Abstract
Numerous and diverse physiological changes occur during fruit ripening, including the development of a specific volatile blend that characterizes fruit aroma. Maturity at harvest is one of the key factors influencing the flavor quality of fruits and vegetables1. The validation of robust methods that rapidly assess fruit maturity and aroma quality would allow improved management of advanced breeding programs, production practices and postharvest handling.
Over the last three decades, much research has been conducted to develop so-called electronic noses, which are devices able to rapidly detect odors and flavors2-4. Currently there are several commercially available electronic noses able to perform volatile analysis, based on different technologies. The electronic nose used in our work (zNose, EST, Newbury Park, CA, USA), consists of ultra-fast gas chromatography coupled with a surface acoustic wave sensor (UFGC-SAW). This technology has already been tested for its ability to monitor quality of various commodities, including detection of deterioration in apple5; ripeness and rot evaluation in mango6; aroma profiling of thymus species7; C6 volatile compounds in grape berries8; characterization of vegetable oil9 and detection of adulterants in virgin coconut oil10.
This system can perform the three major steps of aroma analysis: headspace sampling, separation of volatile compounds, and detection. In about one minute, the output, a chromatogram, is produced and, after a purging cycle, the instrument is ready for further analysis. The results obtained with the zNose can be compared to those of other gas-chromatographic systems by calculation of Kovats Indices (KI). Once the instrument has been tuned with an alkane standard solution, the retention times are automatically converted into KIs. However, slight changes in temperature and flow rate are expected to occur over time, causing retention times to drift. Also, depending on the polarity of the column stationary phase, the reproducibility of KI calculations can vary by several index units11. A series of programs and graphical interfaces were therefore developed to compare calculated KIs among samples in a semi-automated fashion. These programs reduce the time required for chromatogram analysis of large data sets and minimize the potential for misinterpretation of the data when chromatograms are not perfectly aligned.
We present a method for rapid volatile compound analysis in fruit. Sample preparation, data acquisition and handling procedures are also discussed.
Keywords: Plant Biology, Issue 61, zNose, volatile profiling, aroma, Kovats Index, electronic nose, gas chromatography, retention time shift
Protocol
1. Sample Preparation
Harvest fruits at desired maturity stage. Rinse with tap water in order to remove dirt and dust.
Select fruits for analysis based on the absence of external and internal defects, and size homogeneity.
Cut fruits longitudinally into wedges to be used for volatile sampling. If applicable, remove skin, seeds, seed cavity tissue, or pit. Fruit tissue selection must be consistent throughout the experiment and take into account the variability within a single fruit (i.e., obtain sample equally from the equatorial, blossom and stem end parts).
Combine the selected fruit tissue, mix it in order to randomize it, and then weigh out 200 g into a blender.
Add 200 mL of saturated CaCl2 solution (372.5 g at 20 °C, in 500 mL of deionized water) and 50 μL of a 100 mM solution of 2-methylbutyl isovalerate in methanol. The CaCl2 is intended to act as an inhibitor of enzymatic activity, which might occur after cutting and homogenizing the fruit flesh. 2-Methylbutyl isovalerate is added as an internal standard to monitor for any possible losses of volatile compounds during the homogenization process.
Homogenize mixture in a laboratory blender (Waring, USA), for 30 seconds at 18,000 rpm, then immediately pour into glass bottle and seal with Teflon lid. Keep the homogenate in the bottle at room temperature until all samples are prepared.
After pouring the homogenate into the bottle, wait 10 minutes to allow the separation of the foam from the liquid, then pipette 5 mL aliquots of liquid , without foam, into 20 mL glass amber vials and seal the vials with steel screw caps fitted with Teflon/silicone septa. This procedure is suitable for melon and pear homogenate preparation. If other fruits are used for the analysis, a centrifugation step might be required. Therefore, remove the foam and then centrifuge the liquid to pellet particles that might obstruct the pipette. Prepare at least three vials per sample to serve as technical replicates.
At this point, samples may be analyzed immediately or flash-frozen in liquid nitrogen and stored at ultra-low temperature (-80 °C) for later analysis.
For frozen samples, on the analysis day remove the samples from the freezer and allow them to thaw for one hour at room temperature. After thawing, and before analysis, replace the vial cap with a new one that has a clean, dry septum. If the septum is not replaced, water condensed on the septum during thawing can be drawn into the instrument and damage it.
2. Gas Chromatography-surface Acoustic Wave (GC-SAW) Set-up and Data Acquisition
Load the appropriate analysis method on the zNose. For analysis of the ester-rich volatile profile of melon, our parameters in the MicroSense version 5.44.22 software (Newbury Park, CA, USA) are as follows: headspace suction into the inlet for 20 seconds at 30 mL min-1 via pump; inlet temperature at 200 °C; Tenax trap temperature at 225 °C; carrier gas (helium purity 99.999%) flow rate of 2.9 mL min-1; column (DB-5 column, 1 m × 0.25mm I.D.× 0.25 μm film thickness) temperature program from 45 °C to 180 °C at a rate of 10 °C sec-1; sensor temperature at 40 °C; valve at 165 °C. The total analysis time is 1 minute per sample.
Connect a stainless steel needle with non-coring tip to the zNose inlet and purge the system several times with ambient air until the baseline is stable and no peaks larger than 200 Counts (Ct) are detected.
Tune the instrument using a solution of straight chain alkanes (C6-C14). The tune result is used by the instrument software to convert the retention time of the eluted peaks from time units into Kovats Index (KI) units. Consequently, after the system is tuned, the retention times are reported in KI units.
Before the analysis, allow the sample to equilibrate for 30 minutes. To analyze one of the sample vials, insert a needle into the vial septum to relieve the pressure. Then insert the needle connected to the instrument inlet into the vial septum and initiate headspace sampling. Analyze at least three technical replicates per sample.
Manually start the instrument by clicking on "Play" button; the pump activates and withdraws the vapors present above the sample. At the end of the analysis, a chromatogram appears on the screen, and the sensor is automatically heated to 150 °C for 10 seconds to clean it. When the system status box button turns green again, the instrument is ready to analyze another sample.
To ensure a stable baseline and proper system cleaning, run at least one air blank between each sample. To monitor for possible volatile contamination from the vial and cap, analyze two vial-blanks (empty vial with cap), at the beginning and end of the day.
3. Data Export and Analysis
Export the data into a Microsoft excel file after acquisition using the "Peak logging" function in MicroSense software. Once the data are exported, add columns containing labels for variables and replicates.
Transform the data format for easier manipulation using the Python (version 2.6; freely available on-line) script we developed, named "reform_data.py"(see Figure 1 for an example of the data format before and after using the script "reform_data.py"). The name of the source file (xls format) and sheet name for the input data, as well as the desired file name for output (xls format), are edited directly in the script.
Start "kim_interface.py" (also written in Python 2.6; see Figure 2), and import the data from the file generated in the previous step. Specifically, the analysis is based on viewing and analyzing the number of times each KI value was detected ("KI hits"). Thus the program displays a bar graph of KI hits for each KI value.
Evaluate the KI hits of specific subsets of samples, analyzing each group of technical replicates together. To do so, analyze every treatment or variable separately by checking/unchecking the corresponding boxes. See Figure 2 caption for detailed description of the graphical user interface (GUI) features.
After identifying the width of each KI window using the GUI, randomly select some of the corresponding chromatograms in Microsense software and evaluate overlapping peaks among the technical replicates. See Figure 3 for an example of overlaid chromatograms of two technical replicates.
Once the KI window is individuated, use the 'Merge' feature available in the GUI to merge the KIs that fall in the window, into the most populated KI. By using this feature, peaks labeled with a range of KI values are consolidated under a single KI label, allowing treating such peaks as a single variable. To do so, first click on the 'Merge' button to activate the feature and select the most populated KI as the center of the window by left-clicking the corresponding bar. Once the bar has been selected, it changes color and turns green. To merge the KIs that fall within the window into the selected KI, right-click on the corresponding bars; this causes the bars to turn red, while a blue bar of the corresponding length is added on top of the central KI (See Figure 4). Once all the selected KIs have been merged into the appropriate central KI, click on the 'Merge' button again to accept the changes; this causes the 'Merge' button to turn yellow. In case of mistakes, the 'Unmerge' button is also available. To unmerge, click the 'Unmerge' button in the GUI, then right-click on the red bar you want to unmerge. From red, the bar turns blue. Click the 'Unmerge' button again to accept the changes.
If one attempts to incorrectly merge two peaks in a single sample into a single KI value, an error message is printed. In such circumstances, closely check the chromatogram and redefine the KI window in that region.
Once all of the merging operations have been performed, save the file.
Before proceeding with statistical analysis, the chromatograms of the air and vial blanks are analyzed to monitor for possible contaminations. Once the KI of the peaks in the blanks have been identified, subtract the area of the peak detected in the air- and/or vial-blank from the area of the peak present in sample. Then proceed with statistical analysis.
4. Representative Results
The electronic nose was able to detect differences in volatile profiles among melon fruit harvested at different maturity stages (Figure 5). Twenty KI windows were identified across all samples. An analysis of variance showed that 14 peaks detected by the electronic nose varied significantly between maturity stages. In Figure 6, the log of the mean peak areas of these 14 components are plotted to show differences in peak abundances between two maturity stages, early mature and fully ripe fruit.
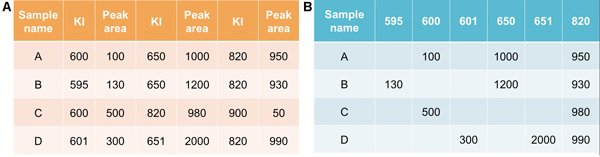 Figure 1. Examples of data format exported from instrument software (A) and after transformation, performed using "reform_data.py" script (B). To facilitate data manipulation and analysis, all the unique KIs are identified across all the samples, then the data are reordered with sample information in rows and peak area in columns, corresponding to unique KIs. If a peak is not detected for a KI value in a sample, the corresponding cell remains empty.
Figure 1. Examples of data format exported from instrument software (A) and after transformation, performed using "reform_data.py" script (B). To facilitate data manipulation and analysis, all the unique KIs are identified across all the samples, then the data are reordered with sample information in rows and peak area in columns, corresponding to unique KIs. If a peak is not detected for a KI value in a sample, the corresponding cell remains empty.
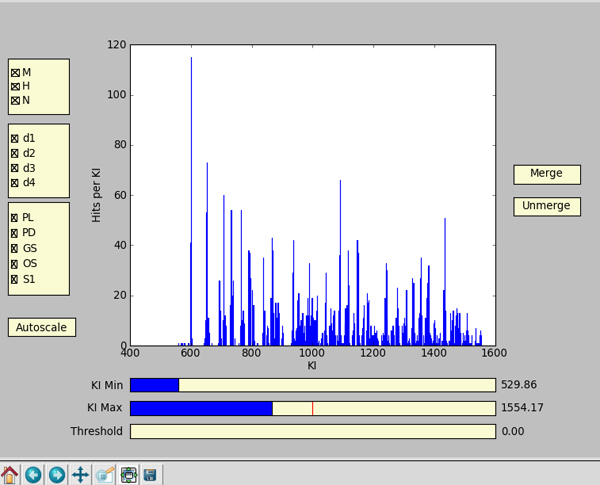 Figure 2. Screen capture from the script file "kim_interface.py". The plot in the center displays number of hits per KI versus KI. 'Hit per KI' is the number of samples in which a peak with that specific KI was detected. On the left side, there are three yellow boxes controlling the selected data. They display parameters to divide the data set (treatments, replicates, qualitative variables, etc.). In this figure, they are (from top to bottom): Variety, Planting date and Maturity stage at harvest. On the bottom: by clicking on the 3 bars and moving the blue bar to the left or to the right, one can select the minimum and the maximum value of the KI range, and the minimum peak area ('Threshold'). On the right: the 'Merge' button allows merging selected KIs by manually clicking on the bars in the plot. The 'Unmerge' button allows one to reverse the process for selected cases.
Figure 2. Screen capture from the script file "kim_interface.py". The plot in the center displays number of hits per KI versus KI. 'Hit per KI' is the number of samples in which a peak with that specific KI was detected. On the left side, there are three yellow boxes controlling the selected data. They display parameters to divide the data set (treatments, replicates, qualitative variables, etc.). In this figure, they are (from top to bottom): Variety, Planting date and Maturity stage at harvest. On the bottom: by clicking on the 3 bars and moving the blue bar to the left or to the right, one can select the minimum and the maximum value of the KI range, and the minimum peak area ('Threshold'). On the right: the 'Merge' button allows merging selected KIs by manually clicking on the bars in the plot. The 'Unmerge' button allows one to reverse the process for selected cases.
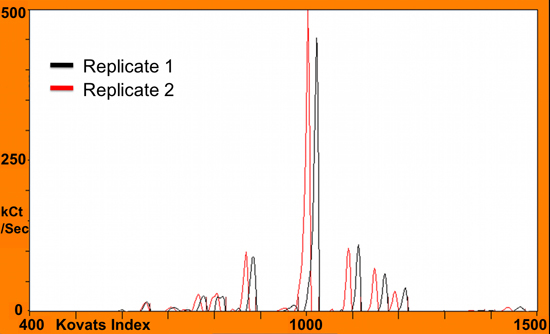 Figure 3. Overlaid chromatograms (in black and red) of two technical replicates from melon volatile headspace to illustrate a shift in retention time.
Figure 3. Overlaid chromatograms (in black and red) of two technical replicates from melon volatile headspace to illustrate a shift in retention time.
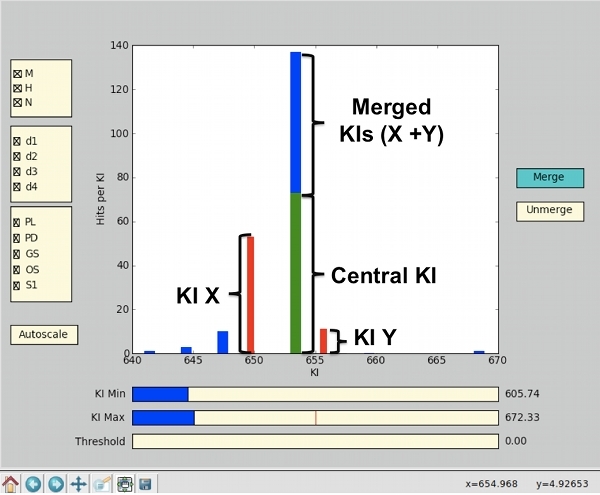 Figure 4. Example of the KI merging procedure. In the central plot, the green bar (Central KI) represents the most populated KI, which has been selected as center of the KI window. KI X and KI Y are KIs falling in the window of interest and they need to be merged into the central KI. By right-clicking on KI X's bar, it turns red and, at the same time, a blue bar of the same length of KI X's bar, appears on top of the green one. By repeating the same procedure for KI Y, the length of the blue bar (Merged KIs) will increase of the corresponding length. Once all the KIs have been added, by clicking on the green 'Merge' button, the merging process ends, the changes are saved, and the button color turns yellow.
Figure 4. Example of the KI merging procedure. In the central plot, the green bar (Central KI) represents the most populated KI, which has been selected as center of the KI window. KI X and KI Y are KIs falling in the window of interest and they need to be merged into the central KI. By right-clicking on KI X's bar, it turns red and, at the same time, a blue bar of the same length of KI X's bar, appears on top of the green one. By repeating the same procedure for KI Y, the length of the blue bar (Merged KIs) will increase of the corresponding length. Once all the KIs have been added, by clicking on the green 'Merge' button, the merging process ends, the changes are saved, and the button color turns yellow.
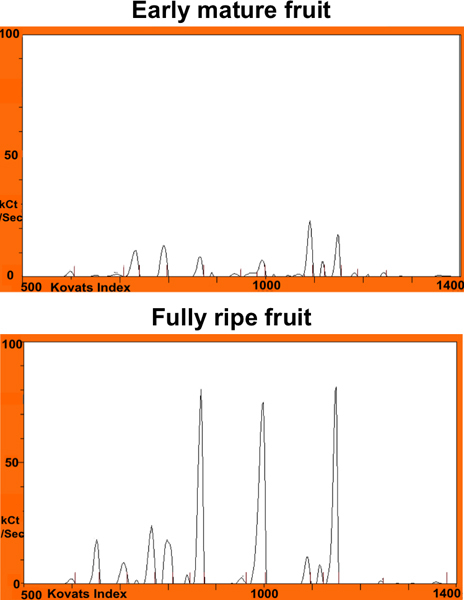 Figure 5. Two chromatograms of melon samples harvested at different maturity stages, early mature (top) and fully ripe (bottom), to illustrate the ability of the electronic nose to detect differences in volatile abundances.
Figure 5. Two chromatograms of melon samples harvested at different maturity stages, early mature (top) and fully ripe (bottom), to illustrate the ability of the electronic nose to detect differences in volatile abundances.
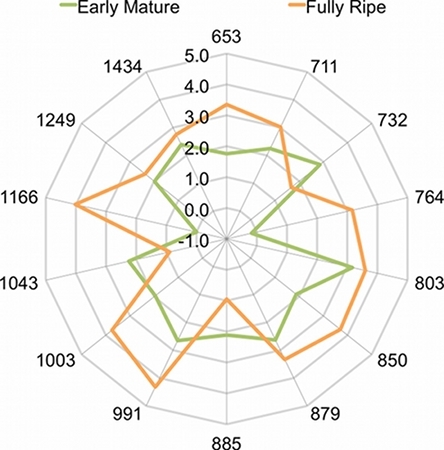 Figure 6. Radar plot showing the peak area of 14 components present in two melon samples at two different maturity stages, early mature and fully ripe. The peak areas are reported in log scale to help visualize the comparison. The numbers at the end of each ray represent the corresponding Kovats Indices.
Figure 6. Radar plot showing the peak area of 14 components present in two melon samples at two different maturity stages, early mature and fully ripe. The peak areas are reported in log scale to help visualize the comparison. The numbers at the end of each ray represent the corresponding Kovats Indices.
Discussion
Electronic noses represent a promising method for the rapid, objective evaluation of aroma profiles from fruits or volatile-rich samples. However, shifts in retention time represent a challenge for peak identification and might lead to misinterpretation of the data when two chromatograms are not perfectly aligned. Visual inspection of chromatograms indicated that the variability of retention times among samples frequently caused the same peak to be labeled with slightly different KI values (approximately ±10). This translated into an exaggerated number of unique KIs detected. In order to take advantage of the facts that (a) different compounds are present at different maturity stages and (b) technical replicates are approximately identical, two computer-based scripts ("kim_merge.py", which contains the routines for handling the data set, and "kim_interface.py", which provides a graphical user interface (GUI)) were developed to systematically compare samples in a semi-automated fashion, greatly reducing the time needed for chromatogram analysis of large data sets. These programs allow the consolidation, where appropriate, of peaks labeled with a range of KI values under a single KI label. This serves two important purposes: (a) it enables a statistical analysis to treat such peaks as a single variable, and (b) it facilitates peak identification and comparison to other systems and published values. Results presented here indicate that melon samples could be discriminated based on maturity and aroma profiling using the zNose system in combination with adequate KI identification. This represents a promising new technology for analysis of volatiles that may be used for quality control programs.
Disclosures
We have nothing to disclose.
Acknowledgments
The authors thank Bill Copes (Harris Moran Seed Company, Davis) for providing melon fruits for this analysis. This project is supported by the Specialty Crops Research Initiative Competitive Grants Program grant no. 2009-51181-05783 from the USDA National Institute of Food and Agriculture.
References
- Kader AA. Flavor quality of fruits and vegetables. Journal of the Science of Food and Agriculture. 2008;88:1863–1868. [Google Scholar]
- Persaud K, Dodd G. Analysis of discriminant mechanisms in the mammalian olfactory system using a model nose. Nature. 1982;299:352–355. doi: 10.1038/299352a0. [DOI] [PubMed] [Google Scholar]
- Gardner JW, Bartlett PN. A brief-history of electronic noses. Sensors and Actuators. 1994;18:211–220. [Google Scholar]
- Rock F, Barsan N, Weimar U. Electronic nose: Current status and future trends. Chem. Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]
- Li C, Heinemann PH, Irudayaraj J. Detection of apple deterioration using an electronic nose and zNose. Transactions of the Asabe. 2007;50:1417–1425. [Google Scholar]
- Li ZF, Wang N, Raghavan GSV, Vigneault C. Ripeness and rot evaluation of 'Tommy Atkins' mango fruit through volatiles detection. J. Food Eng. 2009;91:319–324. [Google Scholar]
- Oh SY, Ko JW, Jeong SY, Hong J. Application and exploration of fast gas chromatography-surface acoustic wave sensor to the analysis of thymus species. J. Chromatogr. A. 2008;1205:117–127. doi: 10.1016/j.chroma.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Watkins P, Wijesundera C. Application of zNose for the analysis of selected grape aroma compounds. Talanta. 2006;70:595–601. doi: 10.1016/j.talanta.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Gan HL, Man YBC, Tan CP, NorAini I, Nazimah SAH. Characterisation of vegetable oils by surface acoustic wave sensing electronic nose. Food Chem. 2005;89:507–518. [Google Scholar]
- Marina AM, Man YBC, Amin I. Use of the SAW Sensor Electronic Nose for Detecting the Adulteration of Virgin Coconut Oil with RBD Palm Kernel Olein. Journal of the American Oil Chemists Society. 2010;87:263–270. [Google Scholar]
- Evans MB, Haken JK. Recent developments in the gas-chromatographic index scheme. Journal of Chromatography. 1989;472:93–127. [Google Scholar]


