the treatment group and Nc is the OD value (colony number) of control group.
Colony formation assay
Forty-eight hours after transfection, NPC cells (200 cells/well) were plated in 6-well plates for growth analysis in RPMI-1640 medium with 8% FBS. The following day, the cells were treated with 0 or 0.5μM cisplatin for 48 hours. After 10 days, the cells were fixed with methanol, stained with 0.1% crystal violet, and scored by counting with an inverted microscope, using the standard definition that a colony consists of 50 or more cells.
Flow-cytometry analysis of the cell cycle and apoptosis
Forty-eight hours after transfection, the cells were collected and fixed overnight in 75% cold ethanol at −20 °C. Cells were washed twice in cold PBS and labeled with propidium iodide (PI) and analyzed immediately after staining using a FACScan flow cytometer (BD Biosciences) and FlowJo software.
Flow cytometric analysis was used to differentiate between living, early apoptotic, late apoptotic/necrotic, and necrotic cells by staining with Annexin V-FITC and PI. Briefly, after treating cells with 5 μM cisplatin for 48 hours, the transfected cells were labeled with Annexin V-FITC and PI according to the manufacturer's recommendations. Quantification of Annexin V-FITC and PI binding was performed using a FACScan flow cytometer.
Statistical analysis
Fisher's exact test was used to compare the expression and localization of Jab1 and p27 with various clinicopathologic variables. The Spearman test was used to analyze the association between cytoplasmic Jab1 and p27. Overall survival was defined as the time from diagnosis to death. Kaplan-Meier analysis was used to examine the association of Jab1 and p27 expression and survival. Statistical analysis for the results was done using Student t test when only two groups, or one-way analysis of variance when more than two groups. Differences between groups were stated to be statistically significant when P<0.05. All computations were carried out with SPSS 16.0 (SPSS, Chicago, IL, USA)
Results
Patient characteristics and demographics
To analyze the Jab1 and p27 expression patterns, we used 45 NPC tissue samples (median age, 41; range, 20–73 years) and 30 nasopharyngeal inflammation tissue samples (median age, 36; range, 14–65 years). The clinicopathologic characteristics of the NPC patients are summarized in Table 1.
Table 1.
Characteristics of 45 NPC patients included in the study
| Characteristics | No. patients (n=45) |
|---|---|
| Gender (male/female) | 36/9 |
| Median age | 41 |
| Tumor histologic type | |
| Squamous cell carcinoma | 32 (71%) |
| Non-keratinizing carcinomas | 13 (29%) |
| Differentiation | |
| Poor | 32 (71%) |
| No differentiation | 13 (29%) |
| Lymph node metastasis | |
| Positive | 40 (89%) |
| Negative | 5 (11%) |
| Stage | |
| I | 0 |
| II | 12 (27%) |
| III | 20 (44%) |
| IV | 13 (29%) |
Jab1 and p27 expression patterns in tissue samples from patients with noncancerous inflamed and nasopharyngeal carcinoma
Immunohistochemical analysis of nasopharyngitis and NPC tissue samples showed that Jab1 was localized to the cytoplasm and nucleus of NPC cells. 74% had positive cytoplasmic staining and 56% had positive nuclear staining for Jab1 in NPC. The staining in these cases was higher than that in the noncancerous inflamed nasopharyngeal tissue, which was 10% in cytoplasmic and 16% in nuclear, respectively, p < 0.01 (Fig. 1A). In contrast, nuclear p27 level was higher in noncancerous inflamed nasopharyngeal tissues (67%) than that in the NPC tissues (58%). However, 64% cytoplasmic p27 staining was observed in NPC cases, which was higher than that in noncancerous tissue (37%), P < 0.05 (Fig. 1B). Furthermore, cytoplasmic Jab1 expression in NPC tissues was inversely associated with nuclear p27 patterns (R=−0.389, P = 0.008), and correlated with cytoplasm p27 (R=0.328, P = 0.028) (Fig. 1C).
Figure 1. Expression patterns of Jab1 and p27 in non-neoplastic tissues and NPC tissues.
(A) Overall Jab1 immunoreactivity was low in non-neoplastic tissue (left) compared to NPC (middle). (Right) Percentage of cases with Jab1 expression in nasopharyngeal tissues and NPC. (B) Nuclear p27 was strongly expressed in non-neoplastic tissue (left). A case of NPC, with the predominantly cytoplasmic staining is shown (middle). (Right) Percentage of cases with p27 expression in non-neoplastic tissues and NPC. Original magnification, ×200; insets ×400. (C) Tumors with cytoplasmic Jab1 expression were associated with nuclear and cytoplasmic p27. R-values and p-values from Spearman's test. (D) Kaplan-Meier analysis of the association of Jab1 expression (left) and survival as well as association of cytoplasmic p27 (right) and survival.
Correlation of Jab1/p27 expression with clinical outcome
Survival analysis by the Kaplan-Meier method showed that high expression of Jab1 tended to correlate with a poor prognosis, p < 0.01 (Fig. 1D). Similar results were obtained from the subsets of patients with Non-keratinizing carcinoma (Supplementary Fig S1A) and Keratinizing squamous carcinoma (Supplementary Fig S1B). The medians for survival time in patients with negative and weakly positive Jab1 tumors was 34 months, compared with 14 months in patients with highly positive Jab1 tumors, (p<0.01). Meanwhile, cytoplasmic p27 positive expression correlate with a poor prognosis, the medians for survival time was 19 months in positive group whereas 36 months in negative group, (p<0.05).
Jab1 promotes p27 degradation through proteolysis and is sensitive to proteasome inhibitors
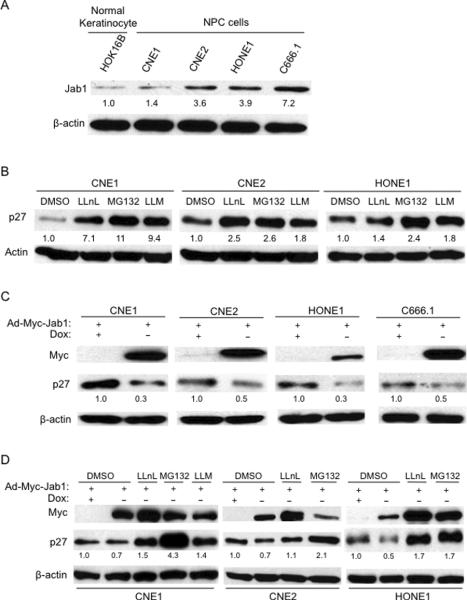
Immunoblotting also showed strong Jab1 expression in NPC cells but not normal keratinocyte cells, where weak Jab1 expression was detected (Fig. 2A). However, there was weaker p27 expression in CNE1 and CNE2 cells. Treatment NPC cells with specific proteasome inhibitors resulted in a significant increase in total p27 levels (Fig. 2B). To investigate whether Jab1 overexpression in NPC cells downregulates p27 levels, we transduced those two cell lines with Jab1 adenovirus in the absence or presence of doxycycline and measured Myc-JAB and p27 levels 48 hours after infection. Using this inducible (Tet-Off) system, Jab1 overexpression led to a significant decrease in p27 levels in all cell lines tested (Fig. 2C). Downregulation of p27 mediated by Jab1 overexpression was inhibited in cells that had been treated with proteasome inhibitors (LLnL, MG132 and LLM) but not in those treated with DMSO (Fig. 2D), indicating that Jab1 promotes p27 degradation through proteolysis and is sensitive to proteasome inhibitors. These data provide direct evidence of a functional relationship between Jab1 and p27 in NPC cells.
Figure 2. Jab1 is overexpressed and regulates p27 levels in NPC cells.
(A) Whole-cell lysates were prepared from the cells as indicated. β-actin was used as a control for protein loading and integrity. The relative Jab1 and p27 intensity from 5 samples is shown. (B) Proteolysis of p27 in NPC cells is sensitive to proteasome inhibitors. (C) Inducible expression of Ad-Myc-Jab1 (Tet-off) downregulates endogenous p27 levels in NPC cell lines. Lysates were prepared from Ad-Myc-Jab1-infected cells in the absence (−) or presence (+) of doxycycline (Dox). (D) NPC cells were transduced with the Ad-Myc-Jab1 virus, and treated as indicated with proteasome inhibitor. The protein levels were quantified using ImageJ software.
Jab1 specifically interacts with p27
To determine whether Jab1 and p27 directly interact, we conducted co-immunoprecipitation analyses. Lysates were immunoprecipitated with anti-Jab1 antibodies and immunoblotted with anti-p27 antibodies (Fig. 3A). We found that endogenous Jab1 indeed interacts with p27 (Fig. 3A). This interaction was specific, because the Jab1-containing lysates were not immunoprecipitated with non-immune rabbit serum. To confirm these results, we examined the ectopic Jab1 and p27 interactions by transfecting Myc-Jab1 and Flag-p27 plasmids into 293T cells. As expected, Myc-Jab1 binds to Flag-p27 (Fig. 3B). Moreover, data showed most p27 in a predominantly nuclear location when Cherry-p27 was transfected into CNE1 cells, but both nuclear and cytoplasmic staining were seen when GFP-Jab1 was induced (Fig. 3C and S3). These data strongly indicate that Jab1 associates with p27 in NPC cells.
Figure 3. Jab1 specifically interacts with p27.
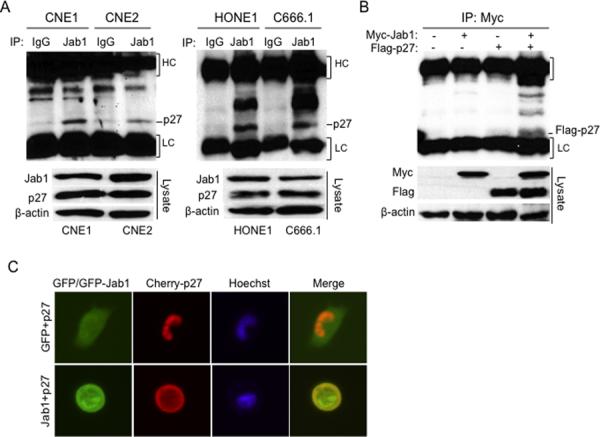
(A) Cell lysates were immunoprecipitated with either non-immune mouse serum or Jab1 and immunoblotted with anti-p27. Immunoglobulin G heavy chain (HC) and light chain (LC) were indicated. (B) 293T cells were transfected with either Myc-Jab1 or Flag-p27 or both for 48 hours, and then cell lysates were immunoprecipitated with Myc and immunoblotted with Flag. Cell lysates immunoblotted with the indicated antibodies are shown in the bottom panel. (C) CNE1 cells were transfected with a GFP vector and Cherry-p27 or GFP-Jab1 and Cherry-p27 for 48 hours, and then examined under a fluorescence microscope. Original magnification ×200.
Depletion of Jab1 by siRNA increases accumulation of p27 and induces cell-cycle arrest and inhibits cell proliferation in NPC cell lines
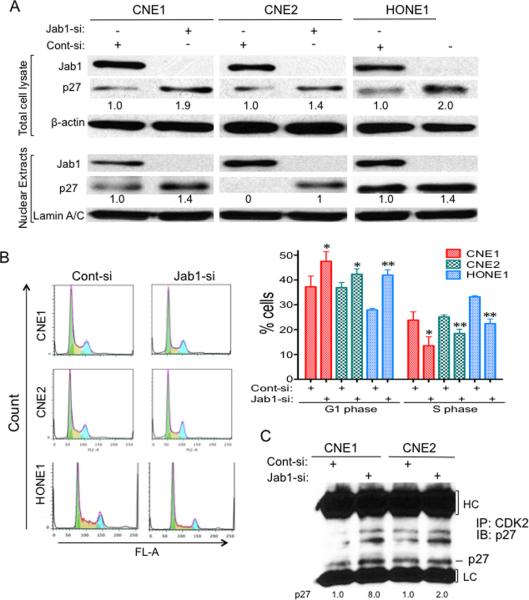
To assess the effect of silencing Jab1 in human NPC cells, we transfected CNE1 and CNE2 cells with Jab1 siRNA oligonucleotides or control siRNA oligonucleotides cloned into the RNAi-vector system. Forty-eight hours after transfection, Jab1 protein levels were substantially decreased (by > 97%) in both Jab1 siRNA-transfected cell lines compared with cells transfected with the control siRNA oligonucleotides (Fig. 4A). The decrease in endogenous Jab1 protein expression was associated with a significant increase in p27 levels and nuclear accumulation of p27 in nucleus (Fig. 4A), suggesting the biological importance of Jab1 in regulating p27 proteolysis in NPC.
Figure 4. Downregulation of Jab1 increases p27 levels and induces cell-cycle arrest in NPC.
(A) Cells were transfected with either a siRNA targeting Jab1 or a scrambled sequence (control); 48 hours later, the total cellular proteins and nuclear proteins were isolated and immunoblotted with anti-Jab1 and anti-p27 antibodies. Anti-β-actin or Lamin A/C antibodies were used as loading controls. (B) NPC cells were transfected as described in (A); 48 hours later, cells were stained with PI and then analyzed by flow cytometry. (Left) Representative results of cell cycle assays with NPC cells. (Right) The percentage of G1 and S phase cells are shown data represent three independent experiments, mean±s.d. * P <0.05, * *P <0.01. (C) Cells were transfected with either a siRNA targeting Jab1 or a scrambled sequence (control); 48 hours later, cell lysates were immunoprecipitated with Cdk2 and immunoblotted with anti-p27.
Next, to determine whether knockdown of Jab1 would lead to an increase in p27 activity, as indicated by inhibition of the G1-to-S phase progression, we used PI staining to analyze the proportion of cells that were in the G1 and S phases of the cell cycle. In CNE1 cells, 24% of the control siRNA-transfected cells were in the S phase compared with 37% of cells in the G1 phase, and 14% of the Jab1 siRNA-transfected cells were in the S phase compared with 47% of cells in the G1 phase (Fig. 4B). Similar results were obtained from CNE2 cells (25% in S phase and 37% in G1 phase for control cells versus 18% in S phase and 42% in G1 phase for Jab1 siRNA-transfected cells) and HONE1 cells (33% in S phase and 28% in G1 phase for control cells versus 22% in S phase and 42% in G1 phase for Jab1 siRNA-transfected cells). To confirm the above conclusion, we next conducted a co-immunoprecipitation experiment measure the amount of p27/cyclin E/Cdk2 inhibitory complex formation. As shown in Fig. 4C, p27 levels in Jab1 knockdown cells were higher when immunoprecipitated with Cdk2, indicating more complex formation. These data suggest that downregulation of Jab1 induces stabilization of p27, therefore increasing p27 levels and activity.
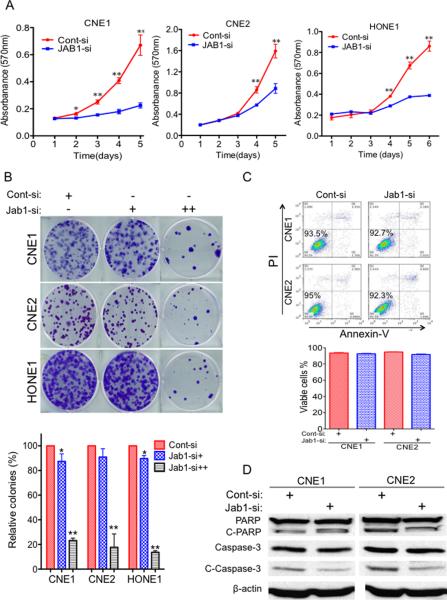
To investigate whether downregulation of Jab1 was involved in growth suppression, we first analyzed whether the siRNA-mediated Jab1 inhibition could recapitulate the tumor-suppressor effect in NPC cell lines. The expression levels of Jab1 protein in Jab1 siRNA-transfected NPC cells were significantly less than those in the control siRNA-transfected cells (Fig. 4A). Moreover, the siRNA-mediated Jab1 knockdown also significantly inhibited the in vitro growth and colony formation of NPC cells (Figs. 5A and B). Notably, the growth inhibitory effects of the Jab1-siRNA knockdown suggest that Jab1 targeting is a mechanism of tumor suppression in NPC cells. In addition, we tested the effect of knocking down Jab1 on apoptosis. Forty-eight hours after the siRNA was transfected, we used Annexin V and PI staining (Fig. 5C) or analysis of cleaved PARP and caspase-3 (Fig. 5D). However, there was no significant difference between the control and Jab1-siRNA treated groups; thus, we cannot conclude that Jab1 knockdown initiates apoptosis under these conditions.
Figure 5. Knockdown of Jab1 inhibits cell proliferation.
(A) NPC cells were transfected with Jab1 siRNA for 48 hours, and then cell growth was determined by an MTT assay. Control-siRNA (Cont-si); Jab1-siRNA (Jab1-si). (B) Representative results of colony formation assays with NPC cells treated with control siRNA or different doses of 1 nM (+) or 5 nM (++) of Jab1 siRNA. Jab1 siRNA. (Bottom) Quantification of the relative number of colonies is shown. Data represent three independent experiments, mean±s.d. *P <0.05, **P <0.01. (C) Cells were treated with control siRNA or Jab1 siRNA for 48 hours, and then stained with Annexin V and PI and measured by flow cytometry. (Bottom) Quantification of the percentage of living cells. Data represent three independent experiments, mean±s.d. (D) Cells were transfected with siRNA for 48 hours, and then cell lysates were immunoblotted with the indicated antibodies for apoptosis analysis.
Jab1 depletion enhances the antitumor effects of cisplatin in NPC
Since cisplatin is the main treatment for NPC, we proposed to investigate Jab1 may involve in the antitumor effects of cisplatin. We firstly tested whether inhibition of Jab1 enhances the antitumor effects of cisplatin by MTT assay. Data showed that 21%, 18%, and 7% of CNE1, CNE2 and HONE1 cells treated with control siRNA and cisplatin were inhibited, whereas, 38%, 38% and 26% cells were inhibited by Jab1 siRNA and cisplatin, suggesting Jab1-knockdown NPC cells were more sensitive to cisplatin than cells treated with control siRNA (Fig. 6A). To test whether Jab1 plays a role in anchorage-independent growth in response to cisplatin, which may reflect in vivo tumorigenicity, we performed a colony formation assay in NPC cells treated with Jab1 siRNA and cisplatin. We found that the Jab1 knockdown cells had significant inhibition of colony formation (73% in CNE1, 90% in CNE2, and 66% in HONE1) compared with the control siRNA-treated cells (22% in CNE1, 60% in CNE2, and 14% in HONE1) in response to cisplatin (Fig. 6B).
Figure 6. Jab1 depletion enhances the antitumor effects of cisplatin in NPC cells.
(A) Forty-eight hours after the cells were treated with control (Cont-si) or Jab1 siRNA (Jab1-si), NPC cells were treated with the 2 μM of cisplatin (CP) for another 48 hours, and the growth-inhibitory effects of cisplatin were quantified by an MTT assay. The inhibition ratio (%) is marked on the graphs. Data represent three independent experiments, mean±s.d. **P <0.01. (B) 1 nM (+) or 5 nM (++) of Jab1 siRNA-treated NPC cells were seeded into 6-well plates and exposed to cisplatin (CP) for 48 hours, and 10 days later the number of colonies formed were counted. (Left) Representative results of colony formation assays. (Right) Quantification of the relative number of colonies. The inhibition ratios (%) are marked on the graphs. Data represent three independent experiments, mean ±s.d. *P <0.05, **P <0.01. (C) siRNA-transfected NPC cells were stained with Annexin V and PI after cisplatin treatment for 48 hours. (Left) Representative results of apoptosis assays in NPC cells. (Right) Quantification of the percentage of apoptotic cells. Data represent three independent experiments, mean±s.d. *P <0.05, **P <0.01. (D) siRNA-transfected NPC cell were exposed to 10 μM of cisplatin for 48h; apoptotic cells were measured by the western blot analysis of cleaved PARP and cleaved caspase-3. Protein levels were quantified using ImageJ software.
We further examined whether Jab1 knockdown could enhance cisplatin-induced apoptosis in NPC cells. We found that cisplatin induced more apoptosis in Jab1 knockdown cells than that in control siRNA-treated cells, which is 1.4 folds in CNE1 and 4.7 folds in CNE2 respectively (Fig. 6C) when using Annexin V and PI staining, and 1.6 in CNE1 and 3.2 in CNE2 when using Hochst33342 staining (Fig. 2S). However, there were no significant differences in apoptosis induced by control and Jab1 siRNA. Proteolytic cleavage of PARP and cleaved caspase-3 are the hallmarks of apoptosis. Thus, we also examined the effect of Jab1 siRNA on the proteolytic cleavage of PARP and cleaved caspase-3 in response to cisplatin. Compared with the control siRNA-treated cells, cisplatin consistently induced more proteolytic cleavage of PARP (12% change in CNE1, 30% change in CNE2, and 6% change in HONE1) and cleaved caspase-3 (11% change in CNE1, 50% change in CNE2, and 31% change in HONE1) in Jab1 knockdown cells (Fig. 6D).
Discussion
The molecular basis of NPC pathogenesis remains poorly defined, and this has hindered the development of new treatments. Therefore, identification of novel mechanisms involved in NPC oncogenesis is needed.
In this study, we identified the role of Jab1-mediated p27 degradation in NPC oncogenesis. First, we found Jab1 was aberrantly expressed in either cytoplasm or nucleus NPC tissues compare to noncancerous inflamed nasopharyngeal tissue. All (14/14) strong Jab1-positive patients died within 41 months whereas 11 of 31 weak or Jab1-negative patients were alive after 60 months. We also showed that Jab1 protein is overexpressed in NPC cell lines but not in paired normal keratinocyte cells. Our findings on p27 expression also confirm those of previous reports. For instance, Hwang et al (11) reported that in NPC patients 47 of 69 cases expressed low levels of p27. In our study, 29 of 45 NPC cases (64%) expressed low or no nuclear p27, and 28 cases (62%) expressed low or no cytoplasmic p27. In addition, p27's nuclear-cytoplasmic translocation has been observed in human tumors and is associated with poor survival (30–33), it was agreement with our studies that NPC patients with cytoplasmic positive p27 had a poor survival.
Furthermore, cytoplasmic Jab1 expression in NPC tissues was inversely associated with nuclear p27 patterns (R=−0.389, P = 0.008), and correlated with cytoplasm p27 (R=0.328, P = 0.028). Our finding of an inverse correlation between Jab1 and p27 expression levels in NPC is in agreement with our previously published results on breast cancer and pancreatic carcinomas (20, 24) and similar associations reported in other epithelial or lymphoid malignancies (21, 22). This suggesting that Jab1 has a physiologic role in controlling p27 levels in NPC. To our knowledge, this is the first study on the expression and function of Jab1 in NPC to be reported.
Jab1 promotes cell proliferation by interacting directly with p27 and induces nuclear export and subsequent p27-degradation (34). p27 is a critical component of the cell-cycle machinery (16). As an inhibitor of cyclin E-Cdk2, p27 plays a pivotal role in controlling cell proliferation and therefore the cell's entry into S phase and exit from G1 phase during development and tumorigenesis (18, 35). We infected NPC cell lines with an inducible Jab1-expressing adenoviral vector and found that p27 levels were significantly reduced. These results are in agreement with those we previously obtained in breast cancer and pancreatic cell lines (20, 24). Furthermore, knockdown of endogenous Jab1 expression with siRNA in NPC cell lines led to a significant increase in total p27 levels and nuclear accumulation of p27 in the nucleus and p27/cyclin E/Cdk2 complex, substantial decreases in the number of S-phase cells, and increases in the number of G1-phase cells. In addition, a direct p27-Jab1 interaction was confirmed by co-immunoprecipitation assays and fluorescent microscopy. These results provide additional evidence that Jab1 controls p27 levels post-translationally by accelerating p27 degradation in NPC.
We also showed that loss of Jab1 resulted in growth inhibition in NPC cell lines. Similarly, a study by Supriatno (36) et al. showed that suppression of Jab1 inhibited the growth of human head and neck cancer cells (HNt and HSY cells) and delayed tumor growth in murine xenografts. It suggests that strong expression of Jab1 not only represents a prognostic marker for malignant transformation but also contributes to cancer cell proliferation and survival. Furthermore, we found that knockdown of Jab1 sensitized NPC cells to cisplatin chemotherapy. Jab1 siRNA-treated cells were more susceptible to apoptotic stimuli than the control siRNA-treated cells. Taken together, the data implicates Jab1 as a potential biomarker and further suggests exploration of whether decreasing Jab1 levels or function would overcome resistance to cisplatin chemotherapy in NPC. The significance of these functions of Jab1 in NPC needs to be explored in future mechanistic studies.
Overall, we found Jab1 to be overexpressed and inversely correlated with p27 expression levels in NPC. Jab1 overexpression associated with low p27 levels or other factors may contribute to uncontrolled cell-cycle progression and tumorigenesis. Depletion of Jab1 by siRNA, which leads to inhibition of proliferation, enhanced cisplatin activity in NPC. Therefore, modulation of the Jab1 gene product is a novel target for therapies in NPC.
Supplementary Material
Acknowledgments
This work was supported by a fellowship from China Scholarship Council (2010638087) (YP), a grant from National Cancer Institute (RO1-CA90853) (FXC); National Natural Science Foundation of China (81071837; 30670627); Natural Science Foundation of Guangdong Province, China (9251008901000005; 06021210) and Scientific and Technological Project of Guangdong, China (2008A030201009; 2010B050700016) (HY). We thank Kate J. Newberry for editing the manuscript.
Footnotes
Conflict of interest statement: The authors have no conflicts of interest to declare.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;365:2041–54. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Spano JP, Busson P, Atlan D, Bourhis J, Pignon JP, Esteban C, et al. Nasopharyngeal carcinomas: an update. Eur J Cancer. 2003;39:2121–35. doi: 10.1016/s0959-8049(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 3.Ong YK, Heng DM, Chung B, Leong SS, Wee J, Fong KW, et al. Design of a prognostic index score for metastatic nasopharyngeal carcinoma. Eur J Cancer. 2003;39:1535–41. doi: 10.1016/s0959-8049(03)00310-1. [DOI] [PubMed] [Google Scholar]
- 4.Yip WK, Leong VC, Abdullah MA, Yusoff S, Seow HF. Overexpression of phospho-Akt correlates with phosphorylation of EGF receptor, FKHR and BAD in nasopharyngeal carcinoma. Oncol Rep. 2008;19:319–28. [PubMed] [Google Scholar]
- 5.Zheng XK, Chen LH, Wang WJ, Ye F, Liu JB, Li QS, et al. Impact of prolonged fraction delivery times simulating IMRT on cultured nasopharyngeal carcinoma cell killing. International journal of radiation oncology, biology, physics. 2010;78:1541–7. doi: 10.1016/j.ijrobp.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–7. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Yau TK, Wong DH, Chan EW, Yeung RM, Ng WT, et al. Treatment of stage IV(A–B) nasopharyngeal carcinoma by induction-concurrent chemoradiotherapy and accelerated fractionation. Int J Radiat Oncol Biol Phys. 2005;63:1331–8. doi: 10.1016/j.ijrobp.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 8.Le QT, Tate D, Koong A, Gibbs IC, Chang SD, Adler JR, et al. Improved local control with stereotactic radiosurgical boost in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:1046–54. doi: 10.1016/s0360-3016(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 9.Bei JX, Li Y, Jia WH, Feng BJ, Zhou G, Chen LZ, et al. A genome-wide association study of nasopharyngeal identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 10.Natasya Naili MN, Hasnita CH, Shamim AK, Hasnan J, Fauziah MI, Narazah MY, et al. Chromosomal alterations in Malaysian patients with nasopharyngeal carcinoma analyzed by comparative genomic hybridization. Cancer Genet Cytogenet. 2010;203:309–12. doi: 10.1016/j.cancergencyto.2010.07.136. [DOI] [PubMed] [Google Scholar]
- 11.Hwang CF, Su CY, Huang SC, Huang CC, Fang FM, Lui CC, et al. Low expression levels of p27 correlate with loco-regional recurrence in nasopharyngeal carcinoma. Cancer Lett. 2003;189:231–6. doi: 10.1016/s0304-3835(02)00508-6. [DOI] [PubMed] [Google Scholar]
- 12.Ko MT, Su CY, Huang SC, Chen CH, Hwang CF. Overexpression of cyclin E messenger ribonucleic acid in nasopharyngeal carcinoma correlates with poor prognosis. J Laryngol Otol. 2009;123:1021–6. doi: 10.1017/S0022215109004848. [DOI] [PubMed] [Google Scholar]
- 13.Mei YP, Zhou JM, Wang Y, Huang H, Deng R, Feng GK, et al. Silencing of LMP1 induces cell cycle arrest and enhances chemosensitivity through inhibition of AKT signaling pathway in EBV-positive nasopharyngeal carcinoma cells. Cell Cycle. 2007;6:1379–85. doi: 10.4161/cc.6.11.4274. [DOI] [PubMed] [Google Scholar]
- 14.Wei N, Deng XW. The COP9 signalosome. Annu Rev Cell Dev Biol. 2003;19:261–86. doi: 10.1146/annurev.cellbio.19.111301.112449. [DOI] [PubMed] [Google Scholar]
- 15.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–7. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 16.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–5. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 17.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 18.Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 19.Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 20.Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1) Cancer Res. 2003;63:2977–81. [PubMed] [Google Scholar]
- 21.Rassidakis GZ, Claret FX, Lai R, Zhang Q, Sarris AH, McDonnell TJ, et al. Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:1121–8. [PubMed] [Google Scholar]
- 22.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tai Y, et al. Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res. 2001;7:4130–5. [PubMed] [Google Scholar]
- 23.Fukumoto A, Ikeda N, Sho M, Tomoda K, Kanehiro H, Hisanaga M, et al. Prognostic significance of localized p27Kip1 and potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep. 2004;11:277–84. [PubMed] [Google Scholar]
- 24.Kouvaraki MA, Korapati AL, Rassidakis GZ, Tian L, Zhang Q, Chiao P, et al. Potential role of Jun activation domain-binding protein 1 as a negative regulator of p27kip1 in pancreatic adenocarcinoma. Cancer Res. 2006;66:8581–9. doi: 10.1158/0008-5472.CAN-06-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berg JP, Zhou Q, Breuhahn K, Schirmacher P, Patil MA, Chen X, et al. Inverse expression of Jun activation domain binding protein 1 and cell cycle inhibitor p27Kip1: influence on proliferation in hepatocellular carcinoma. Hum Pathol. 2007;38:1621–7. doi: 10.1016/j.humpath.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Yoshida H, Matsuzuka F, Matsuura N, Nakamura Y, Nakamine H, et al. Jun activation domain-binding protein 1 expression in malignant lymphoma of the thyroid: its linkage to degree of malignancy and p27 expression. Anticancer Res. 2003;23:4121–5. [PubMed] [Google Scholar]
- 27.Shen L, Tsuchida R, Miyauchi J, Saeki M, Honna T, Tsunematsu Y, et al. Differentiation-associated expression and intracellular localization of cyclin-dependent kinase inhibitor p27KIP1 and c-Jun co-activator JAB1 in neuroblastoma. Int J Oncol. 2000;17:749–54. doi: 10.3892/ijo.17.4.749. [DOI] [PubMed] [Google Scholar]
- 28.Dahinden C, Ingold B, Wild P, Boysen G, Luu VD, Montani M, et al. Mining tissue microarray data to uncover combinations of biomarker expression patterns that improve intermediate staging and grading of clear cell renal cell cancer. Clin Cancer Res. 2010;16:88–98. doi: 10.1158/1078-0432.CCR-09-0260. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Peng G, Parant JM, Leventaki V, Drakos E, Zhang Q, et al. Essential roles of Jab1 in cell survival, spontaneous DNA damage and DNA repair. Oncogene. 2010;29:6125–37. doi: 10.1038/onc.2010.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 31.Masciullo V, Sgambato A, Pacilio C, Pucci B, Ferrandina G, Palazzo J, et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res. 1999;59:3790–4. [PubMed] [Google Scholar]
- 32.Singh SP, Lipman J, Goldman H, Ellis FH, Jr., Aizenman L, Cangi MG, et al. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730–5. [PubMed] [Google Scholar]
- 33.Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, et al. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58:114–22. [PubMed] [Google Scholar]
- 34.Shackleford TJ, Claret FX. JAB1/CSN5: a new player in cell cycle control and cancer. Cell Div. 2010;5:26. doi: 10.1186/1747-1028-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 36.Supriatno Harada K, Yoshida H, Sato M. Basic investigation on the development of molecular targeting therapy against cyclin-dependent kinase inhibitor p27Kip1 in head and neck cancer cells. Int J Oncol. 2005;27:627–35. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.