Abstract
Recent decades have seen a paradigm shift in the treatment of liver tumours from invasive surgical procedures to minimally invasive image-guided ablation techniques. Magnetic resonance-guided high-intensity focused ultrasound (MR-HIFU) is a novel, completely non-invasive ablation technique that has the potential to change the field of liver tumour ablation. The image guidance, using MR imaging and MR temperature mapping, provides excellent planning images and real-time temperature information during the ablation procedure. However, before clinical implementation of MR-HIFU for liver tumour ablation is feasible, several organ-specific challenges have to be addressed. In this review we discuss the MR-HIFU ablation technique, the liver-specific challenges for MR-HIFU tumour ablation, and the proposed solutions for clinical translation.
Keywords: MR-HIFU, focused ultrasound, liver tumour ablation, interventional oncology
Introduction
In the last few decades, the treatment paradigm of both primary and secondary liver tumours has shifted from invasive open surgical procedures to minimally invasive image-guided tumour ablation techniques. The most recent update to the Barcelona Clinic Liver Cancer (BCLC) treatment strategy for hepatocellular carcinoma recommends image-guided radiofrequency ablation (RFA) as the first-line treatment for very early stage (BCLC 0) patients, who do not qualify for liver transplantation[1]. For metastatic liver tumours surgical resection is still the preferred treatment, but minimally invasive image-guided treatment options are gaining ground: in particular, RFA is widely accepted as a potentially curative treatment option for patients who are not eligible for surgery[2]. The main advantages of image-guided tumour ablation techniques are lower peri-procedural morbidity and mortality, shorter hospital stays and improved quality of life[3].
High-intensity focused ultrasound (HIFU) is a novel, completely non-invasive image-guided tumour ablation technique. The principal therapeutic mechanism of HIFU ablation is the thermal energy deposition by a focused ultrasound beam. The ultrasound beam is generated by a high-power transducer; the focalization can be achieved by employing a phased array, bowl-shaped ultrasound transducer. The HIFU beam penetrates skin and other soft tissues without causing damage, but the tissue in the focal region of the beam (an area in the order of millimetres) absorbs significantly more acoustic energy than adjacent areas, resulting in a temperature increase[4,5]. This rapid temperature increase causes a localized coagulation necrosis while the surrounding tissue remains unharmed. Magnetic resonance (MR) imaging is used for image guidance. This provides high-quality anatomical and physiological data for the physician in real time. It also allows for treatment monitoring and assessment of the therapeutic end point.
In this review we discuss the clinical application of MR-HIFU for ablation of malignant liver tumours. We present challenges that are specific to liver tumour ablation, and discuss the proposed solutions that are currently under investigation and development.
MR-HIFU ablation
The first investigations on the therapeutic use of focused ultrasound date back to the 1940s[4]. Although many papers were published, especially during the 1970s and 1980s, the image guidance (using B-mode ultrasound) was of limited value only, since the temperature could not be monitored accurately during the ablation with the prevalent equipment. This issue was addressed when the HIFU ablation technique was combined with MR imaging (Fig. 1), which in turn led to a renewed interest in HIFU ablation and a surge in research papers in the 1990s[6].
Figure 1.
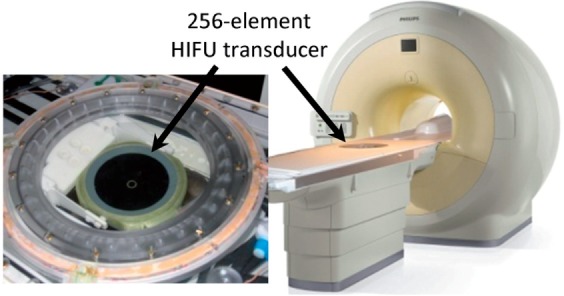
A 256-element HIFU transducer, installed on the table top of a clinical 1.5-T MR scanner.
In the Western world, the main clinical application of MR-HIFU is currently the ablation of uterine fibroids, and more recently the palliative ablation of bone metastases[7,8]. Uterine fibroid ablation has the advantage over oncological applications that complete tumour ablation is not a necessity, as a reduction in tumour bulk usually provides sufficient symptom relief[9]. Because of the widespread acceptance of MR-HIFU ablation of uterine fibroids, the use of MR-HIFU is steadily finding its way into daily clinical practice. However, the most promising clinical application of MR-HIFU is its use for non-invasive and precisely targeted ablation of malignant tumours.
MR-HIFU offers several advantages over conventional thermal ablation techniques such as RFA. Unlike these ablation techniques, MR-HIFU does not require the insertion of a probe in the body and is thus completely non-invasive. Furthermore, MR-HIFU relies much less on thermal conduction, since the entire ablation volume is directly heated by the deposited ultrasound energy[10,11]. The advantage of this ablation mechanism is that the temperature gradient around the ablation lesions can be made very steep, resulting in a sharply demarcated necrotic lesion with virtually no damage to the surrounding tissue[12].
Dedicated MR imaging techniques can be used for dynamic temperature mapping[13]. MR temperature mapping provides the physician with real-time spatio-temporal temperature information that can be used for treatment monitoring during the ablation process[5,6,14]. The most widely used MR temperature-mapping method is the proton resonance frequency shift (PRFS) method. This method relies on the temperature dependence of the electron screening constant of hydrogen nuclei in water. This effect leads to a shift in the observed proton resonance frequency in water-containing tissues when the temperature changes, and can be used to measure the temperature with a precision on the order of ±0.5°C in vitro[15]. The precision in vivo depends on multiple factors such as the tissue proton density and relaxation times, organ movement, receiver coil, voxel size, field of view and the required temporal resolution[13]. The temporal resolution for clinical applications depends mainly on the number of slices needed to cover the target area and to monitor heating in the near and far fields of the ultrasound beam, and is usually between 2 and 6 s. In addition, the combination with anatomical MR imaging provides images with excellent soft-tissue contrasts for tumour delineation during the treatment planning stage.
Temperature mapping and thermal dose
The temperature information obtained by MR temperature mapping can be used to assess the ablated tissue volume in two ways. First, coagulation necrosis is known to occur at temperatures over 57°C. Therefore, any tissue part that has been heated to a temperature above 57°C can be regarded as necrotic. The second frequently used quantity to reflect the inflicted thermal damage is the thermal dose. Thermal dose links the tissue temperature and the duration of the temperature elevation in a non-linear fashion to provide a quantity of tissue damage. Thermal dose is expressed in equivalent minutes at 43°C (CEM43)[16,17]. Pre-clinical studies have shown that the thermal dose required for necrosis ranges from ±50 to 240 CEM43 (depending on tissue type and study); therefore, 240 CEM43 is often used as the “lethal thermal dose limit” in quantifying the necrotic tissue area (Fig. 2). Two remarks should be made regarding thermal dose. First, the lethal thermal dose differs between tissue types and has not been established for tumour tissue of various types[10,18,19]. Second, papers that describe the correlation between thermal dose and necrotic tissue area in vivo generally have no, or very limited, longitudinal follow-up. Therefore, secondary cell death that might take several hours to days to develop (e.g., due to oedema or hypoperfusion) is not taken into account[20]. To characterize these processes further studies are needed, including a comprehensive follow-up of ablated tumours and analyses of MR imaging and histological samples.
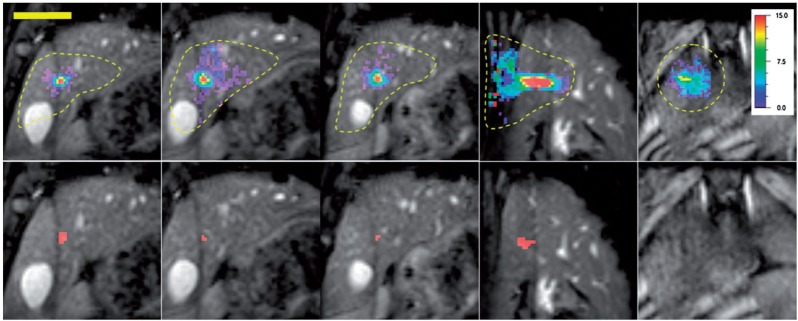
Figure 2.
Temperature (top row) and thermal dose (bottom row) images of a pig liver during MR-HIFU ablation. The temperature-increase scale is displayed on the right. The pixels coloured red correspond to a thermal dose of 240 CEM43 and above. Left to right: three coronal slices centred around the focal point, one sagittal slice centred on the focal point, and one slice located near the skin. The broken contours in the temperature images show the selected regions of interest (ROIs) for displaying the temperature data. The temperature images display the temperature distribution measured at the end of MR-HIFU ablation, and the thermal dose images are the final images in the time series. (Reprinted from Quesson et al.[53] with permission.)
Volumetric ablation
Initially, MR-HIFU ablation was performed on a point-by-point basis: the entire tissue volume was ablated by sequentially targeting multiple small foci of a few millimetres in diameter. Apart from being very time consuming, this method is inefficient because a large part of the absorbed energy is lost by heat diffusion after each point ablation. For this reason volumetric MR-HIFU was introduced, using initially mechanical beam displacements and subsequently electronic beam steering (Fig. 3)[21–23]. With volumetric MR-HIFU ablation, the focal point of the ultrasound beam is steered along multiple points on circular or spiral trajectories perpendicular to the ultrasound beam. The fast (50 ms) switching between different focal points provides a uniform heating of the specified tissue volume, or treatment cell. By starting in the middle of the treatment cell, diffusing heat is effectively used to heat the entire treatment cell. In this way the diameter of the tissue volume that can be ablated at once can be increased from approximately 2 mm to 16 mm or more.
Figure 3.
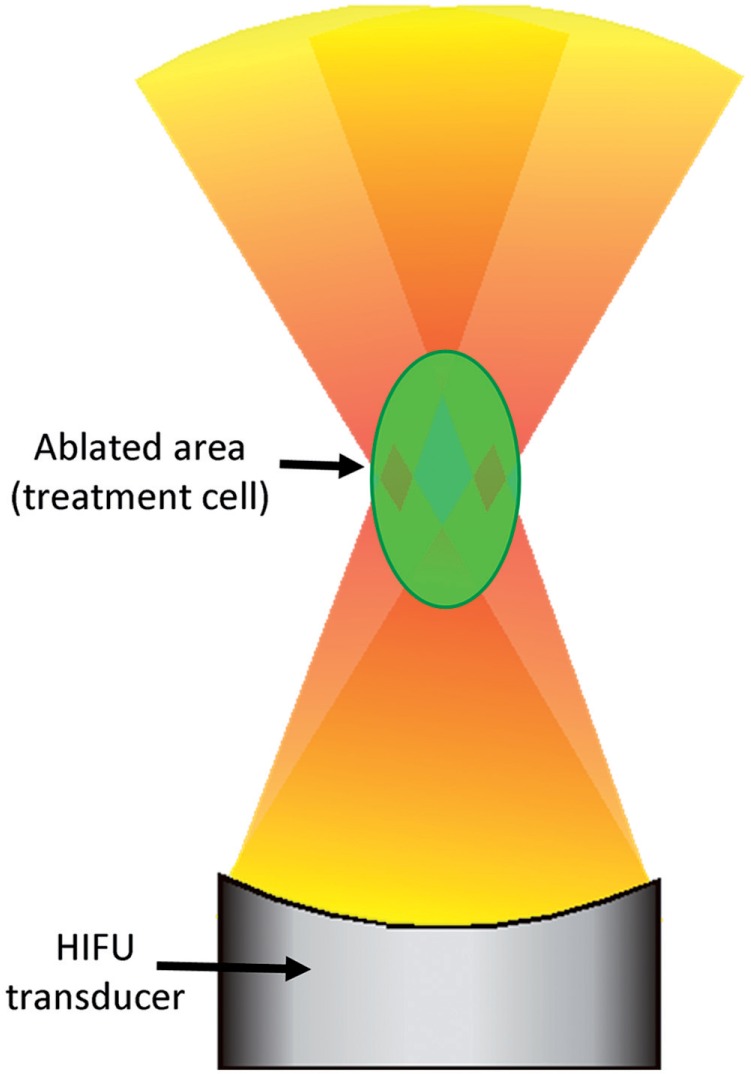
Volumetric ablation works by steering the focal point of the HIFU beam along multiple points in the treatment cell with fast (50 ms) switching, resulting in a homogeneous heating throughout the treatment cell.
Liver tumour ablation
As discussed before, MR-HIFU represents the ideal surgical tool in several ways: HIFU precisely and effectively destroys all tumour cells, while the MR guidance allows for precise targeting of the tumour, real-time control of the ablation procedure and treatment evaluation. It has long been recognized that for patients with liver tumours, these properties make MR-HIFU an attractive treatment option[24].
Pre-clinical studies
The first focused ultrasound ablation of liver tissue was performed by Lynn et al. in 1942[4]. Because of conflicting study results, relatively little attention was paid to HIFU for decades, until the 1980s. Ter Haar and others did valuable research by studying the shape, size and reproducibility of focused ultrasound lesions in liver tissue,[24–27] and the group of Chapelon studied ablation of Vx2 tumour-bearing rabbit livers[28,29]. In these studies, focused ultrasound ablation of liver tumour tissue was effective, but suffered from incomplete tumour destruction and frequent damage to surrounding organs. This was mainly caused by the lack of proper image guidance.
In 1993, Hynynen et al. were the first to use real-time MR guidance for HIFU ablation, thereby renewing the interest in HIFU[6,30]. MR-HIFU ablations of liver tissue have been performed in pig and dog liver by Kopelman et al.[31,32]. This group used general anaesthesia and controlled apnoeas to prevent liver motion during ablation, and reported that the MR guidance was reliable for temperature mapping and predicting the necrotic tumour volume, as assessed at histological evaluation.
Clinical studies
The literature to date is summarized in Table 1. In the 1990s the first liver tumour ablations using ultrasound-guided HIFU were performed in man. The group of Ter Haar evaluated the safety and performance of ultrasound-guided HIFU ablation in 28 patients, and concluded that the ablations were effective, with the only side effects being transient pain and skin burns. However, in neither of these studies was it attempted to ablate tumours entirely. Also, the investigators mentioned the limitations of ultrasound guidance, i.e., difficulties in visualizing the tumour during ablation[33–35]. Wu et al. have the most extensive experience with ultrasound-guided HIFU ablation of liver tumours. They treated 474 patients with primary and secondary liver tumours[36,37]. However, it should be noted that these investigators performed surgical rib resections in patients where the ribs would obstruct the ultrasound beam, to create an acoustic window, thereby eliminating the non-invasive aspect of the procedure. In addition, intra-arterial chemoembolization was performed in many patients before HIFU treatment to reduce tumour size and perfusion, in an attempt to enhance the efficacy of the HIFU ablation[36]. The first and only report of an MR-guided HIFU liver tumour ablation in man was published in 2006 by Okada et al. from Japan[38]. This group treated a patient with a hepatocellular carcinoma of 15 mm in diameter in the left lateral liver segment, thereby avoiding the ribs. A respiratory monitoring system with colour lamp indicator was used to help the patient perform repeated breath holds at the same point in the respiratory cycle. Okada et al. concluded that MR-HIFU ablation of liver tumours is promising but that technical advances are needed for successful clinical implementation, in particular concerning respiratory motion of the liver and planning of an acoustic beam path that avoids ribs and bowel loops.
Table 1.
Overview of clinical (MR- or ultrasound-guided) HIFU liver tumour ablation studies
| Authors[Ref.] | Year | Patients (n) | Image guidance | Study design | Study objective |
|---|---|---|---|---|---|
| Okada et al.[38] | 2006 | 1 | MR-guided | Case report | Feasibility |
| Visioli et al.[33] | 1999 | 6 | Ultrasound-guided | Prospective phase I patient series | Safety and feasibility |
| Wu et al.[36] | 2004 | 474 | Ultrasound-guided | Retrospective patient series | Safety and feasibility |
| Illing et al.[34] | 2005 | 22 | Ultrasound-guided | Prospective patient series | Safety and efficacy |
| Li et al.[54] | 2007 | 151 | Ultrasound-guided | Case-control study | Tumour response and survival |
| Orsi et al.[52] | 2010 | 23 | Ultrasound-guided | Prospective patient series | Safety and efficacy |
| Jung et al.[55] | 2011 | 79 | Ultrasound-guided | Retrospective patient series | Complications |
| Xu et al.[56] | 2011 | 145 | Ultrasound-guided | Prospective patient series | Efficacy and complications |
| Zhang et al.[57] | 2011 | 27 | Ultrasound-guided | Prospective patient series | Imaging response |
| Jin et al.[58] | 2011 | 73 | Ultrasound-guided | Prospective patient series | Long-term follow-up |
| Fukuda et al.[59] | 2011 | 12 | Ultrasound-guided | Prospective patient series | Safety and efficacy |
Challenges for successful MR-HIFU liver tumour ablation
Although MR-HIFU potentially has many advantages for the treatment of liver tumours, there are several organ-specific challenges that so far have hampered clinical adoption. A lot of effort has been made to find technical solutions to these challenges, and much progress has been made. However, most of the proposed solutions are still in pre-clinical development and require validation under clinical conditions. The most essential problems are discussed here.
Motion of the liver
A major difficulty with MR-HIFU liver tumour ablation is the physiological motion of the liver, which is principally caused by the respiratory cycle and, to a lesser extent, by cardiac motion and bowel movements. This means that the targeted tumour moves continuously, which complicates both a precise and effective HIFU energy deposition and artefact-free MR temperature mapping[39]. Although respiratory-gated approaches have been proposed owing to their technical simplicity, this approach significantly impairs the duty cycle of the ablation process[38]. This is particularly unfavourable for organs such as kidney and liver, since the high perfusion rate generally leads to a strong heat evacuation. This limits the realization of a sufficiently high temperature elevation and hampers the ablation of large tumour volumes in a clinically relevant time frame[40]. Consequently, more recent approaches focus on continuous target-tracking techniques, which lock the ultrasound beam to the target during the entire motion cycle and thus allow continuous energy deposition (Fig. 4). Both real-time MR imaging and diagnostic ultrasound have been successfully demonstrated as a viable tracking modality[13,39,41–43].
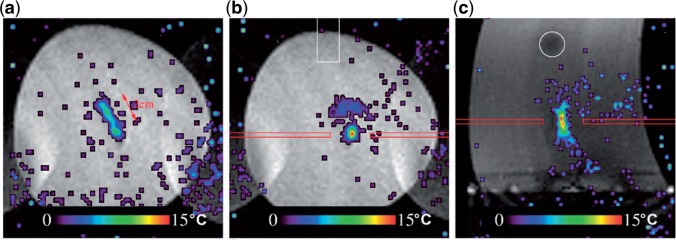
Figure 4.
Real-time MRI target tracking. Temperature maps obtained after 60 s of HIFU ablation on a phantom subjected to periodical motion. The imaging slice was placed either orthogonal to the symmetry axis of the HIFU transducer (a, b) or parallel to the symmetry axis (c). (a) The temperature distribution of non-compensated (i.e., HIFU beam steering disabled, but MR temperature mapping fully motion compensated) HIFU ablation shows the energy dispersion along the motion trajectory. In this example the motion vector of the phantom is parallel to the image plane and is indicated by the red arrow. (b) The fully motion-compensated HIFU ablation shows that the beam energy is deposited at the predefined location. (Reprinted from Ries et al.[39] with permission.)
Obstruction by the ribs
The majority of the liver is covered by the thoracic cage. The high acoustic reflection and attenuation of the ribs form a virtually impassable barrier for the ultrasound waves. The partial obstruction of the ultrasound beam leads to a significant reduction of energy in the focal-point area and can lead to undesired tissue damage of the intercostal muscles and subcutaneous tissue[44,45]. The phased-array design of the HIFU transducer allows for a possible solution to this problem, since the individual transducer elements can be deactivated selectively. The possibility to either detect the reflected ultrasound waves of the obstructed elements directly, or alternatively to identify obstructed elements on anatomical 3-dimensional MR image, allows for identification and subsequent disabling of critical transducer elements (Fig. 5)[43,44]. However, a downside of this solution is that in order to deposit sufficient energy in the HIFU focal region, a higher density of acoustic energy needs to pass through the intercostal space, which in turn increases the risk of burns to the skin and subcutaneous structures. Furthermore, this intercostal HIFU approach is potentially complicated by liver motion. The use of dynamic adaptive intercostal firing strategies for clinical practice is therefore currently under investigation.
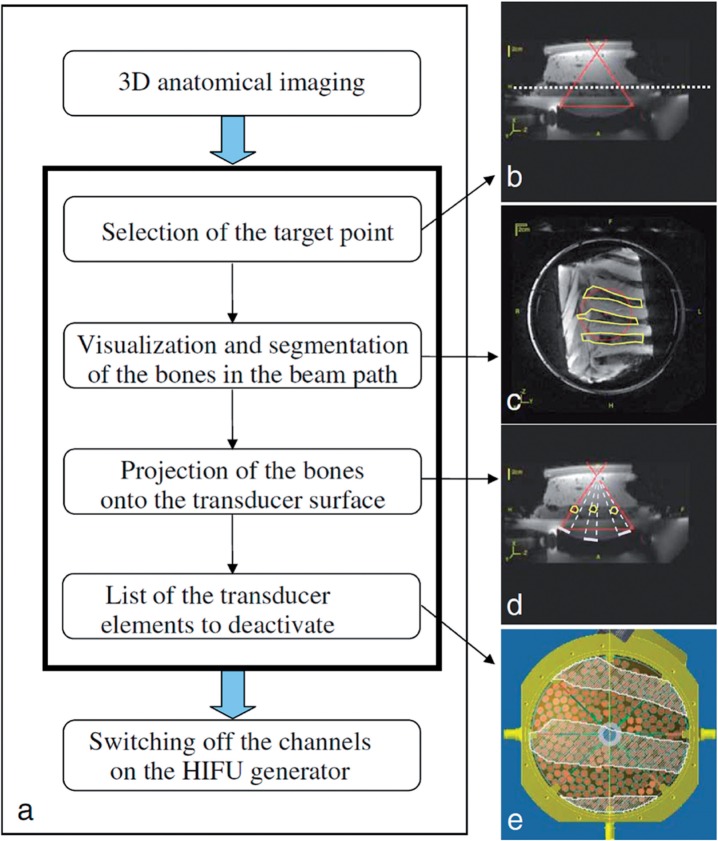
Figure 5.
Selective deactivation of individual transducer elements that have a rib in the beam path. (a) Schematic diagram of the proposed method for selecting the transducer elements to be deactivated. Anatomical images are used for (b) selection of the target point (the solid red lines show the HIFU propagation cone on a transverse slice and the white dashed line shows the horizontal slice displayed in c); (c) Manual segmentation of the bones (ROIs) within the beam path (circle). (d) Projection of the shadow of the ROIs onto the transducer surface (white bars on the transducer surface) by ray tracing from the targeted point (white dashed lines). (e) Visualization of the shadow of the ROIs on the 256 transducer elements distributed on the transducer surface, and determination of the elements to be deactivated. (Reprinted from Quesson et al.[44] with permission.)
Costodiaphragmatic recess
A considerable part of the liver is situated under the diaphragmatic dome, encircled by the inferior border of the right lung in the costodiaphragmatic recess[46]. This implicates an impassable barrier, namely air, for the ultrasound beam. Since the ultrasound transducer can be tilted only to a limited extent, this would render a considerable portion of all liver tumours technically unsuitable for MR-HIFU treatment. This problem has been assessed in several studies for ultrasound guidance of RFA, and the solution of artificial pleural effusion has been proposed[46,47]. Nonetheless, the implementation of this technique for the coupling of a therapeutic HIFU beam has yet to be studied.
Liver perfusion
The relatively high perfusion rate of the liver causes the absorbed energy in the treatment area to be dispersed relatively quickly. Chen et al. studied HIFU ablations in a rat liver with and without ligation of the hepatic artery and portal vein, and found a size reduction of the ablated lesion of more than 20% in a perfused liver, as opposed to a liver with ligated major vessels[48]. Higher acoustical powers can be used to overcome this effect. As a consequence, more acoustic energy will be deposited in the skin and subcutaneous tissue, which are traversed by the ultrasound beam, resulting in a higher risk of burns. Another solution to this problem would be to selectively embolize the liver segment with the tumour in order to decrease the perfusion, a principle used clinically by Wu et al.[36]
Vessels and bile ducts
Since there is a multitude of vessels and ducts that run within the liver parenchyma, liver tumours are often located adjacent to one of these vessels. The blood flow has a cooling effect on tumours adjacent to a larger vessel, known as the heat sink effect. This is a known limitation of RFA[49]. Experimental studies seem to indicate that this problem is less pronounced with HIFU ablation, since the heating process relies less on thermal conduction[10,32,50]. Wu et al. reported no vessel rupture or tumour bleeding in a series of 1038 patients with various solid tumours[36]. Zhang et al. treated 39 patients with hepatocellular carcinoma close (<1 cm) to a major hepatic vein or the inferior vena cava. No vascular complications were seen, although the complete tumour necrosis rate (50%) was somewhat unsatisfactory. This might well have been caused by their broad patient selection criteria, illustrated by the average tumour size of 7.4 cm[51]. Little is published on bile duct or gallbladder damage after HIFU treatment. Orsi et al. studied ultrasound-guided HIFU ablation of tumours in difficult locations, and reported no complications after ablation of 4 tumours located <1 cm from a major bile duct and 2 tumours located <1 cm from the gall bladder[52].
Conclusion
MR-HIFU ablation is a novel technique that offers completely non-invasive image-guided tumour ablation, with accurate treatment planning and real-time treatment control. Although various studies have focused on HIFU for ablation of malignant liver tumours, clinical implementation in this field so far has been hampered by the technical challenges, which arise from the partial obstruction of the target area by the thoracic cage, the continuous motion of the of the liver due to the respiratory cycle, and the required high acoustic power levels attributable to the strong heat dissipation caused by the hepatic perfusion. Several recent pre-clinical studies have successfully demonstrated viable solutions to each of these challenges individually. Future work will need to focus on the integration of these approaches into a robust and integrated clinical package.
Acknowledgement
This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (http://www.ctmm.nl), project VOLTA (grant 05T-201).
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. . PMid:22353262. [DOI] [PubMed] [Google Scholar]
- 2.Wong SL, Mangu PB, Choti Ma, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. . PMid:19841322. [DOI] [PubMed] [Google Scholar]
- 3.Bertot LC, Sato M, Tateishi R, et al. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors; a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. . PMid:21858539. [DOI] [PubMed] [Google Scholar]
- 4.Lynn JG, Zwemer RL, Chick AJ. The biological application of focused ultrasonic waves. Science. 1942;96:119–120. doi: 10.1126/science.96.2483.119. . PMid:17809987. [DOI] [PubMed] [Google Scholar]
- 5.Hynynen K, Freund R, Chung H, et al. A clinical, noninvasive, MR imaging-monitored ultrasound surgery method. Radiographics. 1996;16:185–195. doi: 10.1148/radiographics.16.1.185. PMid:10946699. [DOI] [PubMed] [Google Scholar]
- 6.Hynynen K, Damianou C, Darkazanli A, et al. The feasibility of using MRI to monitor and guide noninvasive ultrasound surgery. Ultrasound Med Biol. 1993;19:91–92. doi: 10.1016/0301-5629(93)90022-G. . PMid:8456533. [DOI] [PubMed] [Google Scholar]
- 7.Voogt MJ, Trillaud H, Kim YS, et al. Volumetric feedback ablation of uterine fibroids using magnetic resonance-guided high intensity focused ultrasound therapy. Eur Radiol. 2012;22:411–417. doi: 10.1007/s00330-011-2262-8. . PMid:21901565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huisman M, van den Bosch MA. MR-guided high-intensity focused ultrasound for noninvasive cancer treatment. Cancer Imaging. 2011;11:S161–166. doi: 10.1102/1470-7330.2011.9041. . PMid:22180520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBlang SD, Hoctor K, Steinberg FL. Leiomyoma shrinkage after MRI-guided focused ultrasound treatment; report of 80 patients. Am J Roentgenol. 2010;194:274–280. doi: 10.2214/AJR.09.2842. [DOI] [PubMed] [Google Scholar]
- 10.Solomon SB, Nicol TL, Chan DY, et al. Histologic evolution of high-intensity focused ultrasound in rabbit muscle. Invest Radiol. 2003;38:293–301. doi: 10.1097/01.RLI.0000066421.79958.96. PMid:12750619. [DOI] [PubMed] [Google Scholar]
- 11.Schmitz AC, Gianfelice D, Daniel BL, et al. Image-guided focused ultrasound ablation of breast cancer; current status, challenges, and future directions. Eur Radiol. 2008;18:1431–1441. doi: 10.1007/s00330-008-0906-0. . PMid:18351348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo W, Zhou X, Gong X, et al. Study of sequential histopathologic changes, apoptosis, and cell proliferation in rabbit livers after high-intensity focused ultrasound ablation. J Ultrasound Med. 2007;26:477–485. doi: 10.7863/jum.2007.26.4.477. PMid:17384045. [DOI] [PubMed] [Google Scholar]
- 13.Rieke V, Butts Pauly K. MR thermometry. J Magn Resonance Imaging. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDannold NJ, King RL, Jolesz FA. Usefulness of MR imaging-derived thermometry and dosimetry in determining the threshold for tissue damage induced by thermal surgery in rabbits. Radiology. 2000;216:517–523. doi: 10.1148/radiology.216.2.r00au42517. PMid:10924580. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Resonance Med. 1995;34:814–823. doi: 10.1002/mrm.1910340606. [DOI] [PubMed] [Google Scholar]
- 16.Sapareto S, Dewey W. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787–800. doi: 10.1016/0360-3016(84)90379-1. . PMid:6547421. [DOI] [PubMed] [Google Scholar]
- 17.Damianou C, Hynynen K. The effect of various physical parameters on the size and shape of necrosed tissue volume during ultrasound surgery. J Acoust Soc Am. 1994;95:1641–1649. doi: 10.1121/1.408550. . PMid:8176064. [DOI] [PubMed] [Google Scholar]
- 18.Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–294. doi: 10.1080/0265673031000119006. . PMid:12745972. [DOI] [PubMed] [Google Scholar]
- 19.Yarmolenko PS, Moon EJ, Landon C, et al. Thresholds for thermal damage to normal tissues; an update. Int J Hyperthermia. 2011;27:320–343. doi: 10.3109/02656736.2010.534527. . PMid:21591897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler MO, Mougenot C, Quesson B, et al. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med Phys. 2009;36:3521. doi: 10.1118/1.3152112. [DOI] [PubMed] [Google Scholar]
- 21.Salomir R, Palussière J, Vimeux FC, et al. Local hyperthermia with MR-guided focused ultrasound; spiral trajectory of the focal point optimized for temperature uniformity in the target region. J Magn Reson Imaging. 2000;12:571–583. doi: 10.1002/1522-2586(200010)12:4<571::AID-JMRI9>3.0.CO;2-2. . PMid:11042639. [DOI] [PubMed] [Google Scholar]
- 22.Palussière J, Salomir R, Le Bail B, et al. Feasibility of MR-guided focused ultrasound with real-time temperature mapping and continuous sonication for ablation of VX2 carcinoma in rabbit thigh. Magn Reson Med. 2003;49:89–98. doi: 10.1002/mrm.10328. [DOI] [PubMed] [Google Scholar]
- 23.Mougenot C, Quesson B, de Senneville BD, et al. Three-dimensional spatial and temporal temperature control with MR thermometry-guided focused ultrasound (MRgHIFU) Magn Reson Med. 2009;61:603–614. doi: 10.1002/mrm.21887. . PMid:19097249. [DOI] [PubMed] [Google Scholar]
- 24.Ter Haar G, Sinnett D, Rivens I. High intensity focused ultrasound - a surgical technique for the treatment of discrete liver tumours. Physics Med Biol. 1989;34:1743–1750. doi: 10.1088/0031-9155/34/11/021. [DOI] [PubMed] [Google Scholar]
- 25.Linke C, Carstensen E, Frizzell L, et al. Localized tissue destruction by high-intensity focused ultrasound. Arch Surg. 1973;107:887–891. doi: 10.1001/archsurg.1973.01350240053015. . PMid:4751833. [DOI] [PubMed] [Google Scholar]
- 26.Yang R, Reilly C, Rescorla F. High-intensity focused ultrasound in the treatment of experimental liver cancer. Arch Surg. 1991;126:1002–1009. doi: 10.1001/archsurg.1991.01410320088012. . PMid:1863205. [DOI] [PubMed] [Google Scholar]
- 27.Rowland IJ, Rivens I, Chen L, et al. MRI study of hepatic tumours following high intensity focused ultrasound surgery. Br J Radiol. 1997;70:144–153. doi: 10.1259/bjr.70.830.9135440. PMid:9135440. [DOI] [PubMed] [Google Scholar]
- 28.Sibille A, Prat F, Chapelon JY, et al. Characterization of extracorporeal ablation of normal and tumor-bearing liver tissue by high intensity focused ultrasound. Ultrasound Med Biol. 1993;19:803–813. doi: 10.1016/0301-5629(93)90096-7. . PMid:8134980. [DOI] [PubMed] [Google Scholar]
- 29.Prat F, Centarti M, Sibille A, et al. Extracorporeal high-intensity focused ultrasound for VX2 liver tumors in the rabbit. Hepatology. 1995;21:832–836. PMid:7875681. [PubMed] [Google Scholar]
- 30.Cline E, Schenck JF, Ettinger RH, et al. Focused US system for MR imaging-guided tumor ablation. Radiology. 1995;194:731–737. doi: 10.1148/radiology.194.3.7862971. PMid:7862971. [DOI] [PubMed] [Google Scholar]
- 31.Kopelman D, Inbar Y, Hanannel A, et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS). Four ablation treatments of a single canine hepatocellular adenoma. HPB (Oxford) 2006;8:292–298. doi: 10.1080/13651820500465212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopelman D, Inbar Y, Hanannel A, et al. Magnetic resonance-guided focused ultrasound surgery (MRgFUS); ablation of liver tissue in a porcine model. Eur J Radiol. 2006;59:157–162. doi: 10.1016/j.ejrad.2006.04.008. . PMid:16725294. [DOI] [PubMed] [Google Scholar]
- 33.Visioli aG, Rivens IH, ter Haar GR, et al. Preliminary results of a phase I dose escalation clinical trial using focused ultrasound in the treatment of localised tumours. Eur J Ultrasound. 1999;9:11–18. doi: 10.1016/S0929-8266(99)00009-9. . PMid:10099162. [DOI] [PubMed] [Google Scholar]
- 34.Illing RO, Kennedy JE, Wu F, et al. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br J Cancer. 2005;93:890–895. doi: 10.1038/sj.bjc.6602803. . PMid:16189519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy JE, Wu F, ter Haar GR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics. 2004;42:931–935. doi: 10.1016/j.ultras.2004.01.089. . PMid:15047409. [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal focused ultrasound surgery for treatment of human solid carcinomas; early Chinese clinical experience. Ultrasound Med Biol. 2004;30:245–260. doi: 10.1016/j.ultrasmedbio.2003.10.010. . PMid:14998677. [DOI] [PubMed] [Google Scholar]
- 37.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China; an overview. Ultrasonics Sonochem. 2004;11:149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Okada A, Murakami T, Mikami K, et al. A case of hepatocellular carcinoma treated by MR-guided focused ultrasound ablation with respiratory gating. Magn Reson Med Sci. 2006;5:167–171. doi: 10.2463/mrms.5.167. . PMid:17139143. [DOI] [PubMed] [Google Scholar]
- 39.Ries M, de Senneville BD, Roujol S, et al. Real-time 3D target tracking in MRI guided focused ultrasound ablations in moving tissues. Magn Reson Med. 2010;64:1704–1712. doi: 10.1002/mrm.22548. . PMid:20878763. [DOI] [PubMed] [Google Scholar]
- 40.Cornelis F, Grenier N, Moonen CT, et al. In vivo characterization of tissue thermal properties of the kidney during local hyperthermia induced by MR-guided high-intensity focused ultrasound. NMR Biomed. 2011;24:799–806. doi: 10.1002/nbm.1624. . PMid:21834004. [DOI] [PubMed] [Google Scholar]
- 41.De Senneville BD, Mougenot C, Moonen CT. Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound. Magn Reson Med. 2007;57:319–330. doi: 10.1002/mrm.21124. . PMid:17260361. [DOI] [PubMed] [Google Scholar]
- 42.Pernot M, Aubry J, Tanter M. High power transcranial beam steering for ultrasonic brain therapy. Physics Med Biol. 2003;48:2577–2589. doi: 10.1088/0031-9155/48/16/301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marquet F, Aubry JF, Pernot M, et al. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Physics Med Biol. 2011;56:7061–7080. doi: 10.1088/0031-9155/56/22/005. [DOI] [PubMed] [Google Scholar]
- 44.Quesson B, Merle M, Köhler MO, et al. A method for MRI guidance of intercostal high intensity focused ultrasound ablation in the liver. Med Phys. 2010;37:2533. doi: 10.1118/1.3413996. . PMid:20632565. [DOI] [PubMed] [Google Scholar]
- 45.Botros Y, Ebbini E, Volakis J. Optimal synthesis of phased array field patterns in the presence of the ribcage. IEEE Ultrasonics Symp. 1997;2:1751–1754. [Google Scholar]
- 46.Koda M, Maeda Y, Matsunaga Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. Am J Roentgenol. 2004;183:583–588. doi: 10.2214/ajr.183.3.1830583. [DOI] [PubMed] [Google Scholar]
- 47.Minami Y, Kudo M, Kawasaki T, et al. Percutaneous radiofrequency ablation guided by contrast-enhanced harmonic sonography with artificial pleural effusion for hepatocellular carcinoma in the hepatic dome. Am J Radiol. 2004;182:1224–1226. doi: 10.2214/ajr.182.5.1821224. [DOI] [PubMed] [Google Scholar]
- 48.Chen L, Ter Haar G, Hill CR, et al. Effect of blood perfusion on the ablation of liver parenchyma with high-intensity focused ultrasound. Phys Med Biol. 1993;38:1661–1673. doi: 10.1088/0031-9155/38/11/011. . PMid:8272440. [DOI] [PubMed] [Google Scholar]
- 49.Berber E, Siperstein A. Local recurrence after laparoscopic radiofrequency ablation of liver tumors; an analysis of 1032 tumors. Ann Surg Oncol. 2008;15:2757–2764. doi: 10.1245/s10434-008-0043-7. . PMid:18618182. [DOI] [PubMed] [Google Scholar]
- 50.Zhang CX, Zhang Z, Chen YZ. Effects of large blood vessel locations during high intensity focused ultrasound therapy for hepatic tumors; a finite element study (conference proceedings) IEEE Engin Med Biol Soc. 2005;1:209–212. doi: 10.1109/IEMBS.2005.1616380. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Zhu H, Jin C, et al. High-intensity focused ultrasound (HIFU); effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur Radiol. 2009;19:437–445. doi: 10.1007/s00330-008-1137-0. . PMid:18795303. [DOI] [PubMed] [Google Scholar]
- 52.Orsi F, Zhang L, Arnone P, et al. High-intensity focused ultrasound ablation; effective and safe therapy for solid tumors in difficult locations. Am J Roentgenol. 2010;195:W245–252. doi: 10.2214/AJR.09.3321. [DOI] [PubMed] [Google Scholar]
- 53.Quesson B, Laurent C, Maclair G, et al. Real-time volumetric MRI thermometry of focused ultrasound ablation in vivo; a feasibility study in pig liver and kidney. NMR Biomed. 2011;24:145–153. doi: 10.1002/nbm.1563. . PMid:21344531. [DOI] [PubMed] [Google Scholar]
- 54.Li YY, Sha WH, Zhou YJ, et al. Short and long term efficacy of high intensity focused ultrasound therapy for advanced hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:2148–2154. doi: 10.1111/j.1440-1746.2006.04719.x. . PMid:18031373. [DOI] [PubMed] [Google Scholar]
- 55.Jung SE, Cho SH, Jang JH, et al. High-intensity focused ultrasound ablation in hepatic and pancreatic cancer; complications. Abdom Imaging. 2011;36:185–195. doi: 10.1007/s00261-010-9628-2. . PMid:20512487. [DOI] [PubMed] [Google Scholar]
- 56.Xu G, Luo G, He L, et al. Follow-up of high-intensity focused ultrasound treatment for patients with hepatocellular carcinoma. Ultrasound Med Biol. 2011;37:1993–1999. doi: 10.1016/j.ultrasmedbio.2011.08.011. . PMid:22036638. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Zhao J, Guo D, et al. Evaluation of short-term response of high intensity focused ultrasound ablation for primary hepatic carcinoma; utility of contrast-enhanced MRI and diffusion-weighted imaging. Eur J Radiol. 2011;79:347–352. doi: 10.1016/j.ejrad.2010.06.039. . PMid:20638211. [DOI] [PubMed] [Google Scholar]
- 58.Jin C, Zhu H, Wang Z, et al. High-intensity focused ultrasound combined with transarterial chemoembolization for unresectable hepatocellular carcinoma; long-term follow-up and clinical analysis. Eur J Radiol. 2011;80:662–669. doi: 10.1016/j.ejrad.2010.08.042. . PMid:20864286. [DOI] [PubMed] [Google Scholar]
- 59.Fukuda H, Ito R, Ohto M, et al. Treatment of small hepatocellular carcinomas with US-guided high-intensity focused ultrasound. Ultrasound Med Biol. 2011;37:1222–1229. doi: 10.1016/j.ultrasmedbio.2011.04.020. . PMid:21645963. [DOI] [PubMed] [Google Scholar]