Abstract
Glioblastoma multiforme (GBM) are the most common and aggressive adult primary brain tumors1. In recent years there has been substantial progress in the understanding of the mechanics of tumor invasion, and direct intracerebral inoculation of tumor provides the opportunity of observing the invasive process in a physiologically appropriate environment2. As far as human brain tumors are concerned, the orthotopic models currently available are established either by stereotaxic injection of cell suspensions or implantation of a solid piece of tumor through a complicated craniotomy procedure3. In our technique we harvest cells from tissue culture to create a cell suspension used to implant directly into the brain. The duration of the surgery is approximately 30 minutes, and as the mouse needs to be in a constant surgical plane, an injectable anesthetic is used. The mouse is placed in a stereotaxic jig made by Stoetling (figure 1). After the surgical area is cleaned and prepared, an incision is made; and the bregma is located to determine the location of the craniotomy. The location of the craniotomy is 2 mm to the right and 1 mm rostral to the bregma. The depth is 3 mm from the surface of the skull, and cells are injected at a rate of 2 μl every 2 minutes. The skin is sutured with 5-0 PDS, and the mouse is allowed to wake up on a heating pad. From our experience, depending on the cell line, treatment can take place from 7-10 days after surgery. Drug delivery is dependent on the drug composition. For radiation treatment the mice are anesthetized, and put into a custom made jig. Lead covers the mouse's body and exposes only the brain of the mouse. The study of tumorigenesis and the evaluation of new therapies for GBM require accurate and reproducible brain tumor animal models. Thus we use this orthotopic brain model to study the interaction of the microenvironment of the brain and the tumor, to test the effectiveness of different therapeutic agents with and without radiation.
Keywords: Medicine, Issue 61, Neuroscience, mouse, intracranial, orthotopic, radiation, glioblastoma
Protocol
I. Cell Preparation
The cells are grown to confluence in DMEM with 10% FBS about one 80% confluent 225 cm3 flask for every 5 mice to be implanted.
Aspirate media off of the cells; wash with 10 ml of PBS without Calcium or Magnesium. Aspirate PBS off cells.
Trypsinize cells with 3 ml of trypsin, wait 10-15 min, and neutralize the trypsin with 15 ml of media.
Pipet all cells and media into a 50 ml conical. Two large flasks fit into one 50 ml conical.
Spin cells at 1600 rpm for 7 min at 4 °C. Completely aspirate supernatant. Resuspend cells in 10 ml of cold PBS, and rinse cells by gently pipetting up and down. Make sure the cell pellet is mixed uniformly with the PBS. The suspension should be cloudy.
At this time a cell count is done using a coulter counter. The cells are centrifuged again at 1600 rpm for 7 min at 4 °C.
After the 2nd centrifugation of the cells, aspirate all PBS and resuspend in 1 ml of PBS.
The cells are spun in the 1ml of PBS at 3000 rpm for 10 min at 4 °C. The PBS is aspirated, and PBS is added to adjust the final concentration to 1x106 per 5 μl for U87 cells, other cell lines may require a different number and concentration of cells for optimal tumor growth.
After the final volume and concentration is obtained the cells are kept on ice in an eppendorf tube.
II. Xenograft
- Preparing the mouse
- The mice are anesthetized using an injection mix of Ketamine/Xylazine/Saline. The mixture is 120 mg/kg of Ketamine, 16 mg/kg of Rompun and Saline. The mice are weighed individually and given 0.1 ml per 10 g based on their weight. The anesthetic is given with 26G needle attached to 1 ml syringe IP. It will take approximately 15 min for the mice to reach a sleeping phase for no pain response. You can check the pain response of the mice by pinching their footpad.
- After the mouse is fully anesthetized, eye ointment is administered to their eyes to prevent them from drying out.
- The mouse is placed on the bed of the stereotaxic with the front upper teeth in the notch on the mouth bar. The bed is then placed in the stereotaxic.
- The nose guard is placed over the mouse's nose to prevent movement of the head, and the ear bars are gently place in the ear canals of the mice. Be careful not to do either placement of the nose bar or ear bars too tight, as the skull may fracture and/or prevent the mouse from breathing.
- Make sure that the head of the mouse is level using the tick marks on the stereotaxic is helpful during this process.
- To prep the mouse, alcohol and providine iodine is applied liberally on the head of the mouse alternating for a total of 3 application of alcohol and 3 applications of iodine. One final application of an alcohol pad with 70% is applied to the head.
- Surgical procedure
- All instruments in the procedure should be sterilized either by autoclaving or soaking instruments in 70% ethanol before beginning the procedure.
- An incision is made on top of the skull behind the right eye diagonally back to the left posterior of the skull. The incision should be about 10 mm to 15 mm long.
- Using a Q-tip, slowly separate the incision to expose the skull and wipe away the membrane that is between the skull and the skin. Use a Q-tip to blot any blood and watch for excessive bleeding.
- Expose the top of the skull to visualize the bregma. Using the Hamilton syringe point, position it over the bregma. The correct position of the hole to be drilled in the skull is 2 mm right and 1 mm anterior of bregma (figure 2). A surgeon's felt-tipped marker, that is purchased sterile, is used to mark the location of the hole to be drilled. Please note that the hole is located close to the anterior suture line.
- Next, drill a small hole in the skull at the marked position using the point of a 22G needle in a pin vase or fed through an 18G needle.
- Load the 10 μl glass Hamilton syringe with 5 μl of cells. The Hamilton syringe is 10 μl with a blunt 30G to 33G fixed needle attached to it. Be sure to mix the cells before loading them, either by pipetting them or using a 1ml syringe with a 25G needle attached. After the Hamilton syringe is loaded, place it back in the arm to line it up with the hole in the skull.
- Lower the bunt end of the needle to the surface of the skull, once the needle is level with the skull; lower the needle until it is at the depth of 3 mm below the surface of the skull.
- After the needle is lowered, it shall remain there for 2 minutes to avoid any major trauma from the needle affecting the brain. Implanting the cells is at a rate of 1 μl per minute for a total of 6-8 minutes, be careful of any backflow. After the last of the cells have been implanted, leave the needle in place for another 2 minutes, to allow all the cells to settle in.
- Remove the needle from the brain, and clean the hole with a Q-tip. Proceed to clean the needle and syringe with 50% Actone, 100% EtOH, and PBS, 3x in each solution and in that order.
- The skin on the skull is ready to be sutured at this point. Using 5-0 PBS close the incision with about 3 stitches.
- Allow the mouse to wake up on a heat pad, and reapply eye ointment if needed. The mouse can be returned to the cage after it shows movement on its own (i.e. Lifting up the head or moving the shoulders).
III. Treatment of the IC implant By Radiation and Drug
- Radiation treatment
- Depending on the cell line, treatment of the mice can take place 5 to 7 days post-implant. Our department uses an orthovoltage radiotherapy unit for radiation treatment.
- Imaging such as BLI (Bioluminescence Imaging), MRI or CT can be used to visualize the tumor prior to randomization (figure 3A).
- The mice are anesthetized with the Ketamine/Xylazine/saline cocktail as before, and eye ointment is applied to their eyes.
- The mice are positioned in a custom made radiation jig that is configured like a pie wheel, in which the heads of the mice point towards the center. The bodies of the mice are shielded from the radiation with lead.
- Drug treatment can be given either before or radiation through different delivery routes, i.e. SQ, IP and IV.
IV. Results
The mouse should wake up from the procedure within 45-60 minutes after anesthesia injection. If the mouse has difficultly waking up after the procedure and time has exceeded 1½ hr after the procedure, the mouse may not recover and euthanasia may be an effective alternative. If there is excessive bleeding or the skull becomes cracked at any point during the procedure euthanasia is advised. If all goes well and depending on the cell line, a tumor should start forming within 2-4 weeks after the procedure has take place. When using intracranial xenograft models, therapeutic efficacy is usually measured as survival post-implantation5. However, as this is an IC model signs and symptoms will occur prior to death. Some of the physical signs the mouse may exhibit are a hunched posture, loss of body weight, and inactivity. Some of the neurological signs may be seizures, head tilt, and loss of balance. Figure 3B shows an HE stain of a tumor at mortality. A Kaplan-Meier plot is used to evaluate the data (figure 4). The number of days of survival at 50% are calculated and compared to the control. If the combination group lived longer than the addition of the individual radiation and drug arms the combination is was successful.
 Figure 1. The Stoelting Stereotaxic equipment.
Figure 1. The Stoelting Stereotaxic equipment.
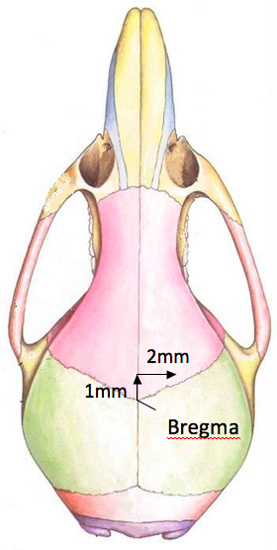 Figure 2. A diagram of the location of the drill hole in the mouse skull.
Figure 2. A diagram of the location of the drill hole in the mouse skull.
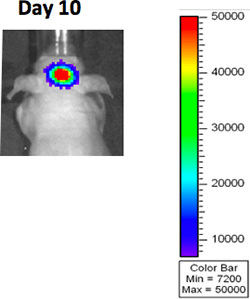 Figure 3A. BLI imaging after GBM implantation.
Figure 3A. BLI imaging after GBM implantation.
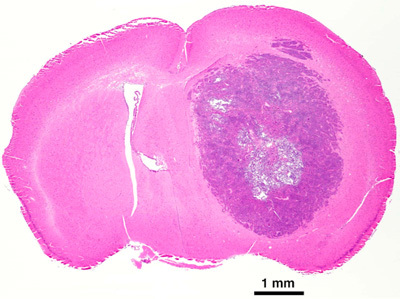 Figure 3B. H and E of GBM tumor at mortality.
Figure 3B. H and E of GBM tumor at mortality.
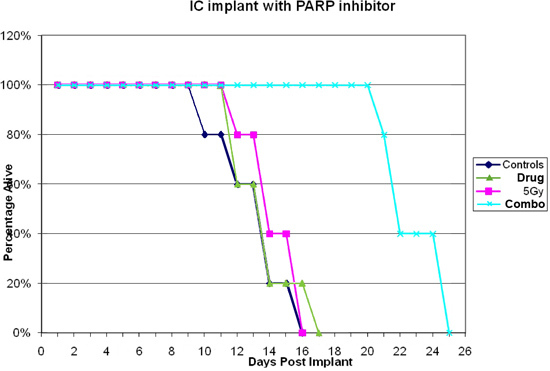 Figure 4. Results of U87 IC implants injected with PARP inhibitor 7 days post surgery.
Figure 4. Results of U87 IC implants injected with PARP inhibitor 7 days post surgery.
Discussion
Malignant gliomas, such as glioblastoma multiforme, represent the most common primary brain tumors and have a dismal prognosis4. Survival of patients affected by GBM has remained virtually unchanged during the last decade (i.e., 9-12 months post-diagnosis) despite advances in surgery, radiation, and chemotherapy5. The features of an appropriate model for the study of glioma treatment should include a reproducible tumor location and growth of the cells as a relatively discrete tumor; the cells should resemble as closely as possible the histological features of human gliomas; there should be a predictable growth rate of the tumor, the ability to grow the tumor in tissue culture and the model should be in a small animal to reduce expense6. Effective treatments depend on experimental models that closely resemble human GBM characteristics for testing new therapy and providing an accurate understanding of the molecular basis of widespread glioma invasion7. Investigations into the biology of brain tumors have also routinely used cell lines grown or maintained in vitro to gain information8. The inhibition of tumor invasion and angiogenesis is a major goal in the development of novel therapeutic strategies against malignant gliomas9. The limited treatments options for glioblastoma have driven an intensive search into the genetic lesions underlying this deadly cancer, with the aim developing more rational and more effective therapies10.
Disclosures
We have nothing to disclose.
Acknowledgments
This research is supported by funding from the Intramural program of the NIH.
References
- Bleau AM, Holland E. Trapping the mouse genome to hunt human alternations. Proc. Nat. Acad. Sci. U.S.A. 2007;104:7737–7738. doi: 10.1073/pnas.0702617104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrady BJ, McCormick D. A murine model of intracranial invasion: morphological observations on central nervous system invasion by melanoma cells. Clin. Exp. Metastasis. 1992;10:387–393. doi: 10.1007/BF00133467. [DOI] [PubMed] [Google Scholar]
- Fei X, Zhang Q, Dong J, Diao y, Wang Z, Li R, Wu Z, Wang A, Lan Q, Zhang S, Huang Q. Development of clinically relevant orthotopic xenograft mouse model of metastatic lung cancer and glioblastoma through surgical tumor tissues injection with trocar. Journal of Experimental & Clinical Cancer Research. 2010;29:84–84. doi: 10.1186/1756-9966-29-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Zhu X, Sasaki K, Ueda R, Low K, Pollack I, Okada H. Inhibition of STAT3 Promotes the Efficacy of Adoptive Transfer Therapy Using Type-1 CTLs by Modulation of the Immunological Microenvironment in a Murine Intracranial Glioma. J. Immunol. 2008;180:2089–2098. doi: 10.4049/jimmunol.180.4.2089. [DOI] [PubMed] [Google Scholar]
- Candolfi M, Curtin JF, Nichols WS, Muhammad AKM. G, King G, Pluhar GD, McNiel GE, Ohlfest EA, Freese JR, Moore PF. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathlogical characterization and tumor progression. J. Neurooncol. 2007;85:133–148. doi: 10.1007/s11060-007-9400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye A, Morstyn G, Gardner I, Pyke K. Development of a xenograft glioma model in mouse brain. Cancer Research. 1986;46:1367–1373. [PubMed] [Google Scholar]
- Zhao Y, Xiao A, diPierro C, Carpenter J, Abdel-Fattah R, Redpath G, Lopez M, Hussaini I. An Extensive Invasive Intracranial Human Glioblastoma Xenograft Model. American Journal of Pathology. 2010;176:3032–3049. doi: 10.2353/ajpath.2010.090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn C, Grossi P, Schmittling R, Xie W, Mitchell D, Karikari I, Wei Z, Dressman H, Sampson J. Genetic Analysis of Intracranial Tumors in a Murine Model of Glioma Demonstrate a Shift in Gene Expression in Response to Host Immunity. J. Neuroimmunol. 2007;182:63–72. doi: 10.1016/j.jneuroim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens T, Laabs Y, Günther H, Kemming D, Zhu Z, Witte L, Hagel C, Westphal M, Lamszus K. Inhibition of Glioblastoma Growth in a Highly Invasive Nude Mouse Model Can Be Achieved by Targeting Epidermal Growth Factor Receptor but not Vascular Endothelial Growth Factor Receptor-2. Clin. Cancer Res. 2008;14:5447–5458. doi: 10.1158/1078-0432.CCR-08-0147. [DOI] [PubMed] [Google Scholar]
- Purow B, Schiff D. Advances in the genetics of glioblastoma: are we reaching critical mass. Nat. Rev. Neurol. 2009;5:419–426. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]


