Abstract
The standardized uptake value (SUV) and other measurements of tumour uptake of fluorodeoxyglucose (FDG) on positron emission tomography (PET) can potentially be supplemented by additional imaging parameters derived either from the PET images or from the computed tomography (CT) component of integrated PET/CT examinations including tumour size, CT attenuation, texture (reflecting tumour heterogeneity) and blood flow. This article illustrates the emerging benefits of such a multiparametric approach. Example benefits include greater diagnostic accuracy in characterization of adrenal masses achieved by using both the SUV and measured CT attenuation. Tumour size combined with the SUV can potentially improve the prognostic information available from PET/CT in oesophageal and lung cancer. However, greater improvements may be realized through using CT measurements of texture instead of size. Studies in breast and lung cancer suggest that combined PET/CT measurements of glucose metabolism and blood flow provide correlates for tumour proliferation and angiogenesis, respectively. These combined measurements can be utilized to determine vascular-metabolic phenotypes, which vary with tumour type. Uncoupling of blood flow and metabolism suggests a poor prognosis for larger more advanced tumours, high-grade lesions and tumours responding poorly to treatment. Vascular-metabolic imaging also has the potential to subclassify tumour response to treatment. The additional biomarkers described can be readily incorporated in existing FDG-PET examinations thereby improving the ability of PET/CT to depict tumour biology, characterize potentially malignant lesions, and assess prognosis and therapeutic response.
Keywords: Fluorodeoxyglucose positron emission tomography/computed tomography, dynamic contrast-enhanced computed tomography, computed tomography texture analysis, oncology imaging, multiparametric
Introduction
Oncological imaging in recent years has had a profound impact from the multiconcerted drive to move imaging into the quantitative realm and away from conventional qualitative or descriptive constraints. This move, formalized by the Quantitative Imaging Biomarker Alliance (QIBA) established by the Radiological Society of North America in 2007, has brought about a wealth of data demonstrating the benefits of extracting and utilizing quantifiable features from imaging in both the research and clinical settings. For positron emission tomography (PET), QIBA have mainly concentrated on measurements of [18F]fluorodeoxyglucose (FDG) uptake within tumours and other tissues, reflecting the revolution in cancer imaging afforded by this radiotracer. Meanwhile, a separate computed tomography (CT) group has initially focused on two basic parameters, namely lesion size and CT attenuation (Hounsfield units). Although as yet to be considered by QIBA, newer quantitative CT techniques such as dynamic contrasted-enhanced (DCE)-CT and CT texture analysis (CTTA) have also emerged as potentially useful modalities in oncology. These advances in oncological imaging can potentially provide useful quantitative biomarkers applicable to almost all aspects of cancer imaging. However, until recently, such quantitative biomarkers have largely been conceptualized and investigated in isolation.
Multiparametric imaging combines quantitative information from a number of techniques to provide a multidimensional (multispectral, multispatial and temporally resolved) depiction of tumour morphology and biology[1]. It enables complex and multifaceted depiction of tumour phenotype, improved characterization of lesion pathology and more accurate assessment of therapeutic response. The introduction of hybrid PET/CT systems has heralded new possibilities to pursue multiparametric imaging in oncology. However, the focus is still largely on the addition of CT for the purpose of anatomical localization and attenuation correction of PET data. This approach fails to make the most of the potential of multifunctional imaging[2].
This article outlines how quantitative measurements of tumour FDG uptake can be combined with CT biomarkers of size, attenuation, DCE-CT and CTTA. Current and future oncological applications of multiparametric PET/CT imaging are reviewed, in particular the utility to depict tumour biology, characterize potentially malignant lesions, assess prognosis and therapeutic response, and also a potential role in development of novel anti-cancer agents.
Quantitative parameters available from FDG-PET/CT
PET images are inherently quantitative as each pixel represents the concentration of activity of FDG in tissue (Bq/ml). Quantitation of tumour metabolism using FDG-PET is complicated by a range of factors apart from tumour glycolysis, including partial volume effects, time, plasma glucose levels and innate differences in biochemical structure between FDG and glucose[3]. The most commonly used parameter for FDG uptake in the standardized uptake value (SUV) which is essentially tumour activity concentration measured at the scanner (Bq/ml) divided by the injected dose of FDG, in proportion to lean body weight. Published consensus guidelines for the standardization of PET image acquisition and analysis have improved the reproducibility of SUV measurements[4,5].
The two basic parameters most readily assessed from the CT component of PET/CT are size and X-ray attenuation. Early quantitative biomarkers in CT were based primarily on tumour size. The continued use of CT measurements of tumour size in clinical staging of many cancers by the TNM system reflects the prognostic significance of such measurements. CT measurements of size are also widely used for anatomically and temporally based therapy response criteria, such as Response Evaluation Criteria in Solid Tumours (RECIST)[6]. On the other hand, attenuation measurements have gained some application in lesion characterization, for example their use in evaluating indeterminate adrenal masses[7]. Attenuation measurements are also the basis of DCE-CT and CTTA.
For DCE-CT, temporal changes in attenuation (proportional to contrast material concentration in tissue or vessels) after bolus intravenous administration of iodinated contrast material are analysed with standard kinetic modelling and software processing to produce a range of parameters reflecting the physiology of tissue microvasculature[8]. In the context of tumour imaging, the following physiological parameters are obtained: (a) regional tumour blood flow of (BF; i.e. perfusion or blood flow per unit volume of tissue); (b) regional tumour blood volume (BV; i.e. proportion of tumour region composed of blood); (c) mean transit time (MTT; time taken for contrast to cross vessels); (d) BF-extraction product (extraction of contrast into extravascular space); and (e) permeability–surface area product (PS; rate of transfer from the intravascular to extravascular space). These parameters have been validated for peripheral tumours against a range of references in animals and humans (reviewed by Miles[2] and Petralia et al.[9]). Although measurement of tumour perfusion from the first pass of FDG has been advocated[10], this approach does not have the spatial resolution of CT and commercial availability of analysis software (approved by the US Food and Drug Administration), which are the advantages of DCE-CT[11,12]. DCE-CT has now gained maturity with recently published consensus guidelines detailing use in therapeutic trials and also clinically[9,10].
CTTA assesses the distribution of attenuation values (heterogeneity) within a tumour or lesion. Filters can be applied to limit the effect of photon noise, while maximizing biological heterogeneity. This enables quantification of attenuation variability, which is unreliably assessed visually on CT images. The computed parameters with CTTA include histogram descriptors such as standard deviation, kurtosis, skewness and statistical derivations of uniformity and entropy[13–19]. Tumour heterogeneity can also be quantified from FDG-PET images[20] but is constrained by the poorer spatial resolution of PET compared with CT.
All of the above CT techniques are readily incorporated into FDG-PET examinations. The ease of adding DCE-CT into standard PET/CT acquisition protocols has been demonstrated[2,21–23] and CTTA data are post-processed, thus extra image acquisition is not required and it can be applied to both pre-acquired PET/CT and DCE-CT data[24,25]. FDG-PET can be combined with conventional CT measurements of tumour size and attenuation, DCE-CT assessments of tumour vascularity and CTTA quantification of tumour heterogeneity in a single examination (Fig. 1).
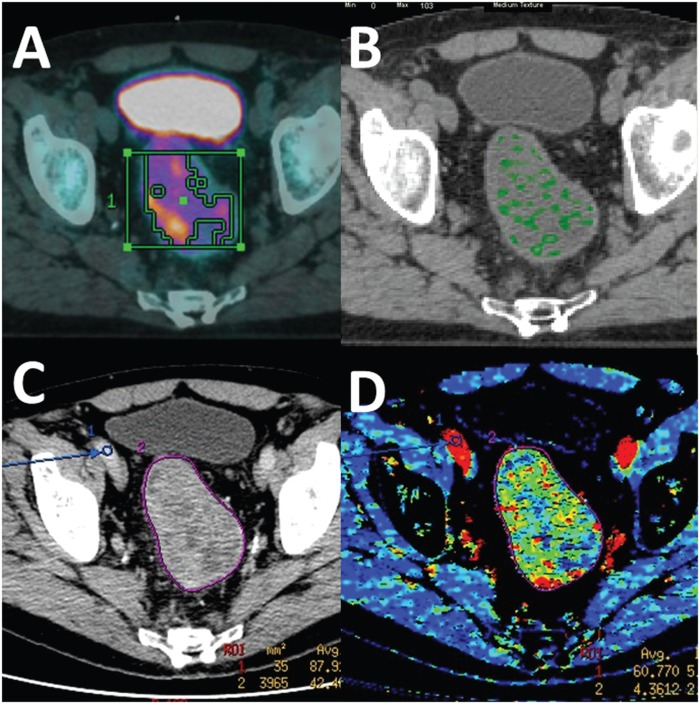
Figure 1.
Multiple parametric PET/CT of rectal cancer comprising (A) fused FDG-PET CT image, (B) CT texture analysis (medium texture image), (C) conventional CT, and (D) DCE-CT (blood volume image). (Courtesy of A.M. Groves, Institute of Nuclear Medicine, University College London.)
Oncological applications of multiparametric PET/CT
Depiction of tumour biology
Multifunctional quantitative imaging is becoming increasingly effective in providing a readily accessible non-invasive method to depict many aspects of biological perturbations occurring within the tumour, in the tumour microenvironment and at the organ and multi-organ system level. Such data can then be extrapolated to understanding targeted therapy response and potential development of novel treatment agents, with a view to modifying or personalizing therapy. Of the various molecular and cellular pathological processes in cancer, multiparametric PET/CT can primarily assess tumour glucose metabolism (FDG-PET), vascularity (DCE-CT) and heterogeneity (CTTA). All of these features are linked to the molecular biology related to hypoxia (Fig. 2).
Figure 2.
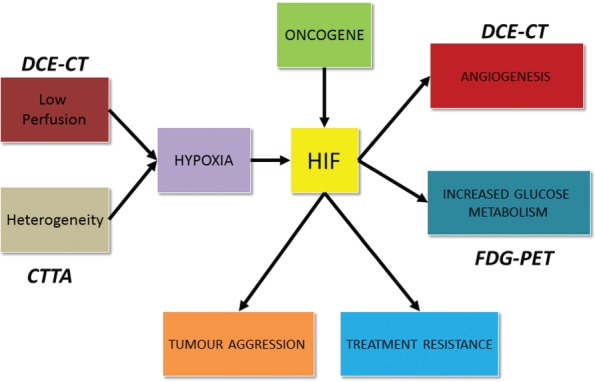
A summary of the biology of tumour hypoxia, highlighting the aspects assessable with multiparametric PET/CT.
FDG-PET imaging relies on the Warburg effect, namely that cancer cells have the fundamental property of switching to anaerobic glycolysis despite the presence of adequate oxygenation, an effect mediated in part by a hypoxia-inducible transcription factor (HIF-1)[26,27]. HIF-1 is a transcription factor that upregulates GLUT-1 and hexokinase, thus increasing the uptake of FDG. In turn, HIF-1 activity can be increased not only by hypoxia but also as a result of mutations in oncogenes such as the p53 and Von Hippel Lindau tumour suppressor genes. For DCE-CT, measurements of tumour perfusion have been shown to reflect tumour oxygenation; tumour vascular density typically correlates with peak enhancement, blood volume and/or PS. Vascular density is also related to HIF-1 activity, which increases angiogenesis via vascular growth factors, thereby supporting tumour growth and survival[28]. HIF-1 also upregulates other biological processes promoting increased tumour aggression and treatment resistance[28].
Combined imaging of glucose metabolism and angiogenesis can allow vascular-metabolic phenotyping of tumours in which a mismatch between tumour vascularity and metabolism reflects adaptation to hypoxia and an adverse tumour biology[26]. Studies using this approach have shown the high metabolic/low vascularity phenotype to be more common in non-small cell lung cancers (NSCLC) greater than 3.9 cm in diameter, in pulmonary adenocarcinomas (compared with squamous cell carcinomas) and in colorectal liver metastases, but uncommon in lymphoma[29–31]. Multiparametric FDG-PET/DCE-CT has also demonstrated preliminary correlations with histological tumour phenotype. Goh et al.[32] report that colorectal tumours with a low flow–high metabolism phenotype show greater expression of vascular endothelial growth factor (VEGF) and HIF-1 thus suggesting an adaptation to hypoxia in these tumours via angiogenesis. A recent small prospective study in NSCLC has also demonstrated that FDG uptake (SUVmean, SUVmax and metabolic tumour volume) correlated with Ki67 expression, a biomarker of tumour invasion capacity, whereas CT perfusion parameters (BV, SPV, Ktrans) correlated with mean vascular density (as expressed by CD34 staining)[33]. Similarly, in a recent prospective study of 40 patients with breast cancer undergoing dynamic first-pass FDG-PET/CT and DCE-CT, SUVmax correlated significantly with the expression of Ki67 in core biopsy samples, whereas tumour blood flow (but not SUVmax) correlated with vascular histological markers including CD105 and CD34[34].
The association between hypoxia and CTTA measurements of tumour heterogeneity arises from the presence of hypoxic voids due to areas of low vascularity in tumours with an inhomogeneous distribution of tumour vessels. These hypoxic voids lead to upregulation of HIF-1 and its downstream transcriptional effects. A heterogeneous blood supply also affects treatment response due to poor delivery of chemotherapeutic agents to areas of low vascularity. More recently, multiparametric assessment using FDG-PET/CTTA has shown potential as a biomarker for tumour angiogenesis and hypoxia. In a prospective study of 53 patients with colorectal cancer undergoing pre-operative FDG-PET/CT, tumour heterogeneity was the best correlate for microvessel density, whereas novel parameters that combined SUVmax and CT heterogeneity were the best predictors of VEGF and Glut-1 expression (Fig. 3)[35].
Figure 3.
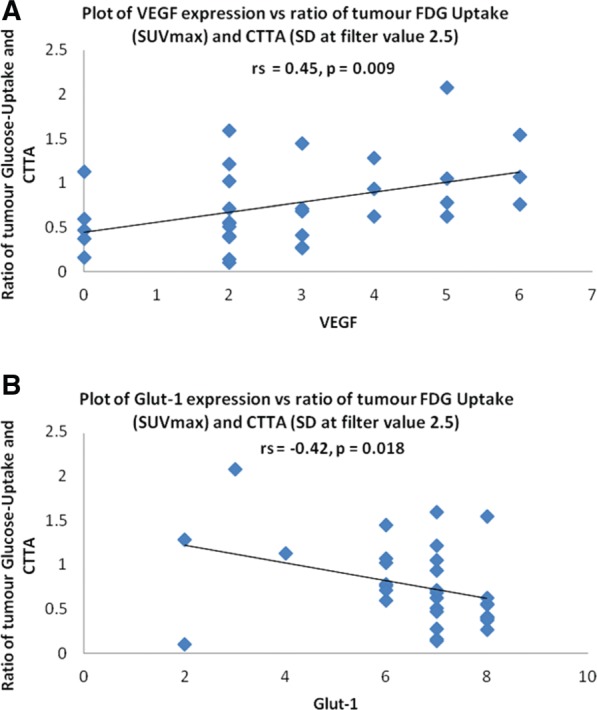
Relationships between a combined FDG-PET/CTTA parameter (the ratio of SUVmax/standard deviation of values in the coarse CT texture image) and tumour expression of VEGF (A) and GLUT-1 (B).
FDG-PET, DCE-CT and CTTA can all be performed in a single examination. A prior study using these techniques to study the liver in patients with colorectal cancer found that, for patients without liver metastases, CTTA parameters correlated with the ratio of hepatic SUV/perfusion, which provides an index of glucose phosphorylation, whereas this correlation was lost when hepatic metastases were present[13].
Lesion characterization: diagnosis (benign versus malignant)
Measurements of FDG uptake in lesions can be used to distinguish a benign pathology from malignancy. However, the incorporation of CT biomarkers into the PET evaluation has the potential to improve diagnostic accuracy. This concept has been clearly demonstrated by the example of adrenal lesions. Recent studies suggest a combined SUV and CT attenuation threshold in adrenal lesions detected in patients presenting for staging of NSCLC by FDG-PET/CT[36]. Furthermore, when performed in conjunction with delayed contrast enhanced CT to allow for washout measurements, diagnostic accuracy was found to increase to up to 100%[37]. Most recently, combining CT histogram analysis with FDG uptake parameters was reported to further increase the diagnostic accuracy of undifferentiated adrenal lesions[38].
Cancer prognostication
Tumour SUV measurements have demonstrated prognostic value for survival in patients with NSCLC and oesophageal cancer[39,40]. CT measurements of tumour size, perfusion and heterogeneity have also been shown to predict survival in a range of tumours. Thus, combining PET and CT imaging biomarkers has the potential to improve estimates of term survival for patients with cancer.
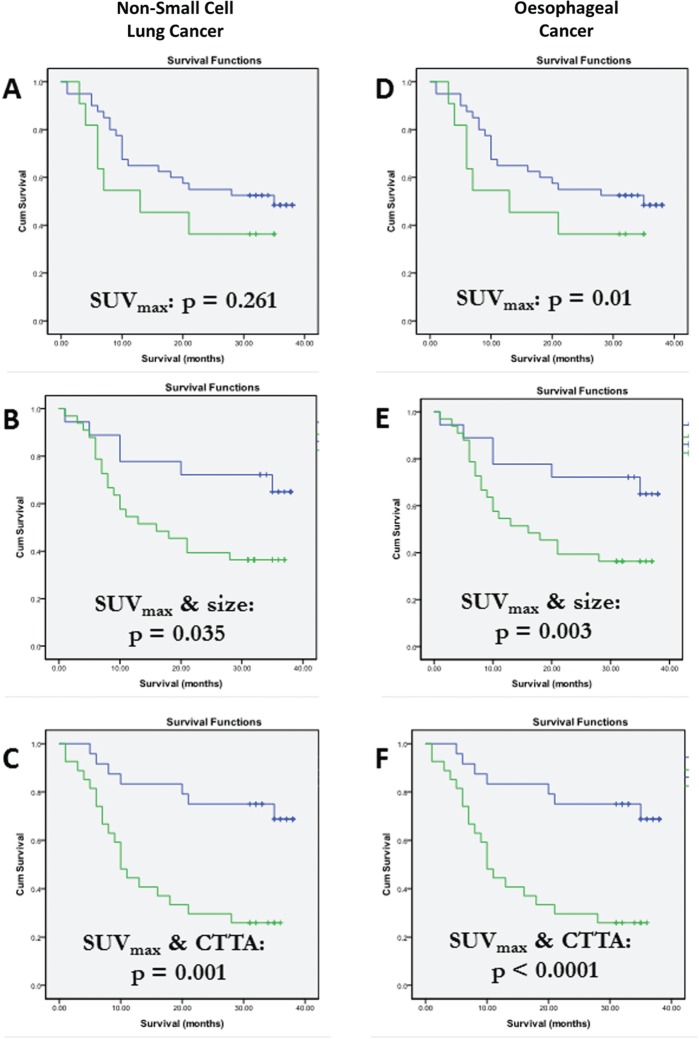
In oesophageal cancer, combining SUV from FDG-PET with CT measurements of tumour length or width was found to have better prognostic value than SUV measurements alone[41,42]. A more recent study using FDG-PET/CTTA in oesophageal cancer demonstrated that the improvements in prognostic value afforded by combining SUV with CT measurements of size and attenuation where out-performed by combinations of SUV with CTTA (Fig. 4D–F)[24]. Similar findings have also been reported for NSCLC (Fig. 4A–C)[24]. These preliminary studies suggest a very promising role for multiparametric PET/CT in identifying tumours with an adverse biological phenotype and predicting survival.
Figure 4.
Kaplan–Meier survival curves for patients with NSCLC (A–C) and oesophageal cancer (D–F) categorized by SUVmax (A,D), SUVmax combined with tumour size (B,E) and SUVmax combined with CT texture (C,F). Incorporation of CT biomarkers improves prognostic performance and is greatest for SUVmax combined with CTTA. (From Ganeshan et al.[24]).
Prediction and evaluation of therapy response in cancer
Assessment of therapeutic response is a major component of cancer imaging and multiparametric PET/CT imaging is emerging as a potentially powerful adjunct to current response assessment criteria. Accurate, comprehensive and reproducible quantitative response biomarkers enable early identification of responders and non-responders during treatment, allowing for modification of therapy regimens with the clear benefit of avoiding cytotoxic and expensive agents and personalizing targeted treatment protocols. Response assessment following completion of a therapy protocol is equally as vital as it can avoid excessive and often more invasive treatments and allow for proven consolidation treatments. The limitations of the widely used conventional response evaluation criteria, namely RECIST (1.0) are now well recognized, especially since the advent of targeted therapies that may prolong survival without producing a measurable change in tumour size. These limitations have led to an increasing increase in alternative imaging techniques for assessment of tumour response.
FDG-PET is now gaining wider acceptance as a biomarker for assessing metabolic response to therapies and a number of consensus guidelines have been published. Complete metabolic response on FDG-PET has been associated with improved survival with a number of malignancies and new metabolic activity indicates progression of disease[43]. However, developing robust quantitative response criteria with SUV measurements has proven difficult. More recently, FDG-PET response criteria using semi-quantitative SUV metrics alongside anatomic size values, have been proposed: PET Response Criteria in Solid Tumours (PERCIST)[43]. Briefly, these criteria require a decline of 30% in SUV along with size reduction, the use of normal liver tissue as background reference and obtaining SUV measurements from a small region of interest within the most active area of tumour.
Other imaging techniques have been shown to be more closely related to clinical outcome than RECIST for particular tumours and/or treatment regimes, including combined measurements of tumour size and enhancement/attenuation (Choi criteria) in patients with gastrointestinal stromal tumours treated with imatinib[44] and in metastatic renal cell cancer treated with tyrosine kinase inhibitors (TKIs)[19,45,46]. CTTA may also offer similar benefits. In a study by Goh et al[19], 87 renal cell carcinoma metastases in 39 patients were analysed with CTTA parameters before and after two cycles of TKI therapy. They found time to progression of disease and prediction of adequate response was better predicted by CTTA parameters than previous RECIST and Choi criteria.
DCE-CT has also demonstrated value in the assessment and prediction of therapy response. A number of small studies have demonstrated that elevated blood volume and flow in head and neck cancer[47–49], higher blood flow in NSCLC[50] and high BF and BV in rectal cancer[51] has strong predictive value in response to either adjuvant or neo-adjuvant chemo/radiotherapy. The pattern emerging from these preliminary clinical data suggests that poor response to chemotherapy or radiotherapy is more likely in tumours with low perfusion, which may relate to relative paucity of tumour vasculature limiting local delivery of cytotoxic agents. Several early phase I and II drug trials in a range of cancers have reported changes in CT perfusion parameters in response to both standard and targeted chemotherapies and radiotherapy in a range of cancers including head and neck cancer[49], oesophageal carcinoma[52], NSCLC[50], rectal adenocarcinoma[51,53] and hepatocellular carcinoma[54].
As discussed above, the use of multiparametric PET/CT to determine the tumour phenotype with regard to adaptation to hypoxia has implications for prediction of response to therapy. Tumours exhibiting flow-metabolic uncoupling represent successful adaptation to a hypoxic environment and are thus more aggressive and resistant to standard treatments. Furthermore, clinical studies that have used multiple imaging markers of response indicate that the various imaging parameters do not necessarily change in parallel following treatment. Mismatched responses have been shown particularly for changes in tumour FDG uptake and perfusion following therapy[26,55]. Frequently it may not be possible to predict which single imaging marker is the best choice for any particular tumour /therapy combination and therefore the use of multiple imaging response markers in a single examination increases the probability of demonstrating response. The power of multiple quantitative biomarkers allows logistic regression analysis to determine whether certain combination of parameters provide more accurate response prediction and assessment. In addition, new methods of classifying differential responses have been proposed in which different response categories may be of distinct biological significance[26]. This approach offers the potential to personalize therapy based on the multiparametric imaging response profile[26].
Improving biological depiction of treatment response and implications for drug development
PET/CT multiparametric imaging may significantly improve standard therapeutic assessment measures to evaluate novel therapies in clinical trials. DCE-CT has now matured to be a standardized and reproducible measure in a number of phase I and II trials evaluating anti-angiogenic agents[11,12]. There is future potential to utilize the full range of parameters offered by PET/CT to provide more comprehensive assessment of drug efficacy. An even more exciting prospect is the ability to provide quantitative surrogate markers of in vivo tumour biology and the tumour microenvironment and therefore a better understanding of the mechanism of action of novel drugs. This is most relevant in assessing drug effects on tumour blood flow–metabolism coupling, which as discussed earlier, may play a crucial role in treatment outcomes.
Limitations and future directions
Multiparametric imaging with PET/CT is an emerging area in oncological imaging and there is potential to significantly improve cancer imaging at all levels, from lesion characterization, non-invasive depiction of complex metabolic and vascular biology of tumour and tumour microenvironment, disease staging and prognosis, prediction and evaluation of response to novel therapies and in vivo assessment for novel drug development. However, large-scale prospective clinical data are still required before these parameters evolve into mature quantitative biomarkers implementable in phase III trials and clinical guidelines. Standardized and reproducible methods have evolved for DCE-CT and consensus guidelines to perform and acquire useful parameters are now available, which will allow wider implementation and future improvements. A current limitation of CTTA is that it utilizes single-section CT data and tumours generally exhibit variability across an entire volume. However, work is underway to evaluate three-dimensional volumetric CTTA analysis[56] in oncological imaging.
Multiparametric imaging with PET/CT can be readily incorporated into current clinical imaging protocols and the techniques described have the potential to improve depiction of tumour biology, lesion characterization, and assessment of prognosis and therapeutic response. A multidisciplinary effort is required to confirm these preliminary results and to encourage greater analysis of multiparametric data from existing hybrid PET/CT imaging systems.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology. 2010;256:348–364. doi: 10.1148/radiol.10091760. . PMid:20656830. [DOI] [PubMed] [Google Scholar]
- 2.Miles KA. PET/CT in oncology: making the most of CT. Cancer Imaging. 2008;8((A)):S87–S93. doi: 10.1102/1470-7330.2008.9015. . PMid:18852084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thie JA. Understanding the standardized uptake value, its methods, and implications for usage. J Nucl Med. 2004;45:1431–1434. PMid:15347707. [PubMed] [Google Scholar]
- 4.Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010;37:181–200. doi: 10.1007/s00259-009-1297-4. . PMid:19915839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med. 2008;49:1804–1808. doi: 10.2967/jnumed.108.054239. . PMid:18927325. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. . PMid:19097774. [DOI] [PubMed] [Google Scholar]
- 7.Sahdev A, Willatt J, Francis IR, Reznek RH. The indeterminate adrenal lesion. Cancer Imaging. 2010;10:102–113. doi: 10.1102/1470-7330.2010.0012. PMid:20299300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miles KA. Molecular imaging with dynamic contrast-enhanced computed tomography. Clin Radiol. 2010;65:549–556. doi: 10.1016/j.crad.2010.04.007. . PMid:20541654. [DOI] [PubMed] [Google Scholar]
- 9.Petralia G, Bonello L, Viotti S, Preda L, d'Andrea G, Bellomi M. CT perfusion in oncology: how to do it. Cancer Imaging. 2010;10:8–19. doi: 10.1102/1470-7330.2010.0001. PMid:20159664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mullani NA, Herbst RS, O'Neil RG, Gould KL, Barron BJ, Abbruzzese JL. Tumor blood flow measured by PET dynamic imaging of first-pass 18F-FDG uptake: a comparison with 15O-labeled water-measured blood flow. J Nucl Med. 2008;49:517–523. doi: 10.2967/jnumed.107.048504. . PMid:18344436. [DOI] [PubMed] [Google Scholar]
- 11.Goh V, Ng QS, Miles K. Computed tomography perfusion imaging for therapeutic assessment: has it come of age as a biomarker in oncology? Invest Radiol. 2012;47:2–4. doi: 10.1097/RLI.0b013e318229ff3e. . PMid:21808202. [DOI] [PubMed] [Google Scholar]
- 12.Miles KA, Lee TY, Goh V, et al. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol. 2012;22:1430–1441. doi: 10.1007/s00330-012-2379-4. . PMid:22367468. [DOI] [PubMed] [Google Scholar]
- 13.Ganeshan B, Miles KA, Young RC, Chatwin CR. In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol. 2007;14:1058–1068. doi: 10.1016/j.acra.2007.05.023. . PMid:17707313. [DOI] [PubMed] [Google Scholar]
- 14.Ganeshan B, Miles KA, Young RC, Chatwin CR. Hepatic entropy and uniformity: additional parameters that can potentially increase the effectiveness of contrast enhancement during abdominal CT. Clin Radiol. 2007;62:761–768. doi: 10.1016/j.crad.2007.03.004. . PMid:17604764. [DOI] [PubMed] [Google Scholar]
- 15.Ganeshan B, Miles KA, Young RC, Chatwin CR. Hepatic enhancement in colorectal cancer: texture analysis correlates with hepatic hemodynamics and patient survival. Acad Radiol. 2007;14:1520–1530. doi: 10.1016/j.acra.2007.06.028. . PMid:18035281. [DOI] [PubMed] [Google Scholar]
- 16.Ganeshan B, Miles KA, Young RC, Chatwin CR. Texture analysis in non-contrast enhanced CT: impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol. 2009;70:101–110. doi: 10.1016/j.ejrad.2007.12.005. . PMid:18242909. [DOI] [PubMed] [Google Scholar]
- 17.Ganeshan B, Panayiotou E, Burnand K, Dizdarevic S, Miles K. Tumour heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: a potential marker of survival. Eur Radiol. 2012;22:796–802. doi: 10.1007/s00330-011-2319-8. . PMid:22086561. [DOI] [PubMed] [Google Scholar]
- 18.Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67:157–164. doi: 10.1016/j.crad.2011.08.012. . PMid:21943720. [DOI] [PubMed] [Google Scholar]
- 19.Goh V, Ganeshan B, Nathan P, Juttla JK, Vinayan A, Miles KA. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker. Radiology. 2011;261:165–171. doi: 10.1148/radiol.11110264. . PMid:21813743. [DOI] [PubMed] [Google Scholar]
- 20.Tixier F, Hatt M, Le Rest CC, Le Pogam A, Corcos L, Visvikis D. Reproducibility of tumor uptake heterogeneity characterization through textural feature analysis in 18F-FDG PET. J Nucl Med. 2012;53:693–700. doi: 10.2967/jnumed.111.099127. . PMid:22454484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veit-Haibach P, Treyer V, Strobel K, et al. Feasibility of integrated CT-liver perfusion in routine FDG-PET/CT. Abdom Imaging. 2010;35:528–536. doi: 10.1007/s00261-009-9559-y. . PMid:19593563. [DOI] [PubMed] [Google Scholar]
- 22.Groves AM, Wishart GC, Shastry M, et al. Metabolic-flow relationships in primary breast cancer: feasibility of combined PET/dynamic contrast-enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36:416–421. doi: 10.1007/s00259-008-0948-1. . PMid:18818917. [DOI] [PubMed] [Google Scholar]
- 23.Bisdas S, Spicer K, Rumboldt Z. Whole-tumor perfusion CT parameters and glucose metabolism measurements in head and neck squamous cell carcinomas: a pilot study using combined positron-emission tomography/CT imaging. AJNR Am J Neuroradiol. 2008;29:1376–1381. doi: 10.3174/ajnr.A1111. . PMid:18483187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganeshan B, Hosur A, Skogen K, Tasker F, Dizdarevic S, Miles K. Multiparametric FDG-PET CT in thoracic malignancies: opportunities for combined prognostic imaging biomarkers. Presented at: UK Radiological Congress, 10–12 June, 2012, Liverpool, UK. PMid:22826419. [Google Scholar]
- 25.Ganeshan B, Burnand K, Young R, Chatwin C, Miles K. Dynamic contrast-enhanced texture analysis of the liver: initial assessment in colorectal cancer. Invest Radiol. 2011;46:160–168. doi: 10.1097/RLI.0b013e3181f8e8a2. . PMid:21102348. [DOI] [PubMed] [Google Scholar]
- 26.Miles KA, Williams RE. Warburg revisited: imaging tumour blood flow and metabolism. Cancer Imaging. 2008;8:81–86. doi: 10.1102/1470-7330.2008.0011. . PMid:18390391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillies RJ, Robey I, Gatenby RA. Causes and consequences of increased glucose metabolism of cancers. J Nucl Med. 2008;49(Suppl 2):24S–42S. doi: 10.2967/jnumed.107.047258. . PMid:18523064. [DOI] [PubMed] [Google Scholar]
- 28.Vaupel P, Harrison L. Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. Oncologist. 2004;9(Suppl 5):4–9. doi: 10.1634/theoncologist.9-90005-4. . PMid:15591417. [DOI] [PubMed] [Google Scholar]
- 29.Miles KA, Griffiths MR, Keith CJ. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur J Nucl Med Mol Imaging. 2006;33:22–28. doi: 10.1007/s00259-005-1932-7. . PMid:16180030. [DOI] [PubMed] [Google Scholar]
- 30.Shastry M, Miles KA, Win T, et al. Integrated 18F-fluorodeoxyglucose-positron emission tomography/dynamic contrast-enhanced computed tomography to phenotype non-small cell lung carcinoma. Mol Imaging. 2012;11:1–8. PMid:22480786. [PMC free article] [PubMed] [Google Scholar]
- 31.Miles KA, Williams RE, Yu D, Griffiths MR. Demonstrating intertumoural differences in vascular-metabolic phenotype with dynamic contrast-enhanced CT-PET. Int J Mol Imaging. 2011 doi: 10.1155/2011/679473. ; article 679473. PMid:21629862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh V, Engledow A, Rodriguez-Justo M, et al. The flow-metabolic phenotype of primary colorectal cancer: assessment by integrated 18F-FDG PET/perfusion CT with histopathologic correlation. J Nucl Med. 2012;53:687–692. doi: 10.2967/jnumed.111.098525. . PMid:22454485. [DOI] [PubMed] [Google Scholar]
- 33.Sauter AW, Winterstein S, Spira D, et al. Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med. 2012;53:521–529. doi: 10.2967/jnumed.111.097865. . PMid:22414637. [DOI] [PubMed] [Google Scholar]
- 34.Cochet A, Pigeonnat S, Khoury B, et al. Evaluation of breast tumor blood flow with dynamic first-pass 18F-FDG PET/CT: comparison with angiogenesis markers and prognostic factors. J Nucl Med. 2012;53:512–520. doi: 10.2967/jnumed.111.096834. . PMid:22343501. [DOI] [PubMed] [Google Scholar]
- 35.Ganeshan B, Ziauddin Z, Goh VJ, et al. Quantitative imaging biomarkers from PET/CT as potential correlates for angiogenesis and hypoxia in colorectal cancer. Presented at: European Congress of Radiology; March 1–5, 2012; Vienna. SS 1801a: B-0876. PMid:22826419. [Google Scholar]
- 36.Brady MJ, Thomas J, Wong TZ, Franklin KM, Ho LM, Paulson EK. Adrenal nodules at FDG PET/CT in patients known to have or suspected of having lung cancer: a proposal for an efficient diagnostic algorithm. Radiology. 2009;250:523–530. doi: 10.1148/radiol.2502080219. . PMid:19188319. [DOI] [PubMed] [Google Scholar]
- 37.Blake MA, Slattery JM, Kalra MK, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy–initial experience. Radiology. 2006;238:970–977. doi: 10.1148/radiol.2383042164. . PMid:16505394. [DOI] [PubMed] [Google Scholar]
- 38.Perri M, Erba P, Volterrani D, et al. Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. AJR Am J Roentgenol. 2011;197:209–216. doi: 10.2214/AJR.10.5342. . PMid:21701032. [DOI] [PubMed] [Google Scholar]
- 39.Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. . PMid:18166834. [DOI] [PubMed] [Google Scholar]
- 40.Pan L, Gu P, Huang G, Xue H, Wu S. Prognostic significance of SUV on PET/CT in patients with esophageal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2009;21:1008–1015. doi: 10.1097/MEG.0b013e328323d6fa. . PMid:19352191. [DOI] [PubMed] [Google Scholar]
- 41.Roedl JB, Sahani DV, Colen RR, Fischman AJ, Mueller PR, Blake MA. Tumour length measured on PET/CT predicts the most appropriate stage-dependent therapeutic approach in oesophageal cancer. Eur Radiol. 2008;18:2833–2840. doi: 10.1007/s00330-008-1078-7. . PMid:18651155. [DOI] [PubMed] [Google Scholar]
- 42.Roedl JB, Sahani DV, Colen RR, Fischman AJ, Mueller PR, Blake MA. Tumour length measured on PET/CT predicts the most appropriate stage-dependent therapeutic approach in oesophageal cancer. Eur Radiol. 2008;18:2833–2840. doi: 10.1007/s00330-008-1078-7. . PMid:18651155. [DOI] [PubMed] [Google Scholar]
- 43.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. . PMid:19403881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–1759. doi: 10.1200/JCO.2006.07.3049. . PMid:17470865. [DOI] [PubMed] [Google Scholar]
- 45.Smith AD, Shah SN, Rini BI, Lieber ML, Remer EM. Morphology, Attenuation, Size, and Structure (MASS) criteria: assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy. AJR Am J Roentgenol. 2010;194:1470–1478. doi: 10.2214/AJR.09.3456. . PMid:20489085. [DOI] [PubMed] [Google Scholar]
- 46.Smith AD, Lieber ML, Shah SN. Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy: importance of size and attenuation on contrast-enhanced CT. AJR Am J Roentgenol. 2010;194:157–165. doi: 10.2214/AJR.09.2941. . PMid:20028918. [DOI] [PubMed] [Google Scholar]
- 47.Bisdas S, Rumboldt Z, Wagenblast J, et al. Response and progression-free survival in oropharynx squamous cell carcinoma assessed by pretreatment perfusion CT: comparison with tumor volume measurements. AJNR Am J Neuroradiol. 2009;30:793–799. doi: 10.3174/ajnr.A1449. . PMid:19351906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zima A, Carlos R, Gandhi D, Case I, Teknos T, Mukherji SK. Can pretreatment CT perfusion predict response of advanced squamous cell carcinoma of the upper aerodigestive tract treated with induction chemotherapy? AJNR Am J Neuroradiol. 2007;28:328–334. PMid:17297007. [PMC free article] [PubMed] [Google Scholar]
- 49.Hermans R, Meijerink M, Van den Bogaert W, Rijnders A, Weltens C, Lambin P. Tumor perfusion rate determined noninvasively by dynamic computed tomography predicts outcome in head-and-neck cancer after radiotherapy. Int J Radiat Oncol Biol Phys. 2003;57:1351–1356. doi: 10.1016/S0360-3016(03)00764-8. . PMid:14630273. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Wu N, Cham MD, Song Y. Tumor response in patients with advanced non-small cell lung cancer: perfusion CT evaluation of chemotherapy and radiation therapy. AJR Am J Roentgenol. 2009;193:1090–1096. doi: 10.2214/AJR.08.1367. . PMid:19770333. [DOI] [PubMed] [Google Scholar]
- 51.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486–493. doi: 10.1148/radiol.2442061189. . PMid:17641369. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi D, Chepeha DB, Miller T, et al. Correlation between initial and early follow-up CT perfusion parameters with endoscopic tumor response in patients with advanced squamous cell carcinomas of the oropharynx treated with organ-preservation therapy. AJNR Am J Neuroradiol. 2006;27:101–106. PMid:16418366. [PMC free article] [PubMed] [Google Scholar]
- 53.Sahani DV, Kalva SP, Hamberg LM, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005;234:785–792. doi: 10.1148/radiol.2343040286. . PMid:15734934. [DOI] [PubMed] [Google Scholar]
- 54.Jiang T, Kambadakone A, Kulkarni NM, Zhu AX, Sahani DV. Monitoring response to antiangiogenic treatment and predicting outcomes in advanced hepatocellular carcinoma using image biomarkers, CT perfusion, tumor density, and tumor size (RECIST) Invest Radiol. 2012;47:11–17. doi: 10.1097/RLI.0b013e3182199bb5. . PMid:21512396. [DOI] [PubMed] [Google Scholar]
- 55.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. . PMid:14745444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganeshan B, Miles KA, Young RC, Chatwin CR, Gurling HM, Critchley HD. Three-dimensional textural analysis of brain images reveals distributed grey-matter abnormalities in schizophrenia. Eur Radiol. 2010;20:941–948. doi: 10.1007/s00330-009-1605-1. . PMid:19760235. [DOI] [PubMed] [Google Scholar]