Abstract
In patients with advanced ovarian cancer, it can be challenging to evaluate response to neoadjuvant chemotherapy on computed tomography (CT) due to disseminated small volume disease and serosal disease. In addition, measuring the change in size of tumour burden takes time in order to allow tumour shrinkage. Despite these challenges, serum CA-125 levels and CT are the standard tools for the assessment of treatment response in ovarian cancer. New functional imaging techniques may allow the identification of response earlier and with higher accuracy. In this review article, we describe the current literature on functional imaging techniques in ovarian cancer response assessment, focusing on fluorodeoxyglucose-positron emission tomography.
Keywords: FDG-PET, FDG-PET/CT, ovarian cancer, response assessment, DWI-MRI, DCE-MRI
Introduction
Ovarian cancer is the most lethal gynaecological cancer in developed countries and the fifth cause of cancer death in women in the United States[1]. The poor prognosis associated with the diagnosis of ovarian malignancy is partly attributed to its detection at advanced stages with disseminated disease, as well as due to the high rates of persistent or recurrent disease following treatment[2–4].
There is a need for significant improvements to be made in the management of ovarian cancer. In this article, we describe advances being made in the early detection of response to chemotherapy that have the potential to identify non-responders who may, in future, benefit from early change of treatment.
Standard method of measuring chemotherapy response
Neoadjuvant chemotherapy (Na-CHT) is a treatment option in patients with stage IV disease or extensive tumour load that is not considered to be suitable for primary optimal cytoreductive surgery[5]. This strategy may lead to improvements in survival and an improved radical resectability rate. For patients undergoing Na-CHT, cytoreductive surgery is usually attempted after 3 or 4 cycles of therapy (interval debulking surgery (IDS)), although in some cases patients undergo definitive cytoreductive surgery after 5 to 6 courses of Na-CHT[5].
Currently, objective response to chemotherapy is first evaluated after 3 cycles of chemotherapy, to allow time for tumour shrinkage. There are two main problems with this approach. First, it can be very challenging to evaluate the extent of tumour shrinkage accurately and reproducibly in patients with diffuse small volume serosal disease. Second, according to RECIST criteria, tumour response is defined as a decrease of the maximum tumour diameter by at least 30%[6], but this can only be identified after several cycles of chemotherapy[7]. Patients may be subjected to multiple cycles of ineffective treatment, with the associated morbidity, in addition to delaying a change in treatment to an alternative therapy.
It would be desirable to have a non-invasive technique to identify those patients who are not responding to Na-CHT after a single cycle of Na-CHT, in order to alter the treatment regime earlier in the course of disease. Functional imaging techniques have been evaluated for this purpose.
Metabolic response assessment
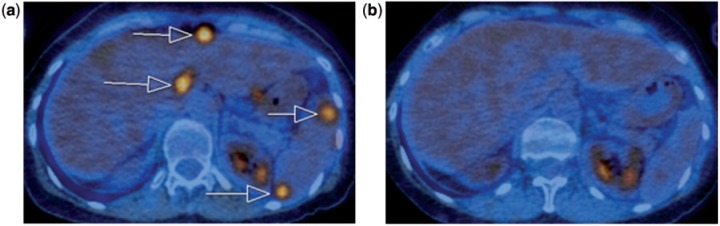
Tumour metabolic response using fluorodeoxyglucose (FDG)-positron emission tomography (PET) has been studied in advanced ovarian cancer and the few reported results suggest that there is potential to improve early detection of response (Fig. 1).
Figure 1.
Axial fused FDG-PET/CT image at baseline scan (a) shows multiple areas of high-grade peritoneal FDG uptake in the upper abdomen (arrows). After the first cycle of chemotherapy, follow-up imaging (b) shows no residual FDG-avid lesions, consistent with a complete metabolic response. Physiological FDG activity is seen in the kidneys.
Early detection of response and prediction of outcome
Avril et al.[7] describe the role of FDG-PET for the non-invasive prediction of response to Na-CHT in untreated advanced ovarian cancer. Their hypothesis was that changes in FDG uptake early in the course of treatment would allow prediction of the effectiveness of chemotherapy and patient outcome. They prospectively studied the use of sequential FDG-PET imaging at baseline, after cycle 1 and after cycle 3 of Na-CHT in 33 patients (although only 26 had PET imaging after cycle 1). Lesions were selected and a circular region of interest (ROI) of 1.5 cm in diameter was placed at the point of maximum uptake, reducing partial volume effects. The mean standardized uptake value (SUV) at this point was recorded. Changes in FDG uptake in the tumour over the course of treatment were then compared with overall patient survival.
They chose a prospectively determined threshold of 20% decrease in FDG uptake after the first cycle of chemotherapy to identify metabolic non-responders, a conservative threshold that would ensure the identification of all potential responders, even at the cost of not identifying all non-responders. Of the 26 patients who were imaged after the first cycle of Na-CHT, 15 were responders according to this definition and this group had a mean decrease in SUV of 59.5 ± 19%, whereas 11 non-responders had a mean decrease in SUV of 4 ± 13.3%.
A threshold of 55% decrease in FDG uptake after the third cycle of chemotherapy was identified retrospectively as the optimal cut-off that produced the most significant difference in metabolic responders (18 patients) versus non-responders (15 patients). Using this definition, they found an overall decrease in FDG uptake of 50.1% after the first cycle of chemotherapy and of 76.2% after the third cycle of chemotherapy in metabolic responders with 65.7% of the metabolic changes occurring within the first 2 weeks after initiation of chemotherapy.
There was a significant correlation between the metabolic response after the first and the third cycles of chemotherapy and overall survival. The median survival for metabolic responders after the first cycle of chemotherapy was 38.3 months, compared with 23.1 months in metabolic non-responders (P = 0.008) and after the third cycle, the median survival was 38.9 months in metabolic responders compared with 19.7 months in metabolic non-responders (P = 0.005).
They also found that clinical response criteria (intraoperative residual tumour <4 cm, regression of peritoneal carcinomatosis, and/or decrease in Ca-125 level >75% from baseline or complete normalization to <35 U/mL) and the histopathological response criteria showed only a weak correlation with overall survival.
Although the number of cases was small, this prospective study showed that sequential FDG-PET predicted patient outcome as early as after the first cycle of Na-CHT and was more accurate than CA-125.
This study also found that surgery in metabolic responders achieved a higher rate of complete tumour resections compared with non-responding patients and macroscopically tumour-free surgery was achieved in 33% of metabolic responders compared with only 13% of non-responders.
Metabolic response to inform the timing of cytoreductive surgery
Martoni et al.[8] focused on whether the timing of surgery following Na-CHT might be informed by metabolic response on FDG-PET. They prospectively measured changes in tumour metabolic activity at baseline, post cycle 3 and post cycle 6 of Na-CHT in 46 patients unsuitable for primary cytoreductive surgery. Patients underwent surgery following 6 cycles and histopathological findings were compared with percentage changes in the maximum SUV (SUVmax). They found that in patients in whom there was a decrease in SUVmax of 100% at 3 cycles, 88% achieved complete pathological response with no post surgical residual disease, whereas in those patients with a decrease in SUVmax of <100% at 3 cycles, only 24% were pathological responders at the end of 6 cycles of Na-CHT.
The authors suggest that patients who achieve good metabolic response may in fact benefit from completing 6 cycles of Na-CHT prior to undergoing definitive surgery, whereas those with only a partial metabolic response should have IDS after 3 cycles to remove potentially chemoresistant tumour[8]. This is an interesting approach and may be the subject of further studies. No patient outcome data are presented in this study.
End of treatment assessment of response
In a retrospective study, using histopathology tumour regression as a gold standard, Nishiyama et al.[9] evaluated the ability of FDG-PET to predict the response of primary tumour to Na-CHT in advanced gynaecological cancer but this study only included 8 patients with ovarian cancer, in addition to 9 patients with cervical cancer and 4 with endometrial cancer. All the patients were submitted to baseline FDG-PET followed by a second FDG-PET about 12 days after the end of therapy. The SUVmax of the primary tumour before and after therapy was measured, the percentage change was calculated and pathology was used as the reference standard. Taking an arbitrary SUVmax after treatment of 3.8 as the cut-off for differentiating between responders and non-responders, FDG-PET showed a sensitivity of 90%, a specificity of 63.6%, and an accuracy of 76.2%. However, it is difficult to interpret the role of FDG-PET in the context of such a mixed group of patients. In addition, this method does not allow assessment of response early in the course of treatment.
Potential role as a predictive biomarker of response
Several authors have studied correlations between tumour FDG uptake and non-imaging biomarkers of response.
Kurokawa et al.[10] found statistically significant positive correlations between FDG uptake in ovarian epithelial tumours (borderline n = 2, benign tumour n = 2, primary ovarian cancer n = 13), and tumour grade (histologic grading score; r = 0.692, P = 0.005), tumour proliferation rate (immunohistochemical measurement of MIB-1, proliferation index marker; r = 0.457, P = 0.014), and glucose metabolism (immunohistochemical measurement of GLUT-1 expression; r = 0.76, P = 0.001), all of which are biomarkers for response to chemotherapy, prognosis and overall survival in primary ovarian cancer patients. Logistic regression analysis revealed that the expression of GLUT-1 transporters was the strongest parameter by which to predict positive FDG uptake (r = 0.76, P = 0.001).
In another study, Canturia et al.[11] reported that GLUT-1 status is an independent prognostic factor of response to chemotherapy in advanced ovarian carcinoma; of the patients with FIGO stage III or IV disease who undergo complete clinical response, those who overexpress GLUT-1 have a significantly shorter disease-free survival rate. Combined with the findings of Kurokawa et al., this would support the use of FDG-PET as a non-invasive biomarker able to predict response to chemotherapy, prognosis and survival[10].
Ovarian cancer response assessment to surgery
Two studies (from the same group) have assessed response to primary cytoreductive surgery followed by chemotherapy, comparing results obtained from FDG-PET/CT with the histologic findings from second-look laparotomy (SLL) as the reference standard[12,13]. The accuracy of FDG-PET/CT in identifying persistent disease (lymph nodal lesion, peritoneal lesion and pelvic lesion) was evaluated. The overall lesion-based sensitivity, specificity and accuracy of PET/CT in the detection of persistent disease are shown in Table 1[12,13]. Picchio et al.[12] have compared the accuracy of FDG-PET/CT versus CT alone, finding a major advantage of PET/CT over CT alone in excluding the presence of residual viable lesions after treatment. Sironi et al.[13], in 31 patients with ovarian cancer (almost all (n = 25) papillary serous adenocarcinoma), found a high positive predictive value of PET/CT in the detection of residual neoplastic lesions (89% lesion-based; 82% patient-based). The authors stressed this finding, supporting the role of PET/CT in identifying patients with macroscopic disease who are candidates for salvage treatment, avoiding the morbidity and expense of invasive surgical second-look procedure. Nevertheless, they report that PET/CT has a low negative predictive value (57% lesion-based; 60% patient-based) with false-negative results occurring in patients with small volume disease. This is likely due to the spatial resolution of the technique (about 5 mm) and the limited capability of PET/CT to depict microscopic or small volume lesions, although the accuracy rate for detection of lesions >1 cm in diameter was higher (90%). Thus, a negative study is limited and in these patients the presence of low volume residual disease cannot be ruled out. The authors suggest that, in the future, a second-look procedure should be recommended only in responders and in patients with stable disease.
Table 1.
Assessment (persistent disease) at the end of primary treatment (cytoreductive surgery followed by platinum-based chemotherapy)
| Study | Year | Design | N | Technique | Compare with | Sensitivity (%) | Specificity (%) | Accuracy (%) | Comment | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Picchio et al.[12] | 2003 | P | 25 | PET/CT | SLL | 82.6 (LB) | 91.7 (LB) | 85.7 (LB) | Imaging within 30 days after the end of treatment. PET/CT findings and CT findings alone were correlated with SLL | |||
| CT alone | 69.56 (LB) | 83.33 (LB) | 74.28 (LB) | |||||||||
| Sironi et al.[13] | 2004 | P | 31 | PET/CT | SLL | 78 (LB) | 53 (PB) | 75 (LB) | 86 (PB) | 77 (LB) | 68 (PB) | Imaging at 21–35 days after the end of treatment. PET/CT findings were correlated with SLL |
P, prospective; LB, lesion-based; PB, patient-based; SLL, second-look laparotomy.
In a retrospective study, Kim et al.[14] compared the prognostic value of post-treatment FDG-PET/CT with SLL. The 55 patients included in the study had all had cytoreductive surgery followed by chemotherapy. At the end of treatment, 30 patients underwent SLL and 25 underwent FDG-PET/CT. There was no significant difference in progression-free interval (28.8 vs 30.6 months, P = 0.29) and no difference in the disease-free interval in the PET-negative patients compared with the SLL-negative patients (40.5 vs 48.6 months, P = 0.12). The authors found that PET imaging had a prognostic value similar to that of second-look surgery suggesting that FDG-PET can be used to replace SLL.
Evaluating response with functional MRI techniques
Functional MRI, including diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE)-MRI, have also been evaluated as tools for assessment of response in advanced ovarian cancer. DWI and the associated quantifiable apparent diffusion coefficient (ADC), which depicts the diffusivity of water within tissues, may change in response to Na-CHT due to cellular disruption and apoptosis. DCE-MRI allows quantification of changes in tissue vascularity, which may be disrupted in response to chemotherapy.
The role of DWI in assessing chemotherapy response has been explored for ovarian cancer by Kyriazi et al.[15] in a prospective study on 42 women with primary or recurrent disease. They used histogram analysis to quantify changes in the tumour before and after treatment. Pre-treatment ADC was not predictive of response. They found that responders had a significant change in ADC histogram parameters after the first and third cycle of Na-CHT, whereas there were no significant changes in non-responders. Changes in the 25th percentile of the histogram performed best in identifying response, and had a better positive predictive value than CA-125 levels and RECIST criteria. However, the technique did not improve the negative predictive value for the identification of non-responders and percent change of the 25th percentile did not predict 6-month progression-free survival.
Response to Na-CHT have been investigated by Sala et al.[16] using both DWI and DCE-MR in 22 patients with primary advanced ovarian cancer. Responders showed a significantly larger increase in the ADC and in the volume of the extravascular extracellular space (V(e)) parameter of DCE as a result of the cytotoxic effect of platinum-based chemotherapy[16]. However this occurred only in the primary ovarian lesion; no significant changes were found in omental or peritoneal deposits. The authors suggest the use of ADC and V(e) parameters as response markers to platinum-based therapy.
Mitchell et al.[17] studied the role of serial DCE-MRI in predicting which patients would have earlier relapse in 23 patients with asymptomatic residual volume ovarian cancer following chemotherapy. They also took plasma samples of a subset of these patients within which a panel of angiogenic biomarkers was quantified (soluble vascular endothelial growth factor receptor-1 and -2). They found that at 4 and 8 weeks post-baseline, the percent change of the enhancing fraction of the tumour differed significantly between patients whose disease remain stable and those who developed progressive disease by 26 weeks, and that the percentage change of whole tumour volume (WTV) correlated with progression-free interval. They also demonstrated a relationship between the circulating angiogenic biomarkers and DCE-MRI imaging biomarkers: a negative relationship with fractional blood plasma volume (Vp) and a positive relationship with volume transfer coefficient of contrast agent across the capillary wall (Ktrans).
Future research
It is not yet clear whether FDG-PET/CT will become a tool for the evaluation of treatment response in ovarian cancer. In some tumour types, such as lymphoma, the use of FDG-PET/CT has become a standard method for response assessment. It is perhaps surprising that there is so little published prospective research studying imaging biomarkers of response in ovarian cancer. Why is this? In patients with ovarian cancer, there is a useful and widely available serum marker, CA-125, and together with CT, this remains the standard method for response assessment. It may be that few functional imaging studies have been done because applying these expensive imaging techniques in multi-centre trials is too complex and expensive an undertaking when perhaps the results, although predictive, would not affect the actual patient outcomes. Another issue is that there are no standard alternative therapies available for platinum-resistant disease and therefore knowledge of early response may have limited impact on the treatment decisions. As new chemotherapy agents are discovered, it is possible that imaging will have an important role to play, as non-responders may then benefit from alternative therapies.
Conflict of interest
AR and NA were involved in a study on response assessment in recurrent ovarian cancer for which Merck paid for the scans in the study. No other financial remuneration was received. AM has no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. . PMid:22237781. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. . PMid:19474385. [DOI] [PubMed] [Google Scholar]
- 3.Palomar A, Nanni C, Castellucci P, et al. Value of FDG PET/CT in patients with treated ovarian cancer and raised CA125 serum levels. Mol Imaging Biol. 2011;14:123–129. doi: 10.1007/s11307-010-0468-9. . PMid:21240639. [DOI] [PubMed] [Google Scholar]
- 4.Gadducci A, Cosio S. Surveillance of patients after initial treatment of ovarian cancer. Crit Rev Oncol Hematol. 2009;71:43–52. doi: 10.1016/j.critrevonc.2008.12.008. . PMid:19179092. [DOI] [PubMed] [Google Scholar]
- 5.Aebi S, Castiglione M, Group EGW. Newly and relapsed epithelial ovarian carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl 4):21–23. doi: 10.1093/annonc/mdp117. PMid:19454452. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. . PMid:19097774. [DOI] [PubMed] [Google Scholar]
- 7.Avril N, Sassen S, Schmalfeldt B, et al. Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluorodeoxyglucose positron emission tomography in patients with advanced-stage ovarian cancer. J Clin Oncol. 2005;23:7445–7453. doi: 10.1200/JCO.2005.06.965. . PMid:16157939. [DOI] [PubMed] [Google Scholar]
- 8.Martoni AA, Fanti S, Zamagni C, et al. [18F]FDG-PET/CT monitoring early identifies advanced ovarian cancer patients who will benefit from prolonged neo-adjuvant chemotherapy. Q J Nucl Med Mol Imaging. 2011;55:81–90. PMid:21068714. [PubMed] [Google Scholar]
- 9.Nishiyama Y, Yamamoto Y, Kanenishi K, et al. Monitoring the neoadjuvant therapy response in gynecological cancer patients using FDG PET. Eur J Nucl Med Mol Imaging. 2008;35:287–295. doi: 10.1007/s00259-007-0627-7. . PMid:17943281. [DOI] [PubMed] [Google Scholar]
- 10.Kurokawa T, Yoshida Y, Kawahara K, et al. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer. 2004;109:926–932. doi: 10.1002/ijc.20057. . PMid:15027127. [DOI] [PubMed] [Google Scholar]
- 11.Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92:1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::AID-CNCR1432>3.0.CO;2-T. . PMid:11571727. [DOI] [PubMed] [Google Scholar]
- 12.Picchio M, Sironi S, Messa C, et al. Advanced ovarian carcinoma: usefulness of [(18)F]FDG-PET in combination with CT for lesion detection after primary treatment. Q J Nucl Med. 2003;47:77–84. PMid:12865867. [PubMed] [Google Scholar]
- 13.Sironi S, Messa C, Mangili G, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology. 2004;233:433–440. doi: 10.1148/radiol.2332031800. . PMid:15516617. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Chung JK, Kang SB, et al. [18F]FDG PET as a substitute for second-look laparotomy in patients with advanced ovarian carcinoma. Eur J Nucl Med Mol Imaging. 2004;31:196–201. doi: 10.1007/s00259-003-1367-y. PMid:15129701. [DOI] [PubMed] [Google Scholar]
- 15.Kyriazi S, Collins DJ, Messiou C, et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging–value of histogram analysis of apparent diffusion coefficients. Radiology. 2011;261:182–192. doi: 10.1148/radiol.11110577. . PMid:21828186. [DOI] [PubMed] [Google Scholar]
- 16.Sala E, Kataoka MY, Priest AN, et al. Advanced ovarian cancer: multiparametric MR imaging demonstrates response- and metastasis-specific effects. Radiology. 2012;263:149–159. doi: 10.1148/radiol.11110175. . PMid:22332064. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell CL, O'Connor JP, Jackson A, et al. Identification of early predictive imaging biomarkers and their relationship to serological angiogenic markers in patients with ovarian cancer with residual disease following cytotoxic therapy. Ann Oncol. 2010;21:1982–1989. doi: 10.1093/annonc/mdq079. . PMid:20351070. [DOI] [PubMed] [Google Scholar]