Abstract
Porcine reproductive and respiratory syndrome (PRRS) is characterized by a delayed and defective adaptive immune response. The viral nonstructural protein 1 (NSP1) of the PRRS virus (PRRSV) is able to suppress the type I interferon (IFN) response in vitro. In this study, recombinant adenoviruses (rAds) expressing NSP1 (rAd-NSP1), glycoprotein 5 (GP5) (rAd-GP5), and the NSP1-GP5 fusion protein (rAd-NSP1-GP5) were constructed, and the effect of NSP1 on immune responses was investigated in pigs. Pigs inoculated with rAd-NSP1 or rAd-NSP1-GP5 had significantly lower levels of IFN-γ and higher levels of the immunosuppressive cytokine IL-10 than pigs inoculated with rAd-GP5, wild-type adenovirus, or cell culture medium alone. The antibody response to vaccination against classic swine fever virus (CSFV) was significantly decreased by inoculation of NSP1 7 d after CSFV vaccination in pigs. Thus, NSP1-mediated immune suppression may play an important role in PRRSV pathogenesis.
Résumé
Le syndrome reproducteur et respiratoire porcin (PRRS) est caractérisé par un délai et un déficit de la réponse immunitaire adaptative. La protéine virale non-structurale 1 (NSP1) du virus PRRS (PRRSV) est capable de supprimer in vitro la réponse de l’interféron (IFN) de type 1. Dans la présente étude, des adénovirus recombinants (rAds) exprimant NSP1 (rAd-NSP1), la glycoprotéine 5 (GP5) (rAd-GP5), et la protéine de fusion NSP1-GP5 (rAd-NSP1-GP5) ont été construits, et l’effet de NSP1 sur les réponses immunitaires étudié chez des porcs. Les porcs inoculés avec rAd-NSP1 ou rAd-NSP1-GP5 avaient des niveaux significativement plus faibles d’IFN-γ et des niveaux plus élevés de la cytokine immunosuppressive IL-10 que les porcs inoculés avec rAd-GP5, l’adénovirus de type sauvage, ou du milieu de culture cellulaire uniquement. La réponse en anticorps à la vaccination contre le virus de la peste porcine classique (CSFV) était réduite de manière significative par l’inoculation de NSP1 sept jours après la vaccination des porcs contre CSFV. Ainsi, la suppression immunitaire causée par NSP1 pourrait jouer un rôle important dans la pathogénie du PRRSV.
(Traduit par Docteur Serge Messier)
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is a small, enveloped, single-stranded, positive-sense RNA virus (1,2) in the genus Arterivirus of the family Arteriviridae (3). It causes economically important disease in pigs that is characterized by a delayed and defective adaptive immune response (4,5). A highly pathogenic PRRSV, which first emerged in China, has caused heavy economic losses in many pig-producing regions (6,7).
The PRRSV genome is approximately 15 kb long and contains 9 open reading frames (ORFs) flanked by untranslated regions at the 5′ and 3′ termini (8–10); ORF1a and ORF1b, situated at the 5′ end, constitute nearly 80% of the viral genome and encode viral nonstructural proteins (NSPs) involved in viral polyprotein processing and replication (11–13). The complete processing of the polyproteins is predicted to yield 12 NSP polypeptides, NSP1 to NSP12 (14–17). Among the polypeptides, NSP1 is critical for subgenomic mRNA synthesis (3). It contains papain-like proteinase α (PCPα), which directs the release of NSP1α (20 kDa), along with PCPβ, which directs the release of NSP1β (27 kDa), depending on the activities of PCPα, and a zinc-finger motif required for subgenomic mRNA transcription (18).
Because type I interferon γ (IFN-γ) is a signature cytokine of the T helper cell Th1-associated response, it is a useful indicator of cell-mediated immunity (CMI) (19). The immunosuppressive cytokine IL-10 can suppress IFN-γ production in peripheral blood mononuclear cells (PBMCs) in pigs (20). The production of IL-10 has been reported to increase after PRRSV infection, the increase correlating with reduced IFN-γ production in virus-infected cells (21). In addition, PRRSV infection can suppress the antibody response to vaccination against classic swine fever virus (CSFV), the most common means of preventing and controlling this important disease of domestic pigs in epidemic areas (22,23), and result in vaccination failure when the pigs are subsequently exposed to CSFV (24,25).
Since NSP1 is expressed early in the virus life cycle, it is available to the macrophage proteosome machinery from the earliest time of infection for degradation and presentation to the immune system in the context of major histocompatibility classes I and II (26,27). This polypeptide is critical to the virus’s life cycle and likely to be toxic to cells owing to its protease activities. It can be processed as NSP1α and NSP1β, and NSP1β is the main protein antagonizing cellular production of type I IFN (28,29). The aim of this study was to determine if PRRSV NSP1 expressed in an adenovirus is able to suppress humoral and CMI responses in pigs.
Materials and methods
Cell cultures and viruses
Recombinant and wild-type adenoviruses (rAd and wtAd) were grown in human embryo kidney (HEK-293A) cells. Highly pathogenic PRRSV strain SY0608 was grown in MARC-145 cells. This strain, belonging to type 2, was first isolated in mideastern China. It caused illness and death in 100% and 25% to 50%, respectively, of pigs 30, 65, and 105 d old, as well as the birth of stillborn and weak piglets. The NSP2 contained 2 discontinuous deletions, 1 and 29 amino acids long, corresponding to strain VR-2332, positions 480 and 531 to 559, respectively (6). Dulbecco’s modified Eagle’s essential medium with 10% heat-inactivated fetal calf serum (FCS) was added to the cell cultures, which were then incubated at 37°C in 5% CO2. Cell lines were inoculated 24 h after seeding.
Amplification and cloning of the PRRSV NSP1 and glycoprotein 5 (GP5) genes
Viral RNA was extracted with the use of TRIzol (Invitrogen, Carlsbad, California, USA). Reverse transcription (RT) was performed at 50°C for 60 min, and 20 μL of the RT mixture was added, for a final concentration of 2 μg of total RNA, along with 200 U of SuperScript III RT (Gibco BRL, Burlington, Ontario), 2.5 μM of oligo(dT)12-18, 5 mM of dithiothreitol, 1× RT buffer, and 0.5 mM of each deoxynucleotide triphosphate (dNTP).
Sequences of primer pairs for amplification of the PRRSV SY0608 NSP1 and GP5 genes were as follows: NSP1, forward 5′-GAGAGATCTATGTCTGGGATACTTG-3′ (BglII site underlined) and reverse 5′-TATAAGCTTACCGTACCACTTATGACTG-3′ (HindIII site underlined); GP5-1, forward 5′-GAAAGATCTATGTT GGGGAAGTGCT-3′ (BglII site underlined) and reverse 5′-GAGAAGCTTGAGACGACCCCATTG-3′ (HindIII site underlined); and GP5, forward 5′-GAAAAGCTTATGTTGGGGAAGTGC-3′ (HindIII site underlined) and reverse 5′-GAGGATATCGAGA CGACCCCATTG-3′ (EcoRV site underlined) (GenBank EU144079).
Amplification was performed in a 50-μL reaction mixture containing 1.5 mM of MgCl2, 1× polymerase chain reaction (PCR) buffer, 0.2 mM of each dNTP, 20 pM of each primer, 1.5 U of Taq DNA polymerase (Promega, Madison, Wisconsin, USA), and 2 μL of complementary DNA. A PTC-150 thermocycler (MJ Research, South San Francisco, California, USA) was used with the following program: denaturation at 94°C for 5 min, 30 cycles composed of denaturation at 94°C for 40 s, annealing at 60°C for 40 s, and extension at 72°C for 1 min, and a final extension at 72°C for 10 min.
The PCR products were cloned into the vector pShuttle-CMV, which produced 3 recombinant plasmids, pShuttle-CMV-NSP1, pShuttle-CMV-GP5, and pShuttle-CMV-NSP1-GP5. The recombinant plasmids were sequenced to confirm tandem in-frame insertion of the NSP1 and GP5 genes.
Construction of rAd expressing NSP1 and/or GP5
Recombinant adenoviruses were generated as described previously (30,31), with some modifications. The recombinant shuttle vector pShuttle-CMV-NSP1 was linearized with PmeI and cotransformed with the adenovirus backbone plasmid pAdEasy-1 (Stratagene, La Jolla, California, USA) into Escherichia coli BJ5183 by electroporation. The rAd vector was generated by homologous recombination, and the positive clones were identified by plasmid extraction and enzyme digestion with PacI. The resulting adenoviral plasmid was linearized with PacI and transfected into HEK-293A cells in 24-well plates with the use of TransFast Transfection Reagent (Promega). The yield of adenovirus was observed under the microscope, and the 50% tissue culture infectious dose (TCID50) was determined in HEK-293A cells by the Reed–Muench method.
Identification of NSP1 and GP5 expressed in the constructs
Indirect immunofluorescence assay (IFA)
Expression of NSP1 and GP5 in HEK-293A cells infected with rAd was determined as described previously (31). The monoclonal antibody against PRRSV NSP1 was produced in our laboratory and had high specificity for PRRSV, as determined by IFA (1:200 dilution). The polyclonal antibody against PRRSV GP5 was produced in our laboratory by vaccination of mice with purified truncated GP5 expressed by pGEX-6P-1 in E. coli BL21 [1:200 diluted in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (PBS-T)].
The HEK-293A cells, seeded in 96-well plates, were fixed in ethanol. After 3 washes in PBS (pH 7.4) the fixed cells were incubated with antibodies against NSP1 and GP5 for 1 h at 37°C. Unbound antibodies were washed 3 times with PBS-T. Fluorescein-conjugated goat antibody against mouse antigen (Boston Bio-Tech, Boston, Massachusetts, USA) was added and the mixture incubated for 1 h at 37°C. After 3 washes with PBS, positive signals were sought by fluorescence microscopy with a Zeiss Axiovert 200 (Carl Zeiss Microscopy, Göttingen, Germany).
Western blot analysis
Western blot analysis was performed as described previously, with some modifications (32). Lysates of HEK-293A cells infected with rAd or wtAd were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Pall Life Sciences, Ann Arbor, Michigan, USA). The membranes were incubated with PRRSV-specific antiserum (porcine anti-PRRSV, produced in our laboratory; 1:100 diluted in PBS-T) at 37°C for 2 h and then at 37°C for 1 h with horseradish peroxidase-conjugated staphylococcal protein A (Boshide, Wuhan, China). Positive signals were detected with the use of luminal-based chemiluminescence reagents (SuperSignal West Pico Trial Kit; Thermo Fisher Scientific Pierce Protein Research Products, Rockford, Illinois, USA).
Effect of PRRSV NSP1 on CMI and humoral immunity
All experimental protocols were approved by the Institutional Animal Care and Ethics Committee of Nanjing Agricultural University (permit IACECNAU20090905) and met the International Guiding Principles for Biomedical Research Involving Animals (33).
Cell-mediated immunity
Twenty-five 3-week-old piglets free of PRRSV, Porcine circovirus-2, and adenovirus infections (serum antibody tests and PCR for these organisms all gave negative results) were divided into 5 equal groups and housed in separate rooms. Three groups were inoculated with 107 TCID50 of rAd-NSP1, rAd-GP5, or rAd-NSP1-GP5 and given a booster 14 d later. The other 2 groups were inoculated with either wtAd or the cell culture medium (mock control) with the same protocol.
On days 0 (day of inoculation), 7, 14, 21, and 28, PBMCs were isolated from 5 mL of blood collected from the pigs into heparin-containing tubes. Viable cells were counted by the trypan blue exclusion method. The cells were resuspended in RPMI 1640 (Gibco BRL) supplemented with 10% FCS, 2 mM of L-glutamine, 50 mM of 2-mercaptoethanol, 100 U/mL of penicillin, and 100 mg/mL of streptomycin solution (Gibco BRL) and seeded in triplicate in 96-well flat-bottom plates, 100 mL per well. After stimulation with concanavalin A (5 μg/mL) for 24 h, supernatant was collected and the concentrations of IFN-γ and IL-10 were determined quantitatively with the use of commercially available porcine cytokine enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen).
Humoral immune response
Thirty 3-week-old piglets free of PRRSV, adenovirus, and CSFV infections were divided into 6 equal groups and housed in separate rooms. Four groups were inoculated intramuscularly with 2 mL of a modified live lapinized Chinese-strain CSFV vaccine and then with 107 TCID50 of rAd-NSP1, rAd- GP5, rAd-NSP1-GP5, or wtAd 7 d later. The other 2 groups were inoculated with either CSFV vaccine only or the cell culture medium only (mock control) with the same inoculation protocol. At 14, 21, and 28 d after the 1st inoculation, blood samples were collected and the levels of antibody to CSFV determined in the serum with a commercially available blocking ELISA kit (HerdChek CSFV Ab; IDEXX Laboratories, Westbrook, Maine, USA). Samples were considered to be positive at a calculated blocking percentage ≥ 40% and negative at a blocking percentage ≤ 30%.
Statistical analysis
The differences in the level of humoral responses and cytokine production between the groups were determined by 1-way repeated-measures analysis of variance and Duncan’s test as a post-test with SPSS software, version 17.0 (SPSS, Chicago, Illinois, USA). Differences were considered to be significant at P < 0.05.
Results
The titers of rAd-NSP1 (expressing NSP1), rAd-GP5 (expressing GP5), and rAd-NSP1-GP5 (expressing NSP1-GP5) were 109.0, 108.0, and 109.0 TCID50/mL, respectively. Expression of NSP1 was confirmed by IFA (Figure 1) and Western blot analysis (Figure 2). The PRRSV NSP1 was cleaved into α and β subunits of approximately 20 and 27 kDa, respectively.
Figure 1.
Results of indirect immunofluorescence assay of monolayers of human embryo kidney (HEK-293A) cells infected with recombinant adenoviruses (rAds) expressing nonstructural protein 1 (NSP1) of the Porcine reproductive and respiratory syndrome virus (PRRSV) (rAd-NSP1), PRRSV glycoprotein 5 (GP5) (rAd-GP5), and the NSP1-GP5 fusion protein (rAd-NSP1-GP5). The assays were done with NSP1-specific monoclonal antibody (left panels) and mouse anti-GP5 serum (right panels). Noninfected HEK-293A cells and cells infected with wild-type adenovirus (wtAd) were used as controls.
Figure 2.
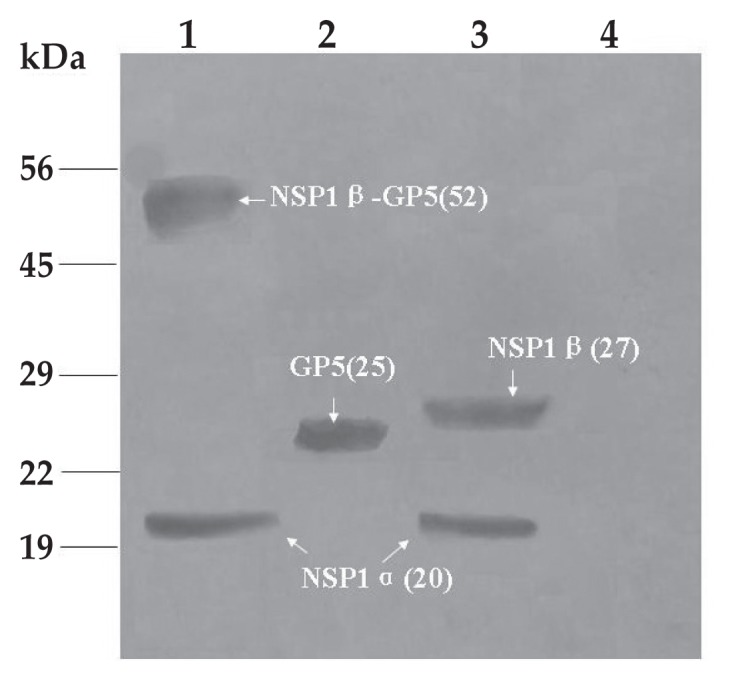
Western blot analysis of cell lysates infected with the same rAds and incubated with PRRSV-specific porcine antiserum. Lane 1 — cell lysates of rAd-NSP1-GP5; lane 2 — cell lysates of rAd-GP5; lane 3 — cell lysates of rAd-NSP1; lane 4 — cell lysates of wtAd. Arrows indicate the expected molecular sizes of NSP1β-GP5, GP5, NSP1β, and NSP1α. Proteins standards are shown on the left.
At 21 and 28 d after inoculation the mean concentration of IFN-γ produced by PBMCs was significantly lower and the mean concentration of IL-10 significantly higher in the pigs inoculated with rAd- NSP1 and rAd-NSP1-GP5 than in those inoculated with rAd-GP5, wtAd, or cell culture medium (P < 0.05) (Figure 3).
Figure 3.
Concentrations of interferon (IFN)-γ and interleukin (IL)-10 in the supernatants of peripheral blood mononuclear cells (PBMCs) isolated from pigs at various times after inoculation with the rAds as well as cell culture medium (“Mock”) and wtAd as controls. After stimulation with concanavalin A in triplicate, enzyme-linked immunosorbent assays (ELISAs) were done with the use of commercial kits. Data are shown as means ± standard errors froms 3 independent experiments. Arrows indicate time of initial and booster inoculations. Different letters indicate a significant difference at P < 0.05.
Anti-CSFV antibodies were detected in serum from the pigs inoculated with CSFV vaccine alone or the cell culture medium alone, as well as in those that were inoculated with rAd-NSP1, rAd-GP5, or rAd-NSP1-GP5 7 d after CSFV vaccination. The antibody levels increased rapidly in the CSFV-vaccinated groups in the 2 wk after vaccination (Figure 4). The levels were significantly lower (P < 0.05) in the CSFV + rAd-NSP1 and CSFV + rAd-NSP1-GP5 groups than in the CSFV alone and CSFV + rAd-GP5 groups during the period 2 to 3 wk after vaccination.
Figure 4.
Levels of serum antibodies, measured with a commercial ELISA kit, against classic swine fever virus (CSFV) after CSFV vaccination and after inoculation 7 d later with the rAds or with wtAd. Mock vaccination with cell culture medium was used as a control procedure. Data are shown as means ± standard errors from 3 independent experiments. The dashed line indicates the cut-off blocking percentage (40%). Different letters indicate a significant difference at P < 0.05.
Discussion
Previous studies have shown that PRRSV infection elicits a poor innate response of antiviral type I IFN, which was postulated to result in a weak adaptive immune response, as demonstrated by a short duration of CMI responses (34,35) and slow development of a virus-specific IFN-γ response (36). In vitro, PRRSV infection of porcine alveolar macrophages (PAMs), monocyte-derived macrophages, and MARC-145 cells inhibited IFN-α and IFN-β production (5,18,21). In transfection experiments using recombinant plasmids expressing the 10 individual PRRSV NSPs, Beura et al (29) showed that 4 PRRSV NSPs (NSP1, NSP2, NSP4, and NSP11) contribute to IFN antagonism. However, there is little information on the immune response to PRRSV NSPs in vivo. In this study, rAds expressing NSP1, the amino terminal protein in a polyprotein encoded by PRRSV, that were derived from highly pathogenic PRRSV were constructed and immune responses determined in pigs. The animals inoculated with rAd-NSP1 and rAd-NSP1-GP5 had significantly lower levels of IFN-γ than those inoculated with rAd-GP5, wtAd, or cell culture medium alone. Pigs inoculated with rAd-NSP1 and rAd-NSP1-GP5 had greater secretion of IL-10 than those inoculated with rAd-GP5, wtAd, or medium alone.
The self-cleavage products of NSP1 during virus infection, NSP1α and NSP1β, could moderate inhibitory effects on IFN-β promoter activation. When expressed stably in cell lines, they strongly inhibited double-stranded RNA signalling pathways (28). Moreover, NSP1β is the main protein antagonizing cellular production of type I interferon, and it inhibits both IFN regulatory factor 3 and NF-κB-dependent gene induction by double-stranded RNA and Sendai virus (29). Beura et al (29) proposed that NSP1β modulates the host innate immune response by antagonizing IRF3 activation. In this study we found that NSP1, which plays a key role in CMI responses (37), could significantly inhibit the secretion of IFN-γ in pigs inoculated with rAd-NSP1 or rAd-NSP1-GP5 compared with pigs inoculated with rAd-GP5 or wtAd. To clarify the role of NSP1α and NSP1β in CMI responses in pigs in vivo, these proteins should be expressed individually with the adenovirus and their effects determined. In addition, to confirm the innate immunity of NSP1 found in vitro (29), other innate cytokines, such as IFN-1β and IL1, should be assayed in pigs.
The immunomodulatory cytokine IL-10 inhibits the synthesis and release of other cytokines (38). Increased production of IL-10 induced by PRRSV infection might be one of the strategies used by the virus to modulate the host’s immune responses, thereby contributing to the unique clinical picture observed after PRRSV infection (39). This cytokine contributed to significantly reduced IFN-γ and TNF-α expression by T lymphocytes (40,41). In our study, NSP1 significantly enhanced secretion of IL-10 in pigs inoculated with rAd-NSP1 and rAd-NSP1-GP5 compared with those inoculated with rAd-GP5 and wtAd. Therefore, the high expression of IL-10 observed in the present study may be responsible for the reduced expression of IFN-γ, which in turn may prolong viral replication in pigs.
The antibody response to CSFV plays an important role in protective immunity (22). To understand the effect of NSP1 on the humoral immune responses, 3-wk-old piglets free of PRRSV, CSFV, and adenovirus infections were inoculated with rAds 7 d after CSFV vaccination and the CSFV-specific antibodies assayed. The levels of antibodies to CSFV were negatively affected by inoculation with rAd-NSP1 and rAd-NSP1-GP5. This finding may be relevant to vaccination programs for the prevention and control of CSF and PRRS.
Previously it was shown that GP5 is the most important glycosylation protein of PRRSV involved in the generation of humoral and CMI responses (42) and that fusion expression of PRRSV GP3 and GP5 delivered by a human adenovirus vector enhances immune responses in pigs (43). Usually neutralizing antibodies play an important role in immune protection. Although GP5 proteins have neutralizing epitopes, only weak and delayed neutralizing antibodies could be induced by constructs expressing GP5 alone (44,45). In this study, GP5 was used as a positive control to examine the effect of NSP1 on adaptive immunity. Neutralizing antibodies to PRRSV were not detected during the period of observation (data not shown).
Conclusion
The PRRSV NSP1 suppressed IFN-γ secretion and increased IL-10 secretion in pigs. It also inhibited the antibody response to CSFV when inoculated 7 d after CSFV vaccination. Thus, NSP1-mediated immune suppression may play an important role in the pathogenesis of PRRS.
Acknowledgments
This work was supported by grants from the National Key Genomic Engineering Programme (2009ZX08009-143B), the National Natural Science Foundation (30871868), the National Key Technology R&D Programme (2007BAD86B02-3) and priority academic program development of Jiangsu higher education institutions (PAPD).
References
- 1.Bennfield DA, Nelson E, Collins JE, et al. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332) J Vet Diagn Invest. 1992;4:127–133. doi: 10.1177/104063879200400202. [DOI] [PubMed] [Google Scholar]
- 2.Wensvoort G, Terpstra C, Pol JMA, et al. Mystery swine disease in the Netherlands: The isolation of Lelystad virus. Vet Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- 3.Gorbalenya AE, Enjuanes L, Ziebuhr J, Snijder EJ. Evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller LC, Laegreid WW, Bono JL, Chitko-McKown CG, Fox JM. Interferon type I response in porcine reproductive and respiratory syndrome virus-infected MARC-145 cells. Arch Virol. 2004;149:2453–2463. doi: 10.1007/s00705-004-0377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LC, Lager KM, Kehrli ME. Role of Toll-like receptors in activation of porcine alveolar macrophages by porcine reproductive and respiratory syndrome virus. Clin Vaccine Immunol. 2009;16:360–365. doi: 10.1128/CVI.00269-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Wang X, Bo K, et al. Emergence of a highly pathogenic porcine reproductive and respiratory syndrome virus in the mid-eastern region of China. Vet J. 2007;174:577–584. doi: 10.1016/j.tvjl.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Tian K, Yu X, Zhao T, et al. Emergence of fatal PRRSV variants: Unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE. 2007;2:1–10. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conzelmann KK, Visser N, Van Woensel P, Thiel HJ. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruce CB, Akrigg A, Sharpe SA, Hanke T, Wilkinson GWG, Cranage MP. Replication-deficient recombinant adenoviruses expressing the human immunodeficiency virus Env antigen can induce both humoral and CTL immune responses in mice. J Gen Virol. 1999;80:2621–2628. doi: 10.1099/0022-1317-80-10-2621. [DOI] [PubMed] [Google Scholar]
- 10.Wu HW, Fang Y, Rowland RR, et al. The 2b protein as a minor structural component of PRRSV. Virus Res. 2005;114:177–181. doi: 10.1016/j.virusres.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bautista EM, Faaberg KS, Mickelson D, McGruder ED. Functional properties of the predicted helicase of porcine reproductive and respiratory syndrome virus. Virology. 2002;298:258–270. doi: 10.1006/viro.2002.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meulenberg JJ, Hulst MM, de Meijer EJ, et al. Lelystad virus, the causative agent of porcine epidemic abortion and respiratory syndrome (PEARS), is related to LDV and EAV. Virology. 1993;192:62–72. doi: 10.1006/viro.1993.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snijder EJ, Meulenberg JJM. The molecular biology of arteriviruses. J Gen Virol. 1998;79:961–979. doi: 10.1099/0022-1317-79-5-961. [DOI] [PubMed] [Google Scholar]
- 14.Nelsen CJ, Murtaugh MP, Faaberg KS. Porcine reproductive and respiratory syndrome virus comparison: Divergent evolution on two continents. J Virol. 1999;73:270–280. doi: 10.1128/jvi.73.1.270-280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Aken D, Zevenhoven-Dobbe J, Gorbalenya AE, Snijder EJ. Proteolytic maturation of replicase polyprotein pp1a by the nsp4 main proteinase is essential for equine arteritis virus replication and includes internal cleavage of nsp7. J Gen Virol. 2006;87:3473–3482. doi: 10.1099/vir.0.82269-0. [DOI] [PubMed] [Google Scholar]
- 16.Wootton S, Yoo D, Rogan D. Full-length sequence of a Canadian porcine reproductive and respiratory syndrome virus (PRRSV) isolate. Arch Virol. 2000;145:2297–2323. doi: 10.1007/s007050070022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziebuhr J, Snijder EJ, Gorbalenya AE. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J Gen Virol. 2000;81:853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Lawson S, Sun Z, et al. Identification of two auto-cleavage products of nonstructural protein 1 (NSP1) in porcine reproductive and respiratory syndrome virus infected cells: NSP1 function as interferon antagonist. Virology. 2010;398:87–97. doi: 10.1016/j.virol.2009.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overend C, Mitchell R, He D, Rompato G, Grubman MJ, Garmendia AE. Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with Porcine reproductive and respiratory syndrome virus. J Gen Virol. 2007;88:925–931. doi: 10.1099/vir.0.82585-0. [DOI] [PubMed] [Google Scholar]
- 20.Turner J, Gonzalez-Juarrero M, Ellis DL, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 21.Charerntantanakul W, Platt R, Roth JA. Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol. 2006;19:646–661. doi: 10.1089/vim.2006.19.646. [DOI] [PubMed] [Google Scholar]
- 22.Díaz I, Darwich L, Pappaterra G, Pujols J, Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259. doi: 10.1016/j.virol.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Moennig V. Introduction to classical swine fever virus, disease and control policy. Vet Microbiol. 2000;73:93–102. doi: 10.1016/s0378-1135(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 24.Van Oirschot JT. Vaccinology of classical swine fever: From lab to field. Vet Microbiol. 2003;96:367–384. doi: 10.1016/j.vetmic.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Yang H. Infection of porcine reproductive and respiratory syndrome virus suppresses the antibody response to classical swine fever virus vaccination. Vet Microbiol. 2003;95:295–301. doi: 10.1016/s0378-1135(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 26.Brown E, Lawson S, Welbon C, et al. Antibody response to porcine reproductive and respiratory syndrome virus (PRRSV) nonstructural proteins and implications for diagnostic detection and differentiation of PRRSV types I and II. Clin Vaccine Immunol. 2009;16:628–635. doi: 10.1128/CVI.00483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson CR, Yu W, Murtaugh MP. Cross-reactive antibody responses to NSP1 and NSP2 of porcine reproductive and respiratory syndrome virus. J Gen Virol. 2007;88:1184–1195. doi: 10.1099/vir.0.82587-0. [DOI] [PubMed] [Google Scholar]
- 28.Fang Y, Snijder EJ. The PRRSV replicase: Exploring the multifunctionality of an intriguing set of nonstructural proteins. Virus Res. 2010;154:61–76. doi: 10.1016/j.virusres.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beura LK, Sarkar SN, Kwon B, et al. Porcine reproductive and respiratory syndrome virus nonstructural protein 1β modulates host innate immune response by antagonizing IRF3 activation. J Virol. 2010;84:1574–1584. doi: 10.1128/JVI.01326-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang W, Jiang P, Li Y, Tang J, Wang X, Ma S. Recombinant adenovirus expressing GP5 and M fusion proteins of porcine reproductive and respiratory syndrome virus induce both humoral and cell-mediated immune responses in mice. Vet Immunol Immunopathol. 2006;113:169–180. doi: 10.1016/j.vetimm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Qiu HJ, Tian ZJ, Tong GZ, Zhou YJ, Ni JQ, Luo YZ. Protective immunity induced by a recombinant pseudorabies virus expressing the GP5 of porcine reproductive and respiratory syndrome virus in piglets. Vet Immunol Immunopathol. 2005;106:309–319. doi: 10.1016/j.vetimm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Council for International Organizations of Medical Sciences. International Guiding Principles for Biomedical Research Involving Animals. 1985. [Last accessed July 16, 2012]. Available from http://cioms.ch/publications/guidelines/1985_texts_of_guidelines.htm.
- 34.Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao Z, Batista L, Dee S, Halbur P, Murtaugh MP. The level of virus-specific T-cell and macrophage recruitment in porcine reproductive and respiratory syndrome virus infection in pigs is independent of virus load. J Virol. 2004;78:5923–5933. doi: 10.1128/JVI.78.11.5923-5933.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meier WA, Galeota J, Osorio FA, Husmann RJ, Schnitzlein WM, Zuckermann FA. Gradual development of the interferon-γ response of swine to porcine reproductive and respiratory syndrome virus infection or vaccination. Virology. 2003;309:18–31. doi: 10.1016/s0042-6822(03)00009-6. [DOI] [PubMed] [Google Scholar]
- 37.Kroese MV, Zevenhoven-Dobbe JC, Bos-de Ruijter JN, et al. The NSP1 α and nsp1 papain-like autoproteinases are essential for porcine reproductive and respiratory syndrome virus RNA synthesis. J Gen Virol. 2008;89:494–499. doi: 10.1099/vir.0.83253-0. [DOI] [PubMed] [Google Scholar]
- 38.Batista L, Pijoan C, Dee S, et al. Virological and immunological responses to porcine reproductive and respiratory syndrome virus in a large population of gilts. Can J Vet Res. 2004;68:267–273. [PMC free article] [PubMed] [Google Scholar]
- 39.Costers S, Lefebvre DJ, Goddeeris B, Delputte PL, Nauwynck HJ. Functional impairment of PRRSV-specific peripheral CD3+CD8high cells. Vet Res. 2009;40:46. doi: 10.1051/vetres/2009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suradhat S, Kesdangsakonwut S, Sada W, Buranapraditkun S, Wongsawang S, Thanawongnuwech R. Negative impact of porcine reproductive and respiratory syndrome virus infection on the efficacy of classical swine fever vaccine. Vaccine. 2006;24:2634–2642. doi: 10.1016/j.vaccine.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Charerntantanakul W, Platt R, Johnson W, Roof M, Vaughn E, Roth JA. Immune responses and protection by vaccine and various vaccine adjuvant candidates to virulent porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2006;109:99–115. doi: 10.1016/j.vetimm.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Bastos RG, Dellagostin OA, Barletta RG, et al. Immune response of pigs inoculated with Mycobacterium bovis BCG expressing a truncated form of GP5 and M protein of porcine reproductive and respiratory syndrome virus. Vaccine. 2004;22:467–474. doi: 10.1016/s0264-410x(03)00572-3. [DOI] [PubMed] [Google Scholar]
- 43.Cao J, Wang XL, Du YJ, Li YF, Wang XW, Jiang P. CD40 ligand expressed in adenovirus can improve the immunogenicity of the GP3 and GP5 of porcine reproductive and respiratory syndrome virus in swine. Vaccine. 2010;28:7514–7522. doi: 10.1016/j.vaccine.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Pirzadeh B, Dea S. Immune response in pigs vaccinated with plasmid DNA encoding ORF5 of porcine reproductive and respiratory syndrome virus. J Gen Virol. 1998;79:989–999. doi: 10.1099/0022-1317-79-5-989. [DOI] [PubMed] [Google Scholar]
- 45.Jiang WM, Jiang P, Wang XL, Li YF, Wang XW, Du YJ. Influence of porcine reproductive and respiratory syndrome virus GP5 glycoprotein N-linked glycans on immune responses in mice. Virus Genes. 2007;35:663–671. doi: 10.1007/s11262-007-0131-y. [DOI] [PubMed] [Google Scholar]