Abstract
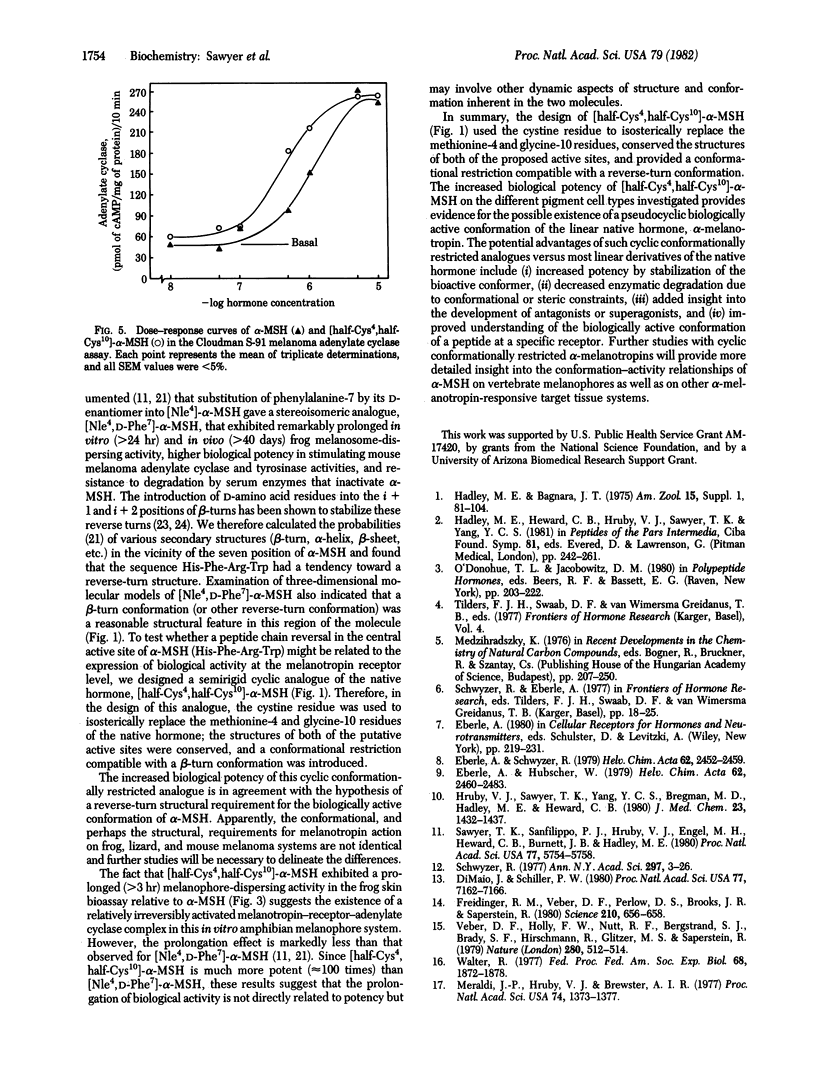
alpha-Melanocyte-stimulating hormone (alpha-melanotropin; alpha-MSH) is a linear tridecapeptide (Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2) that reversibly darkens amphibian skins by stimulating melanomsome (pigment granule) dispersion within melanophores. By using a number of in vitro melanocyte assays, we have examined the conformational requirements for alpha-MSH activity. Synthesis of [half-Cys4,half-Cys10]-alpha-MSH, a cyclic, conformationally restricted, "isosteric" analogue of alpha-MSH, provided a melanotropin with a potency greater than 10,000 times that of the native hormone in stimulating frog (Rana pipiens) skin darkening. The cyclic analogue also showed substantially prolonged activity relative to the native hormone. [half-Cys4,half-Cys10]-alpha-MSH was approximately 30 times more potent than alpha-MSH in stimulating lizard (Anolis carolinensis) skin melanophores in vitro. By using a cell-free Cloudman S-91 mouse melanoma plasma membrane preparation, we found the cyclic analogue to be approximately 3 times as potent as the native hormone in stimulating adenylate cyclase activity. These results provide insight into the conformational requirements for biological activity of alpha-MSH, and the comparative conformational requirements of alpha-MSH at a number of pigment cell receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bregman M. D., Sawyer T. K., Hadley M. E., Hruby V. J. Adenosine and divalent cation effects on S-91 melanoma adenylate cyclase. Arch Biochem Biophys. 1980 Apr 15;201(1):1–7. doi: 10.1016/0003-9861(80)90480-4. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran R., Lakshminarayanan A. V., Pandya U. V., Ramachandran G. N. Conformation of the LL and LD hairpin bends with internal hydrogen bonds in proteins and peptides. Biochim Biophys Acta. 1973 Mar 23;303(1):14–27. doi: 10.1016/0005-2795(73)90143-8. [DOI] [PubMed] [Google Scholar]
- Chipens G. I., Mutulis F. K., Katayev B. S., Klusha V. E., Misina I. P., Myshlyiakova N. V. Cyclic analogue of bradykinin possessing selective and prolonged biological activity. Int J Pept Protein Res. 1981 Sep;18(3):302–311. doi: 10.1111/j.1399-3011.1981.tb02985.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- DiMaio J., Schiller P. W. A cyclic enkephalin analog with high in vitro opiate activity. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7162–7166. doi: 10.1073/pnas.77.12.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidinger R. M., Veber D. F., Perlow D. S., Brooks J. R., Saperstein R. Bioactive conformation of luteinizing hormone-releasing hormone: evidence from a conformationally constrained analog. Science. 1980 Nov 7;210(4470):656–658. doi: 10.1126/science.7001627. [DOI] [PubMed] [Google Scholar]
- Hadley M. E., Anderson B., Heward C. B., Sawyer T. K., Hruby V. J. Calcium-dependent prolonged effects on melanophores of [4-norleucine, 7-D-phenylalanine]-alpha-melanotropin. Science. 1981 Aug 28;213(4511):1025–1027. doi: 10.1126/science.6973820. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., Sawyer T. K., Yang Y. C., Bregman M. D., Hadley M. E., Heward C. B. Synthesis and structure-function studies of melanocyte stimulating hormone analogues modified in the 2 and 4(7) positions: comparison of activities on frog skin melanophores and melanoma adenylate cyclase. J Med Chem. 1980 Dec;23(12):1432–1437. doi: 10.1021/jm00186a026. [DOI] [PubMed] [Google Scholar]
- Meraldi J. P., Hruby V. J., Brewster A. I. Relative conformational rigidity in oxytocin and (1-penicillamine)-oxytocin: a proposal for the relationship of conformational flexibility to peptide hormone agonism and antagonism. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1373–1377. doi: 10.1073/pnas.74.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K., Sanfilippo P. J., Hruby V. J., Engel M. H., Heward C. B., Burnett J. B., Hadley M. E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyzer R. ACTH: a short introductory review. Ann N Y Acad Sci. 1977 Oct 28;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Holly F. W., Nutt R. F., Bergstrand S. J., Brady S. F., Hirschmann R., Glitzer M. S., Saperstein R. Highly active cyclic and bicyclic somatostatin analogues of reduced ring size. Nature. 1979 Aug 9;280(5722):512–514. doi: 10.1038/280512a0. [DOI] [PubMed] [Google Scholar]
- Venkatachalam C. M. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of three linked peptide units. Biopolymers. 1968 Oct;6(10):1425–1436. doi: 10.1002/bip.1968.360061006. [DOI] [PubMed] [Google Scholar]
- Walter P. Indentification of sites in oxytocin involved in uterine receptor recognition and activation. Fed Proc. 1977 May;36(6):1872–1878. [PubMed] [Google Scholar]
- Yang Y. C., Hruby V. J., Heward C. B., Hadley M. E. Synthesis of alpha- and beta-melanocyte stimulating hormones. Int J Pept Protein Res. 1980 Feb;15(2):130–138. [PubMed] [Google Scholar]


