Abstract
Cancer is a major cause of illness and death in Western society and is associated with a heavy concomitant economic burden. Although use of imaging comprises only a small proportion of the fiscal impact of cancer, its use has been increasing over recent decades, causing concern amongst funders of health care and efforts to constrain the use of new imaging tests with a relatively high unit cost. In clinical practice, positron emission tomography/computed tomography (PET/CT) is generally performed when less expensive tests have left some uncertainty regarding appropriate management. In this setting, its utility relates to provision of incremental diagnostic information. However, given that superior diagnostic information can positively affect patient management, wherein the majority of costs reside, it may be both more efficient and cost effective to go directly to the most accurate investigation in certain situations. For PET/CT, the ability to provide more accurate assessment of metastatic status than is available from conventional diagnostic paradigms provides a rationale for its independent rather than incremental use in patients presenting with either a high likelihood of malignancy or proven malignancy of a locally advanced nature and an accordingly high risk of metastatic disease. A randomized trial design is described that could be used to test this hypothesis.
Keywords: Randomized trial, positron emission tomography/computed tomography, oncology, cost-effectiveness
The challenge of an ageing population
Advances in the treatment of cardiovascular disease and reduction in other causes of premature death have progressively increased the longevity of individuals in Western Society. However, a corollary of this has been an increase in the incidence of cancer, which is strongly associated with ageing[1]. Improvements in the care of cancer patients has been associated with a reduction in death rates in many types of cancer, but has also led to the prevalence of cancer increasing in society[2]. This has been associated with a resulting increase in the economic burden of this disease. The combination of an increased proportion of elderly people who are less effectively contributing to the productivity of society and increased direct health-care costs is a source of growing concern in many countries. This has stimulated an active debate on the need to rationalize cancer management and reduce expenditure on it[3].
The use of diagnostic imaging in cancer has increased progressively over recent years[4], reflecting the increased prevalence of cancer and an increasing range of effective targeted therapies that can be brought to bear on even advanced malignancies[5]. Much of this growth in imaging has been fuelled by advanced imaging techniques such as computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET), which are often used serially and in combination to diagnose, stage and monitor treatment efficacy. Confronted by this apparently profligate use of expensive technologies, funding bodies have sought to restrict access to new imaging techniques through application of health technology assessment. Although founded on principles of evidence-based medicine with the primacy of quality patient care at its core, there is increasing evidence that this process has been corrupted to become an instrument of fiscal management with the objective of reducing the burgeoning cost of health care, particularly with respect to the use of PET in cancer[6].
How does imaging affect costs of cancer care?
Despite the increase in imaging for the evaluation of cancer patients, a study from the United States, where there has been relatively unconstrained access to advanced imaging technologies, has estimated that it contributes less than 6% of total health-care expenditure, with the vast majority of direct costs of health care being related to the delivery of treatment[4]. Therefore, the impact of imaging on direct health-care costs needs to be considered in the context of its ability to influence therapeutic choices. Fundamental to the selection of appropriate treatment options is accurate diagnosis and staging of cancer. Indeed, cancer stage is the best validated prognostic factor in most cancers, and almost all treatment guidelines incorporate use of this parameter when making treatment recommendations[7].
Given the high cost of surgical oncology procedures, radiotherapy and systemic treatments, appropriate allocation of patients to receive these therapies has the potential to have a major influence of health-care expenditure. For example, performing surgery with curative intent in the setting of unrecognized metastatic disease subjects the patient to the risks of mortality inherent in such operations, the related convalescence and any ongoing morbidity, as well as any direct costs or loss of earnings related to time off work, without any chance of effecting a cure (Fig. 1). Furthermore it may delay introduction of more appropriate treatments, some of which may have a higher likelihood of providing a progression-free or overall survival advantage if introduced at an earlier time point in the progression of disease. Conversely, recognizing false-positive results on conventional staging can allow patients to enter curative treatment pathways. Although these may increase direct costs of care in the short term, there can be major societal advantages in delivering efficient cures. These include reduction in the loss of productive life-years and reduced palliative care costs in the longer term. In the restaging setting, prior surgery, radiotherapy or chemotherapy can be associated with residual morphological abnormalities that may or may not contain viable tumour. These are a source of considerable anxiety for patients and often prompt ongoing imaging surveillance, with its attendant cost and loss of productivity. Furthermore, they may induce invasive procedures to determine their nature or introduction of empiric treatment in the hope of salvaging a cure. These interventions carry cost and morbidity, which are clearly unwarranted if the patient is already cured of cancer. On the other hand, early detection of residual disease after attempted curative treatment may allow timely salvage treatment.
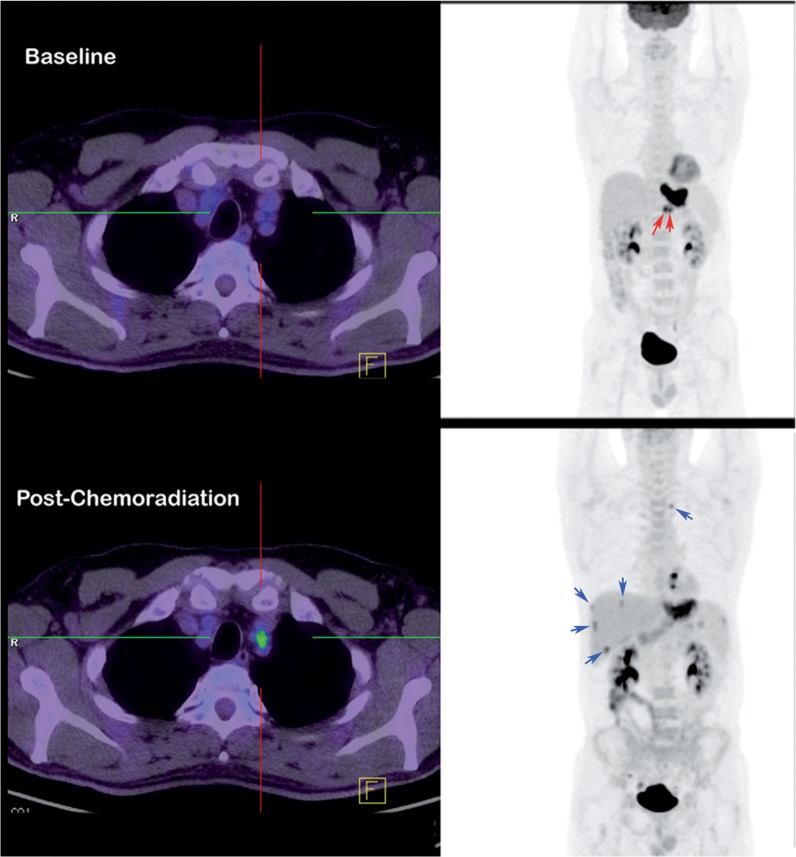
Figure 1.
Primary staging. In patients being staged for locally advanced cancer, more sensitive detection of metastatic disease by positron emission tomography/computed tomography (PET/CT) provides both avoidance of expensive and toxic therapies and a baseline for therapeutic response assessment. In this patient with locally advanced gastro-oesophageal cancer, baseline [18F]fluorodeoxyglucose (FDG) PET/CT demonstrated high uptake in normal-sized lesser curve nodes (red arrows) and therefore neoadjuvant chemoradiation was delivered. Follow-up FDG PET/CT prior to planned oesophagectomy demonstrated a poor local response to treatment and development of increased activity in the superior mediastinum in a non-enlarged node, as well as multiple small liver metastases (blue arrows) that were not apparent on CT but were confirmed by later progression.
Beyond the consequential medical cost of the imaging procedure itself and its flow-on effect on treatment choices that influence health-care expenditure, there are many less obvious sources of economic impact[8]. The time invested by patients and their social support network in undergoing imaging procedures can be substantial. Loss of productivity through attendance for investigations or interventions that may be unnecessary ought to be factored into assessment of the efficiency of imaging paradigms. More efficient staging approaches wherein a single test may replace several investigations can have benefits that far offset the net expenditure on the procedures themselves. For patients who live at a distance from their medical centre, these reductions in the time and cost of travel and sometimes accommodation expenses, lost earnings for themselves and anyone accompanying them, and the psychological impact of dislocation from family need to be considered. For the community, the environmental and resource-allocation imposts must also be factored into the microeconomic analysis[9].
Even more difficult to quantify is the impact of diagnostic uncertainty on patients. When less accurate investigations are performed, clinicians are often placed in a position where they must convey a significant degree of uncertainty regarding the significance of the scan result. This is particularly the case in the setting of a post-treatment residual mass. While the clinician may be relatively comfortable with the prospect of monitoring this with serial imaging to confirm stability or regression as indicators of a benign process, the intervening period is necessarily filled with anxiety for the patient, particularly if further investigation by way of biopsy is recommended.
In a medical environment that is increasingly constrained by resources, resulting in lengthening surgical and radiotherapy waiting lists[9,10], and where the increasing cost of drugs is challenging personal and hospital budgets[11], more accurate diagnosis can lead patients into more appropriate treatment pathways. By preventing patients who have no chance of cure from surgery or definitive chemoradiotherapy, patients who can are likely to access these resource-intensive and, therefore, expensive treatments in a more timely manner, increasing their own chances of local control and cure[12]. For patients receiving chemotherapy, earlier assessment of response may provide either reassurance that the expenditure and any attendant toxicity are worthwhile, whereas lack of response or progression may allow a change in treatment before significant progression or toxicity have accrued.
Is there evidence that PET can provide these benefits?
There is a large body of published literature indicating that both PET[13] and, more recently, PET/CT[14] are significantly more accurate for defining disease stage than are conventional staging investigations. As well as generally including diagnostic CT with use of appropriate contrast agents, many of the diagnostic paradigms with which PET has been compared have involved a combination of investigations.
In the case of PET, seminal studies performed shortly after the introduction of whole-body imaging capability for evaluation of cancer demonstrated the ability of this superior diagnostic performance to positively influence patient management. For example, Valk et al. reported a high clinical impact and potential cost savings in a retrospective analysis of patients undergoing PET after conventional cancer-staging paradigms[15]. The ability of PET to affect management selection in a beneficial way has been supported by many other subsequent studies. My own group has performed a number of studies that have applied a similar methodology to assess and validate changes in management occasioned by PET. In these studies we evaluated the concordance between a management plan indicated prospectively by the referring clinician based on all information available prior to the PET (pre-PET plan) and that made after the PET became available (post-PET plan). The change in management was assessed based on standardized criteria wherein impact was rated as high when treatment intent (curative versus palliative) or treatment modality were altered, medium if PET influenced treatment delivery but not intent or modality, low if PET indicated that the pre-PET plan was appropriate, and none if the post-PET plan was incompatible with the PET findings. The validity of these management changes was confirmed in a high proportion of cases and was supported by demonstration of superior prognostic stratification by PET compared with conventional staging in several series (Table 1). The impact of PET or PET/CT can be summarized as primarily avoiding aggressive but inappropriate loco-regional therapies, or better targeting them in the staging setting, wherein detection of previously occult sites of disease is the most common cause of discordant imaging findings; or prevention of unnecessary investigations or procedures in the restaging setting, where residual masses can more reliably be identified as non-viable scar tissue on metabolic imaging.
Table 1.
Studies assessing the management impact of PET or PET/CT, and prognosis
| First author[Ref.] |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| MacManus[26] | Hicks[27] | Hicks[28] | Ware[29] | Duong[30] | Duong[31] | Connell[32] | Barber[33] | Gregory[34] | |
| Year | 2001 | 2001 | 2001 | 2004 | 2006 | 2006 | 2007 | 2012 | 2012 |
| Cancer type | NSCLC | NSCLC | NSCLC | SCC H&N | Oesophageal | Oesophageal | SCC H&N | Oesophageal | NSCLC |
| Instrumentation | PET | PET | PET | PET | PET | PET | PET/CT | PET/CT | PET/CT |
| Design | Prospective | Prospective | Prospective | Prospective | Prospective | Prospective | Prospective | Prospective | Prospective |
| Recruitment years | 1996–1999 | 1996–1998 | 1996–1998 | 1996–1999 | 1996–2002 | 2002–2003 | 2002–2005 | 2002–2003 | |
| Patients (n) | 153 | 153 | 63 | 53 | 68 | 53 | 76 | 139 | 168 |
| Indication | Staging | Staging | Restaging | Restaging | Staging | Restaging | Staging/ Restaging | Staging | Staging |
| Median follow-up (months) | 9 | 17 | 12 | 55 | 22 | 19 | 28 | 60 | 60 |
| Management impact (%) | 52 | 60 | 63 | 40 | 47 | 36 | 40/34 | 34 | 48 |
| High | 30 | 35 | 50 | 40 | 40 | 36 | 11/NA | 26 | 42 |
| Medium | 22 | 25 | 13 | 0 | 7 | 0 | 29/NA | 8 | 5.4 |
| Superior stratification | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
PET, positron emission tomography; CT, computed tomography; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; H&N, head and neck; NA, no data available.
A similar methodology was also adopted as part of the Australian PET Data Collection Project with a series of prospective trials[16–20]. Even though there was less rigorous validation of the appropriateness of management changes in these studies given the difficulty of detailed patient follow-up in a multi-centre trial, these data confirmed that the results previously obtained at the Peter MacCallum Cancer Centre could be generalized. Similarly, although again not validated in individual patients, the scale of the clinical impact of PET on management in various cancers has been demonstrated by prospective studies in the United States as part of the National Oncologic PET Registry (NOPR)[21].
In all of these studies, patients have been referred for PET or PET/CT on the basis of prior imaging findings and, not infrequently, to clarify equivocal results. Accordingly they have, in essence, evaluated the incremental diagnostic utility of PET in a group of patients in whom there is an intrinsic pre-test selection bias. Furthermore, the validation of test accuracy is often dependent on behaviours induced by the results of PET, leading to a potential post-test validation bias. Although these facts are frequently ignored by applying retrospective blinded reading to obtain an assessment of comparative sensitivity, specificity and accuracy, a scientifically more valid approach is to define the prevalence of disease in the population being studied and the negative and positive predictive value of the PET result in this group of patients. Such information helps to define for clinicians the likely benefit of adding a PET scan in a patient with a similar clinical presentation or with an equivalent a priori likelihood of disease.
Because of the design of most trials involving PET, especially those of a retrospective nature, many patients with advanced metastatic disease have been excluded from analysis. The exception to this has been in therapeutic monitoring trials. Such studies have demonstrated the ability of PET, with [18F]fluorodeoxyglucose (FDG) in particular, to more robustly assess early treatment response, particularly to novel targeted agents[22]. However, PET is still infrequently performed for this purpose in clinical practice and even in most clinical trials where anatomical imaging with application of the Response Evaluation Criteria in Solid Tumours (RECIST)[23] remains the most commonly used surrogate of survival.
Could PET be used to provide independent rather than incremental diagnostic value?
Disease stage is used not only for prognostic stratification within groups of patients with a given malignancy, but also for determining the most appropriate treatment. Of the factors considered crucial to outcome and treatment selection, the presence of distant metastatic disease carries the worst prognosis and generally leads to either systemic therapies or supportive care. In the absence of distant metastatic disease, the presence of nodal metastasis is a manifestation of the ability of cancer cells to invade, migrate and colonize remote sites. Accordingly, it is now believed that most cancers require a combined therapeutic response wherein aggressive loco-regional therapies, such as surgery and radiotherapy, are often combined with systemic treatment as a neoadjuvant or adjuvant therapy. When either systemic or nodal metastasis is present, these findings generally override the extent of the primary tumour in assigning disease stage. For example, stage IV disease definitions generally contain the statement “any T-stage”.
In comparing the relative diagnostic performance of PET with that of conventional imaging, a consistent benefit of the former is its ability to detect nodal disease even in the absence of nodal enlargement, to exclude disease in enlarged nodes due to non-malignant causes, and to more sensitively and specifically detect systemic metastases. Where PET often performs less well is in the characterization of T-stage, which is usually determined by size and anatomical relations that are not necessarily defined well on functional imaging. However, many of these limitations have been overcome using hybrid PET/CT technology, especially if contrast enhancement is added to the acquisition protocol in appropriate cases. Thus, the aspects of tumour stage that are most critical to prognosis and treatment selection are best assessed by PET/CT. Therefore, it would be reasonable to suggest that this investigation should be done in all patients other than in those with a very low likelihood of metastatic spread. In these latter patients, in whom surgery is the most likely therapeutic option, detailed T-staging is most appropriate.
Nevertheless, most cancer-staging paradigms still recommend the use of CT for systemic staging based on cost and accessibility relative to PET, often reasoning that if widespread distant metastatic disease is identified then the treatment would be unlikely to be modified by detection of additional metastatic sites. However, this argument against the use of PET as the initial staging test is countered by the evidence that PET can provide additional characterization of tumour biology and act as a baseline for therapeutic response assessment based, on modulation of cellular biology early during treatment (Fig. 2). In an era of increasingly personalized medicine, both these features may influence treatment choices.
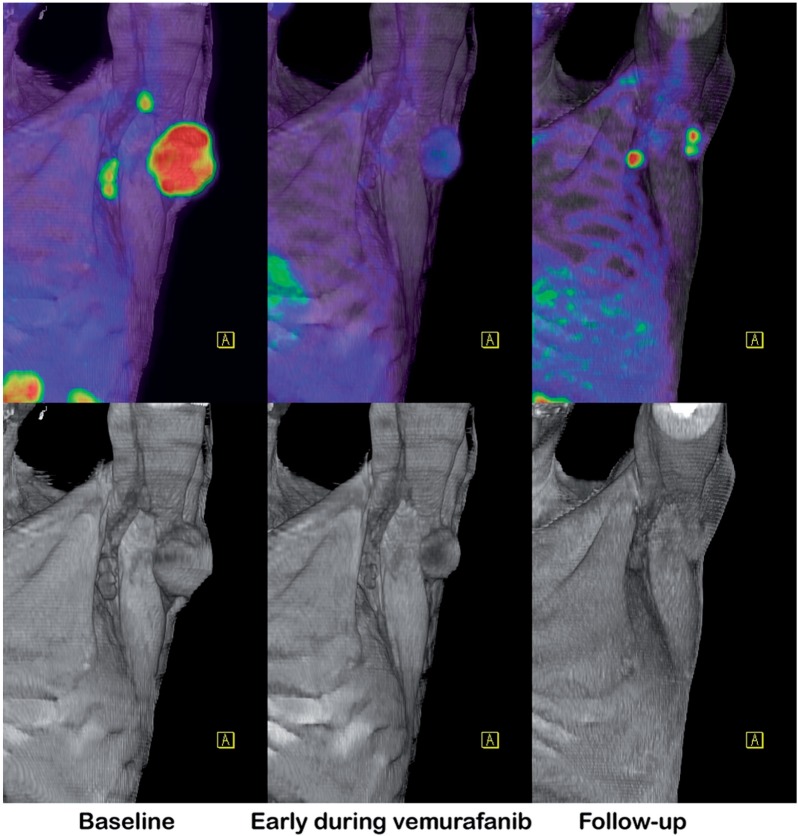
Figure 2.
Therapeutic response assessment. Early demonstration of response to expensive targeted therapies can either reassure a patient of the value of this treatment or allow a change in management. This patient with mutant BRAF-expressing melanoma was started on vemurafanib, with an excellent early metabolic response and partial radiologic response. Although this was subsequently matched by further significant regression of disease on CT, reactivation of glycolytic metabolism was an early manifestation of developing resistance to treatment.
Accordingly, a case can be made for routine PET/CT for at least baseline assessment of the vast majority of cancers, with relatively few cancers currently presenting at a stage where the likelihood of metastatic disease is very low. Those that do are generally detected by direct visual inspection, e.g., cutaneous or mucosal lesions, or incidentally during imaging performed for other purposes including cancer screening.
How might this hypothesis be proved?
There is a significant impediment to the implementation of such an approach related to the inertia of medical establishment, which is slow to react to changes in evidence. It must also battle vested interests in imaging practice and the reluctance of funding bodies to embrace new technologies such as PET that are perceived to be expensive. Therefore, it will be necessary to establish a strong evidence base in favour of such a change. This is difficult to achieve when most cancer patients already present to oncology facilities having had a CT scan done to evaluate symptoms or signs of cancer; therefore, the opportunity to use PET/CT as the primary staging tool is lost. Nevertheless, there are situations where cancer can be diagnosed, or strongly suspected, without recourse to imaging and wherein the opportunity arises to make a choice of staging paradigm. Examples of such situations include tumours that are diagnosed by physical examination or endoscopy, including locally advanced breast cancer, lymphoma with palpable lymphadenopathy, head and neck cancer, appendicular sarcoma, oesophageal cancer, gastric malignancy, colorectal cancer and cervical carcinoma. In these cases the diagnosis can be directly confirmed by biopsy prior to definitive staging. In other cases, regional imaging or blood biomarkers may indicate a high likelihood of malignancy prior to a pathological diagnosis and confirmation of the nature of the disease. In such situations, selection of biopsy site and definitive staging could be performed by either conventional imaging including CT or by using PET/CT. Situations might include locally advanced lung cancer diagnosed on chest X-ray, or patients with highly elevated tumour markers.
In situations where there is a potential choice between performing conventional staging or proceeding directly to PET/CT, there is a potential opportunity to use a randomized trial design to assess both the independent and incremental value of PET/CT. We are currently performing such a study in patients with locally advanced primary or recurrent prostate cancer diagnosed on digital rectal examination, rectal ultrasound or elevated levels of prostate-specific antigen. This study compares the combination of bone scan and contrast-enhanced abdomino-pelvic CT as the conventional imaging (CI) paradigm to PET/CT using [18F]fluoro-ethyl-choline (FCH)[24] as a first-line staging technique. This study was stimulated by demonstration of significant incremental impact of FCH PET/CT in our facility[25].
A randomized trial design to assess the independent versus incremental value of PET/CT
The trial design (Fig. 3) involves three phases, as follows:
Following randomization, participants undergo either PET/CT or CI as the first-line staging procedure. Using these results the treating clinician records an assessment of disease extent and a provisional management plan, based on all information up to that time. These results are used as the primary data to determine the independent value of each test in determining stage accuracy and the appropriateness of management.
Cross-over to the alternative imaging strategy is performed only if first-line imaging is negative or equivocal for systemic metastasis beyond the site of the known primary. Using these results, the treating clinician records a secondary assessment of extent of disease and a revised management plan, if required. These results are used to evaluate the incremental value of adding PET/CT to CI and vice versa.
Optional confirmatory investigations (biopsy or any other imaging modality not already performed) can be performed at the discretion of the treating clinician. Using these results, the referring physician records the final extent of clinical disease and the final management plan, based on all available information.
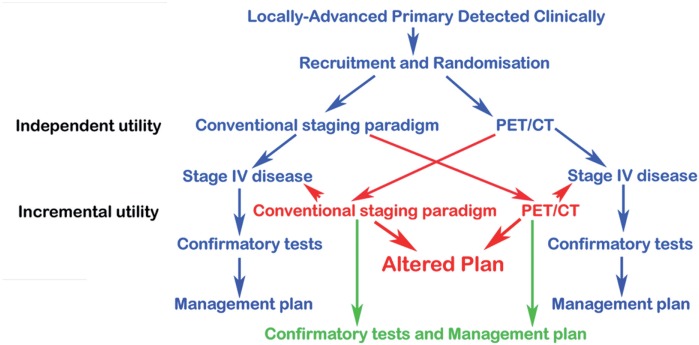
Figure 3.
Schema for a randomized controlled trial of PET/CT versus conventional imaging. Patients with distant metastatic disease are deemed to have sufficient information to proceed to systemic therapy and are not randomized to second-line imaging (blue-arrow pathway). All other patients cross over to have the other testing paradigm (red-arrow pathway). Independent diagnostic utility is determined by the ability of imaging to identify distant metastatic disease when done as a first-line investigation or when the management plan after the first-line investigations is unchanged by adding the second-line investigation (green-arrow pathway). Incremental diagnostic utility is defined by a change in management plan after second-line investigation compared with the plan defined after the first-line investigation (red-arrow pathway).
Reporting imaging specialists have unrestricted access to clinical and radiological data available up to the time of the scan as per usual clinical practice, and the clinical report produced by the readers are transmitted to the referring physician. Therefore, the trial design reflects, as closely as possible, usual clinical practice.
Upon completion of the diagnostic phases and after the final management plan is implemented, follow-up is performed using the standard surveillance protocols pertinent to the disease in question.
The primary end point of such studies is the independent utility of each first-line imaging paradigm. This is defined as the proportion of participants who, following a second-line work-up, do not require any change to be made to the management plan that was initially formulated. The secondary end point is the incremental utility, which is defined as the proportion of participants who receive the second-line imaging modality when the first-line imaging is negative or equivocal for distant metastasis, leading to a change in the management plan formulated following the first-line imaging. A “change in management plan” is defined as high when there is a change in treatment intent (between curative, palliative or expectant management) or treatment modality, or medium when there is a change in the delivery of a chosen treatment modality (e.g., a change in radiotherapy field). An important potential validation of the value of each test will be the ability of each first-line imaging technique to stratify overall survival.
This trial design also lends itself to many combinations of conventional staging and PET-based staging. For example, in the staging of locally advanced breast cancer, the combination of mammography, sentinel node biopsy, CT and bone scintigraphy could be compared with FDG PET/CT. For patients with symptoms of carcinoid syndrome and elevated chromogranin-A levels, triple-phase CT could be compared with 68Ga DOTA-octreotate PET/CT, and so on.
Furthermore, it is possible to build into such studies an assessment of patient satisfaction, efficiency and cost. A survey regarding the time taken to complete the staging tests, their comfort and convenience can be incorporated at each phase of investigation. The total cost of the staging strategy can be determined from the sum of the first-line work-up plus the cost of any mandated second-line work-up, and any optional validating procedures that are determined to be necessary by the treating clinician before arriving at a definitive treatment plan.
Conclusion
Although this may seem a radical, and some would say irresponsible, suggestion, there are cogent reasons to consider the proposition that patients deserve access to information regarding the most efficient and effective imaging paradigm whatever its cost. While challenging to health-funding authorities, there is a moral obligation to both patients and the community to assess dispassionately whether it may be less expensive in the long run to spend more on the initial phases of diagnosis and staging to ensure rational use of expensive and toxic therapies, especially if the results obtained in providing these treatments is improved by better selection and delivery.
Conflict of interest
The author has no conflicts of interest to declare.
References
- 1.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. . PMid:20644100. [DOI] [PubMed] [Google Scholar]
- 2.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. doi: 10.1093/jnci/djr077. . PMid:21454908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. . PMid:21958503. [DOI] [PubMed] [Google Scholar]
- 4.Dinan MA, Curtis LH, Hammill BG, et al. Changes in the use and costs of diagnostic imaging among Medicare beneficiaries with cancer, 1999–2006. JAMA. 2010;303:1625–1631. doi: 10.1001/jama.2010.460. . PMid:20424253. [DOI] [PubMed] [Google Scholar]
- 5.Villanueva MT. Targeted therapies: smart tumor, smarter treatment. Nat Rev Clin Oncol. 2012;9:127. doi: 10.1038/nrclinonc.2012.4. . PMid:22310739. [DOI] [PubMed] [Google Scholar]
- 6.Ware RE, Hicks RJ. Doing more harm than good? Do systematic reviews of PET by health technology assessment agencies provide an appraisal of the evidence that is closer to the truth than the primary data supporting its use? J Nucl Med. 2011;52(Suppl 2):64S–73S. doi: 10.2967/jnumed.110.086611. . PMid:22144557. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL, Sobin LH. The staging of cancer: a retrospective and prospective appraisal. CA Cancer J Clin. 2008;58:180–190. doi: 10.3322/CA.2008.0001. . PMid:18460593. [DOI] [PubMed] [Google Scholar]
- 8.Hicks RJ, Borland J. Are health economics making us sick? J Nucl Med. 2010;51:1665–1667. doi: 10.2967/jnumed.110.079772. . PMid:20956473. [DOI] [PubMed] [Google Scholar]
- 9.Devbhandari MP, Bittar MN, Quennell P, et al. Are we achieving the current waiting time targets in lung cancer treatment? Result of a prospective study from a large United Kingdom teaching hospital. J Thorac Oncol. 2007;2:590–592. doi: 10.1097/JTO.0b013e318070ccf0. . PMid:17607113. [DOI] [PubMed] [Google Scholar]
- 10.Williams MV, Drinkwater KJ. Geographical variation in radiotherapy services across the UK in 2007 and the effect of deprivation. Clin Oncol (R Coll Radiol) 2009;21:431–440. doi: 10.1016/j.clon.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey SD. How should we pay the piper when he's calling the tune? On the long-term affordability of cancer care in the United States. J Clin Oncol. 2007;25:175–179. doi: 10.1200/JCO.2006.08.9805. . PMid:17210936. [DOI] [PubMed] [Google Scholar]
- 12.Chen Z, King W, Pearcey R, Kerba M, Mackillop WJ. The relationship between waiting time for radiotherapy and clinical outcomes: a systematic review of the literature. Radiother Oncol. 2008;87:3–16. doi: 10.1016/j.radonc.2007.11.016. . PMid:18160158. [DOI] [PubMed] [Google Scholar]
- 13.Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(5 Suppl):1S–93S. PMid:11483694. [PubMed] [Google Scholar]
- 14.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med. 2007;48(Suppl 1):78S–88S. PMid:17204723. [PubMed] [Google Scholar]
- 15.Valk PE, Pounds TR, Tesar RD, Hopkins DM, Haseman MK. Cost-effectiveness of PET imaging in clinical oncology. Nucl Med Biol. 1996;23:737–743. doi: 10.1016/0969-8051(96)00080-7. . PMid:8940715. [DOI] [PubMed] [Google Scholar]
- 16.Scott AM, Gunawardana DH, Bartholomeusz D, Ramshaw JE, Lin P. PET changes management and improves prognostic stratification in patients with head and neck cancer: results of a multicenter prospective study. J Nucl Med. 2008;49:1593–1600. doi: 10.2967/jnumed.108.053660. . PMid:18794254. [DOI] [PubMed] [Google Scholar]
- 17.Scott AM, Gunawardana DH, Kelley B, et al. PET changes management and improves prognostic stratification in patients with recurrent colorectal cancer: results of a multicenter prospective study. J Nucl Med. 2008;49:1451–1457. doi: 10.2967/jnumed.108.051615. . PMid:18703607. [DOI] [PubMed] [Google Scholar]
- 18.Chatterton BE, Ho Shon I, Baldey A, et al. Positron emission tomography changes management and prognostic stratification in patients with oesophageal cancer: results of a multicentre prospective study. Eur J Nucl Med Mol Imaging. 2009;36:354–361. doi: 10.1007/s00259-008-0959-y. . PMid:18931839. [DOI] [PubMed] [Google Scholar]
- 19.Scott AM, Gunawardana DH, Wong J, et al. Positron emission tomography changes management, improves prognostic stratification and is superior to gallium scintigraphy in patients with low-grade lymphoma: results of a multicentre prospective study. Eur J Nucl Med Mol Imaging. 2009;36:347–353. doi: 10.1007/s00259-008-0958-z. . PMid:18931840. [DOI] [PubMed] [Google Scholar]
- 20.Fulham MJ, Carter J, Baldey A, Hicks RJ, Ramshaw JE, Gibson M. The impact of PET-CT in suspected recurrent ovarian cancer: A prospective multi-centre study as part of the Australian PET Data Collection Project. Gynecol Oncol. 2009;112:462–468. doi: 10.1016/j.ygyno.2008.08.027. . PMid:19150121. [DOI] [PubMed] [Google Scholar]
- 21.Hillner BE, Liu D, Coleman RE, et al. The National Oncologic PET Registry (NOPR): design and analysis plan. J Nucl Med. 2007;48:1901–1908. doi: 10.2967/jnumed.107.043687. . PMid:17942807. [DOI] [PubMed] [Google Scholar]
- 22.McArthur GA, Puzanov I, Amaravadi R, et al. Marked, homogeneous, and early[18F]fluorodeoxyglucose-positron emission tomography responses to vemurafenib in BRAF-mutant advanced melanoma. J Clin Oncol. 2012;30:1628–1634. doi: 10.1200/JCO.2011.39.1938. . PMid:22454415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. . PMid:16616487. [DOI] [PubMed] [Google Scholar]
- 24.DeGrado TR, Baldwin SW, Wang S, et al. Synthesis and evaluation of (18)F-labeled choline analogs as oncologic PET tracers. J Nucl Med. 2001;42:1805–1814. PMid:11752077. [PubMed] [Google Scholar]
- 25.Beauregard JM, Williams SG, Degrado TR, Roselt P, Hicks RJ. Pilot comparison of F-fluorocholine and F-fluorodeoxyglucose PET/CT with conventional imaging in prostate cancer. J Med Imaging Radiat Oncol. 2010;54:325–332. doi: 10.1111/j.1754-9485.2010.02178.x. . PMid:20718912. [DOI] [PubMed] [Google Scholar]
- 26.Mac Manus MP, Hicks RJ, Ball DL, et al. F-18 fluorodeoxyglucose positron emission tomography staging in radical radiotherapy candidates with nonsmall cell lung carcinoma: powerful correlation with survival and high impact on treatment. Cancer. 2001;92:886–895. doi: 10.1002/1097-0142(20010815)92:4<886::AID-CNCR1397>3.0.CO;2-V. . PMid:11550162. [DOI] [PubMed] [Google Scholar]
- 27.Hicks RJ, Kalff V, MacManus MP, et al. (18)F-FDG PET provides high-impact and powerful prognostic stratification in staging newly diagnosed non-small cell lung cancer. J Nucl Med. 2001;42:1596–1604. PMid:11696627. [PubMed] [Google Scholar]
- 28.Hicks RJ, Kalff V, MacManus MP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42:1605–1613. PMid:11696628. [PubMed] [Google Scholar]
- 29.Ware RE, Matthews JP, Hicks RJ, et al. Usefulness of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with a residual structural abnormality after definitive treatment for squamous cell carcinoma of the head and neck. Head Neck. 2004;26:1008–1017. doi: 10.1002/hed.20097. . PMid:15459925. [DOI] [PubMed] [Google Scholar]
- 30.Duong CP, Demitriou H, Weih L, et al. Significant clinical impact and prognostic stratification provided by FDG-PET in the staging of oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:759–769. doi: 10.1007/s00259-005-0028-8. . PMid:16470369. [DOI] [PubMed] [Google Scholar]
- 31.Duong CP, Hicks RJ, Weih L, et al. FDG-PET status following chemoradiotherapy provides high management impact and powerful prognostic stratification in oesophageal cancer. Eur J Nucl Med Mol Imaging. 2006;33:770–778. doi: 10.1007/s00259-005-0040-z. . PMid:16550384. [DOI] [PubMed] [Google Scholar]
- 32.Connell CA, Corry J, Milner AD, et al. Clinical impact of, and prognostic stratification by, F-18 FDG PET/CT in head and neck mucosal squamous cell carcinoma. Head Neck. 2007;29:986–995. doi: 10.1002/hed.20629. . PMid:17563906. [DOI] [PubMed] [Google Scholar]
- 33.Barber TW, Duong CP, Leong T, Bressel M, Drummond EG, Hicks RJ. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53:864–871. doi: 10.2967/jnumed.111.101568. . PMid:22582047. [DOI] [PubMed] [Google Scholar]
- 34.Gregory DL, Hicks RJ, Hogg A, et al. Effect of PET/CT on management of patients with non-small cell lung cancer: results of a prospective study with 5-year survival data. J Nucl Med. 2012;53:1007–1015. doi: 10.2967/jnumed.111.099713. PMid:22677701. [DOI] [PubMed] [Google Scholar]