Abstract
The effects of selenium (Se) supplementation and source on equine immune function have not been extensively studied. This study examined the effects of oral Se supplementation and Se source on aspects of innate and adaptive immunity in horses. Fifteen horses were assigned to 1 of 3 groups (5 horses/group): control, inorganic Se (sodium selenite), organic Se (Se yeast). Immune function tests performed included: lymphocyte proliferation in response to mitogen concanavalin A, neutrophil phagocytosis, antibody production after rabies vaccination, relative cytokine gene expression in stimulated lymphocytes [interferon gamma (IFNγ), interleukin (IL)-2, IL-5, IL-10, tumor necrosis factor alpha (TNFα)], and neutrophils (IL-1, IL-6, IL-8, IL-12, TNFα). Plasma, red blood cell Se, and blood glutathione peroxidase activity were measured. Plasma and red blood cell Se were highest in horses in the organic Se group, compared with that of inorganic Se or control groups. Organic Se supplementation increased the relative lymphocyte expression of IL-5, compared with inorganic Se or no Se. Selenium supplementation increased relative neutrophil expression of IL-1 and IL-8. Other measures of immune function were unaffected. Dietary Se content and source appear to influence immune function in horses, including alterations in lymphocyte expression of IL-5, and neutrophil expression of IL-1 and IL-8.
Résumé
Les effets d’un supplément de sélénium (Se) ainsi que sa source sur la fonction immunitaire équine n’ont pas été étudiés à fond. On examina dans la présente étude les effets d’un supplément oral en Se et les sources de Se sur des éléments de l’immunité innée et adaptative de chevaux. Quinze chevaux ont été assignés à un de trois groupes (5 chevaux/groupe); témoin, Se inorganique (sélénite de sodium), et Se organique (Se provenant de levures). Les tests de fonctions immunitaires effectués étaient : prolifération lymphocytaire en réponse au mitogène concanaviline A, phagocytose par les neutrophiles, production d’anticorps après vaccination anti-rabique, expression relative des gènes des cytokines de lymphocytes stimulés [interferon gamma (IFNγ), interleukine (IL)-2, IL-5, IL-10, facteur de nécrose tumorale alpha (TNFα)], et de neutrophiles (IL-1, IL-6, IL-8, IL-12, TNFα). Le Se plasmatique et des globules rouges, ainsi que l’activité de la glutathion peroxydase ont été mesurés. Le Se plasmatique et des globules rouges étaient plus élevés chez les chevaux dans le groupe de Se organique, comparativement au groupe recevant le Se inorganique ou le groupe témoin. Un supplément de Se organique entraîna une augmentation d’expression relative d’IL-5 par les lymphocytes, comparativement au Se inorganique ou aucun Se. Un supplément de Se augmenta l’expression relative d’IL-1 et IL-8 par les neutrophiles. Les autres mesures des fonctions immunitaires n’étaient pas affectées. Le contenu et les sources de Se alimentaire semblent influencer les fonctions immunitaires des chevaux, incluant des altérations dans l’expression d’IL-5 par les lymphocytes, et l’expression d’IL-1 et IL-8 par les neutrophiles.
(Traduit par Docteur Serge Messier)
Introduction
Selenium is an essential micronutrient that has diverse roles in mammalian metabolism, but is best known as a component of antioxidant enzymes such as glutathione peroxidase and thioredoxin reductase (1). Selenoproteins act in concert with other antioxidants such as vitamin E to prevent oxidative damage to cell membranes and intracellular structures. The classical example of a Se and vitamin E-responsive disease in large animals is white muscle disease (WMD), which mainly affects neonates and young animals (2,3).
In addition to its role in antioxidant defense, Se has been associated with many subclinical disorders including changes in immune function in humans and many animal species; thus it appears to play a role in inflammatory processes and susceptibility to infectious disease. Selenium deficiency has been shown to alter both innate and adaptive immunity, including neutrophil and macrophage function, lymphocyte function, and antibody production in humans and animals (4–8). Two studies in adult horses and ponies investigating the relationship between Se status of the animal and the humoral immune response have shown that Se status may also affect immune function of horses (4,5). Both studies used an inorganic Se source.
Forages and grains grown in eastern Canada and many other parts of North America contain insufficient amounts of Se to meet the dietary requirements of livestock (9,10). Animals receiving feed-stuffs grown in Se-deficient areas require Se supplementation. For Se supplementation of equine feeds, no regulation exists; however, guidelines are provided by the National Research Council (NRC) (11). The Se requirement for mature idle horses was estimated by the NRC to be 0.1 ppm of diet dry matter, but it has been suggested that 0.3 ppm would be a more appropriate recommendation for exercising horses (12). Selenium requirements for optimal immune function are currently unknown and may also be higher than present NRC recommendations (11).
Selenium can be supplemented using either inorganic or organic forms. Bovine and porcine studies have demonstrated organic Se to be overall more effective in increasing common measures of Se adequacy (further referred to in this text as “Se status”), specifically plasma/serum Se concentration and whole blood Se concentration when compared to inorganic Se (13,14). In horses, one study reported a tendency for greater plasma Se concentrations in horses receiving organic Se compared with those receiving inorganic Se, but did not find this difference to be statistically significant (15). Recently, another equine study found that Se yeast was more effective than sodium selenite at increasing total Se in blood, but it did not modify glutathione peroxidase-1 activity (16); however, the effect of Se supplementation or source on immune function was not studied.
To date, the effects of Se supplementation and Se source on immune function have not been extensively studied in horses. Therefore, it was the purpose of this study to examine the effects of inorganic and organic Se on aspects of innate and adaptive immune function in adult horses. The effects of Se supplementation and source on measures of Se status in blood were also investigated. We hypothesized, that supplementary Se will result in higher blood Se concentrations and GPx activity as well as differences in measures of immune response, when compared to no supplementation; and that supplementation of horses with an organic form of Se affects measures of Se status and immune response differently when compared to horses receiving inorganic supplementary Se.
Materials and methods
Experimental animals and study design
Fifteen adult, non-pregnant, non-lactating Standardbred horses (13 mares and 2 geldings) were used in this study. The experimental animals had been kept as a herd for several years, with minimal contact with other horses. All horses were vaccinated annually against tetanus, influenza, and equine herpes viruses 1 and 4. The use of animals in this study was consistent with the Guide to the Care and Use of Experimental Animals and approved by the Animal Care Committee of the University of Prince Edward Island.
The horses enrolled in the study were randomly assigned to 1 of 3 experimental groups, with 5 horses per group. Selenium supplementation was withheld for 3 mo prior to beginning the feeding trial. During this period the horses were turned out onto Se-deficient (< 0.05 ppm) pasture. The horses had continuous access to non-Se-containing trace mineral blocks (Sifto; Canadian Stockman, Mississauga, Ontario).
During the study, horses were housed in individual stalls to which they were well-adapted. All horses were fed a basal diet of timothy hay and locally grown whole oats. Pasture samples, core samples of the hay, and composite samples of the oats were analyzed for Se content at the beginning of the study; analyses were performed using fluorimetric determination of Se (17). The Se concentrations in the pasture, hay, and oat samples were 0.043, 0.047, and 0.050 ppm DM, respectively.
The ration fed to each horse was based on liveweight and calculated to meet NRC maintenance requirements for energy (18). Continuous access to a non-Se-containing trace mineral block (Sifto; Canadian Stockman) was provided during the study period. Dietary treatments were assigned as follows: control group: Se-deficient diet; inorganic Se group: sodium selenite supplement (feed grade sodium selenite); organic Se group: Se yeast supplement (SelPlex; Alltech, Kentucky, USA). A small amount of barley was used as a carrier to deliver the respective Se supplements, while the control group received an equal amount of barley without the addition of a Se supplement.
To ensure that the horses received the entire Se supplement it was hand-fed separately in a small feed bowl once a day before the morning feeding. Each bowl was checked to ensure that there was no residual feed or supplement remaining in the bowl. There were no problems associated with feed consumption or the suspension of the Se in the barley. Selenium was added to deliver 0.3 ppm [mg/kg body weight (BW)] supplementary Se on a dry matter basis in the total ration.
Blood samples were obtained from the jugular vein of each horse using EDTA and heparinized vacuum tubes prior to onset of treatments, and 1, 2, 3, and 4 mo after beginning of treatments, for determination of plasma and whole blood Se concentrations (EDTA samples) and whole blood glutathione peroxidase activity (heparinized samples). After collection, the blood was transferred into several aliquots of 2 mL Eppendorf microfuge tubes and frozen at −20°C until analysis.
Blood selenium concentrations and blood glutathione peroxidase activity
Plasma and whole blood Se concentrations were measured using atomic absorption spectrophotometry (19) and erythrocyte Se concentration was estimated using the following formula after sub-sampling for estimation of hematocrit:
as previously described (20).
Glutathione peroxidase activity in blood was measured using a commercial assay (Ransel; RANDOX laboratories, Mississauga, Ontario) based on the method by Paglia and Valentine (21). Briefly, this is a UV method which measures the decrease in adsorbance of light at 340 nm when glutathione is oxidized by cumene hydroperoxide catalyzed by glutathione peroxidase. Results were reported in U/g hemoglobin (Hb). All measurements were performed at the Atlantic Veterinary College at the University of Prince Edward Island.
Lymphocyte blastogenesis in response to the mitogen Concanavalin A (ConA)
Two heparinized blood samples were collected from each horse before beginning the feeding trial, and again at 1 and 3 mo after the beginning of the trial. Preparations for the lymphocyte blastogenesis were started immediately after the blood was collected. During the first step, the blood was mixed with an equal volume of RPMI-1640 (Sigma, Oakville, Ontario). The blood-RPMI mixture was layered onto Ficoll-Paque Plus (Amersham Biosciences, Baie d’Urfé, Quebec) and centrifuged at 400 × g for 30 min at 4°C to separate the lymphocyte fraction from the whole blood. After separation, the lymphocyte layer was harvested and the cells were washed twice with sterile saline (700 × g for 5 min); the cell pellet was resuspended in 1 mL of RPMI+ (RPMI-1640 with 100 Units/mL of Penicillin and 100 μg/mL of Streptomycin, 2 mM glutamine, and 10% heat inactivated fetal calf serum). The cells were counted and cell viability was evaluated using the trypan blue exclusion method (22). The average cell viability was greater than 90 percent.
The cell number was adjusted to 2 × 106/mL and the cells were dispensed at 100 μL/well in a 96-well plate (BD Falcon, VWR, Mississauga, Ontario) to which an equal volume of ConA (Sigma) diluted in RPMI+ was added to give a final concentration of 5, 2, and 0 μg/mL of ConA. All samples were run in quadruplicate. Cellular proliferation in response to mitogen stimulation was determined using [3H]thymidine incorporation into cellular DNA. The cells were incubated at 37°C for 48 h in a 5% CO2 air humidified atmosphere, after which 1 μCi of [3H]thymidine (GE Healthcare, Baie d’Urfé, Quebec) was added to each well. The cells were incubated for 18 h with the [3H]thymidine and then harvested onto glass fiber filtermats (Skatron Instruments, Sterling, Virginia, USA) using a Skatron semi-automatic cell harvester. Finally, the amount of radioactivity [counts per minute (CPM)] incorporated into cellular DNA was detected with a Wallac Microbeta Trilux 11450 (Perkin Elmer, Woodbridge, Ontario) liquid scintillation counter. Stimulation indices (SI) were calculated using the following formula: the CPM of the stimulated cells divided by the CPM of the unstimulated cells. The optimal number of cells per well and concentration of ConA used was established in a pilot trial preceding this study.
Neutrophil phagocytosis of fluorescent beads measured by flow cytometry
Neutrophils were isolated from the red blood cell fraction after Ficoll-Paque separation of lymphocytes. First, the red blood cells were washed twice with saline at 700 × g for 5 min. This was followed by red blood cell lysis modified from Raidal et al (23). To lyse the red blood cells, 19 mL of lysis solution (0.8% ammonium chloride, 0.08% sodium EDTA, 0.08% sodium carbonate) were added to 1 mL of blood at room temperature. After 10 min, 19 mL of saline were added and the mixture was pipetted through a 50 μm nylon mesh to eliminate remaining cellular debris. This was then centrifuged at 700 × g for 5 min. The cell pellet was resuspended in 10 mL of lysis solution, after which 19 mL of saline were added. This mixture was again centrifuged at 700 × g for 5 min and the cell pellet was then washed twice with saline. Finally, the cell pellet was resuspended in 1 mL of saline. After isolating the neutrophils, the cells were counted and their viability was assessed using the trypan blue exclusion method (22). The average cell viability was > 90%.
To evaluate phagocytosis, isolated neutrophils (0.5 × 106/mL) were incubated with 2 μm polystyrene fluorescene (FITC) labeled beads (10 beads/neutrophil; Polysciences, Warrington, Pennsylvania, USA). Incubation lasted for 30 min at 37°C in the dark on a shaker. To differentiate beads that were attached to the outside of the cell from engulfed beads (evidence of phagocytosis activity), a negative control was included for each sample. This control was prepared on ice and 0.2% phosphate-buffered saline — ethylenediamine tetraacetic acid (PBS-EDTA) (0.2 g EDTA in 100 mL PBS) was added to inhibit phagocytosis.
Fluorescence of each sample was measured using flow cytometry (FACSCalibur; Becton-Dickinson, San José, California, USA) to determine the percentage of cells that engulfed fluorescent beads. The phagocytic activity was reported as the difference between the sample, incubated at 37°C, and the control sample, in which phagocytosis was inhibited as described (% gated cells). Before the study, a pilot trial was performed to assess different methods to inhibit phagocytosis.
Rabies antibody production in response to vaccination
Rabies titers in serum for each horse were measured on three occasions: immediately prior to initial rabies vaccination (IMrab; Mérial, Baie d’Urfé, Quebec), administered one month after the beginning of the feeding trial; prior to booster vaccination (8 wk later); and at the end of the trial (1 mo after booster). Serum for rabies titers was frozen and maintained at −80°C until all analyses could be performed at one time. Records for the experimental horses indicated that they had not been vaccinated for rabies since they had entered the herd (at least 2 y in all cases; for most horses, several years).
The Canadian Food Inspection Agency Rabies Laboratory measured rabies antibody titers in serum using a competitive enzyme-linked immunosorbent assay (ELISA) (24). In this test, both serum and a labeled monoclonal antibody (Mab) were applied to virus-coated plates at the same time, and antibodies in the serum competed with the labeled Mab for antigen binding sites. The results were reported as percent (%) inhibition of binding of a rabies virus glycoprotein-specific peroxidase-labeled Mab to microtiter plates coated with ERA (Evelyn-Rokitnicki-Abelseth) variant rabies virus. An inhibition value of 20% or greater was considered positive for detection of glycoprotein-specific antibodies in the horse serum, indicative of seroconversion.
Relative cytokine gene expression measured by real-time polymerase chain reaction (RT)-PCR
Relative cytokine gene expression was measured in mitogen-stimulated lymphocytes and LPS-stimulated neutrophils. Lymphocytes were suspended in 1 mL of RPMI+ at a concentration of 0.5 × 106/mL. An unstimulated sample was analyzed at baseline before the beginning of the feeding trial. A stimulated and an unstimulated sample for each animal were analyzed at 1 and 3 mo after beginning the feeding trial. Following analysis, a stimulation index (SI) was calculated for each cytokine using the following formula:
Lymphocytes were stimulated with 2 μg/mL of ConA in RPMI+ and an equal volume of RPMI+ was added to the unstimulated cells. All cells were incubated at 37°C for 24 h in a 5% CO2 air humidified atmosphere. Following incubation, the sample was centrifuged at 16 000 × g in a microfuge for 3 min, after which time the cell pellet was resuspended in RNAlater (Sigma). The samples were frozen at −20°C and stored for further analysis.
Neutrophils from each animal were also suspended in 1 mL of RPMI+ at a concentration of 0.5 × 106/mL. The sampling time points and sample processing was identical to the procedure used for the lymphocytes except that the neutrophils were stimulated with lipopolysaccharide (LPS) at 50 ng/mL for 4 h.
Gene expression assays were performed at the Gluck Equine Research Center at the University of Kentucky. All samples from an individual horse were analyzed in one run. During the first step of the analyses, the samples were thawed and the RNA was purified using the RNeasy Micro Kit (Qiagen; Valencia, California, USA). This commercial kit is specially designed for the purification of RNA from small samples. A carrier RNA, supplied with the kit, was also used to increase the total yield of RNA.
Following RNA purification, reverse transcription reactions were performed as previously described (25) using 12 μL of each RNA sample and reverse transcription master mix (Promega, Madison, Wisconsin, USA). The reactions were incubated at 42°C for 15 min and 95°C for 5 min. The cDNA was stored at −20°C until analyzed by real-time polymerase chain reaction (RT-PCR).
Cytokine gene expression was measured by RT-PCR as described previously (25), using equine specific intron-spanning primer/probe sets for IFNγ, IL-2, IL-5, IL-10, and tumor necrosis factor alpha (TNFα) for the lymphocyte samples and IL-1, IL-6, IL-8, IL-12, and TNFα for the neutrophil samples. The cytokines were chosen based on their importance in immunological processes involving the cell types of interest.
Each reaction contained 4.5 μL of cDNA and 5 μL TaqMan Gene Expression Master Mix (ABI) and 0.5-μL primer and probes. All reactions were incubated in duplicate wells at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s in an Applied Biosystems 7900 sequence detection system. Beta-glucuronidase (β-GUS) was used as the housekeeping gene for the lymphocyte samples and beta-2-microglobulin (B2M) was the housekeeping gene for the neutrophil samples. After recording the cycle threshold (CT) for each lymphocyte cytokine, the ΔΔCT method was used to calculate relative changes in cytokine gene expression (26), where:
The same formula was applied to the neutrophil cytokine results, using the results for B2M instead of β-GUS. The relative changes in gene expression before (baseline sample, used for calibrator calculation) and after initiation of treatment were measured for each gene of interest. The calibrator was calculated from the mean ΔCT obtained from the baseline samples for each individual gene. Through this method, the baseline (pre-treatment) value was included in the calculation of the relative quantification (RQ) value for the one- and three-month samples. The use of a calibrator helps to reduce the effect of between horse variations. Results are expressed as RQ of each cytokine after stimulation calculated as 2−ΔΔCT, at 1 and 3 mo after initiation of dietary treatment. Results are also expressed as the stimulation index (SI), calculated as RQ stimulated/RQ unstimulated for the same two time periods.
Statistical analysis
Statistical analysis was performed with commercial software (Intercooled Stata 9.2; StataCorp, College Station, Texas, USA), using repeated measures analysis of variance (ANOVA). Comparisons between means were made using the following pre-planned contrasts: Se-supplemented versus Se-unsupplemented, and organic versus inorganic. Values for neutrophil phagocytosis, lymphocyte blastogenesis, relative cytokine gene expression, and the stimulation indices for gene expression were log10-transformed before statistical analysis to normalize their distribution; where the time × treatment interaction was not significant, values are presented for the treatment main effect. Lymphocyte blastogenesis and gene expression medium controls were analyzed using ANOVA. Following ANOVA, a priori contrasts were tested or means were compared using Bonferroni correction. Results are presented as means with standard error (SE), unless otherwise indicated. Significance was set at P ≤ 0.05.
Results
The mean (± SE; range) age (in years) of horses from each experimental group was as follows: control group, 9.4 (4.93; 7 to 18); inorganic group, 12.4 (4.28; 8 to 17); and organic group, 12.0 (5.24; 6 to 18), with no significant differences in age between groups. The average daily intake of Se per kg body weight (BW) was 0.0040 mg/kg BW for horses in the inorganic and organic Se groups and 0.0006 mg/kg BW in the control group. No systemic illness was observed in any of the horses during the study period, except for an impaction colic in a horse in the organic Se group during the last month of the study. This horse was treated medically and the problem resolved within 2 d; data for this horse were retained.
Blood selenium concentrations and glutathione peroxidase activity
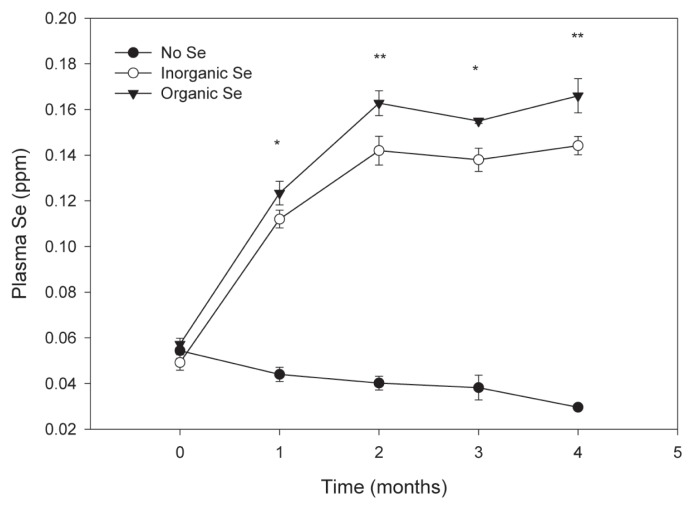
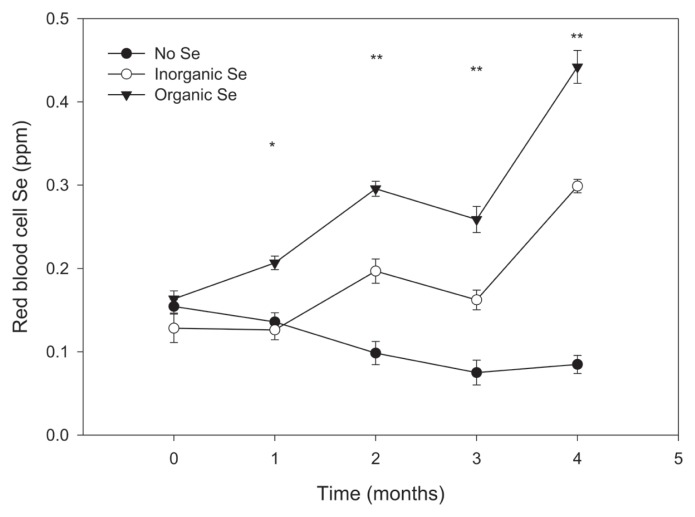
At the end of the experimental period, all measures of blood Se were significantly higher in horses receiving supplementary Se, regardless of source, when compared to horses in the control group (Figures 1 and 2). Furthermore, horses in the organic Se group had significantly higher plasma and red blood cell Se concentrations compared with horses in the inorganic Se group, with the greater difference noted in red blood cell Se concentrations. The effects of time and time × group interaction were significant for each measure of Se status (P < 0.01). Mean (± SE) Se concentrations at the end of the study for the control, inorganic, and organic groups were 0.030 (0.001), 0.144 (0.004), and 0.166 (0.008) ppm Se in plasma, and 0.085 (0.011), 0.299 (0.008), and 0.442 (0.020) ppm Se in red blood cells, respectively (all pairwise group comparisons significantly different, P < 0.05).
Figure 1.
Mean (± SE) plasma Se concentration in adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); * the mean of horses receiving supplementary Se is significantly different from the mean of the control group (P < 0.05); ** all pairwise mean comparisons are significant (P < 0.05).
Figure 2.
Mean (± SE) red blood cell Se concentration in adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); * the mean of horses receiving organic Se is significantly different from the mean of the horses receiving inorganic or no Se (P < 0.05); ** all pairwise mean comparisons are significant (P < 0.05).
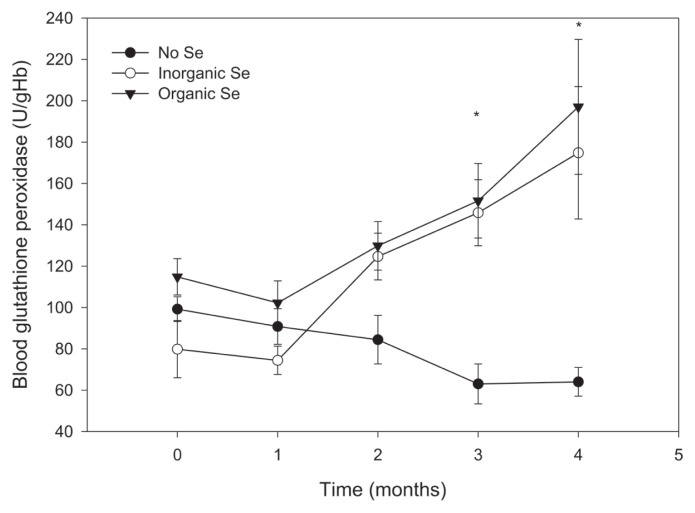
Mean glutathione peroxidase activity at the end of the trial was higher in the Se-supplemented horses compared with horses in the control group (P < 0.001); however, there was no significant difference between horses in the 2 Se-supplemented groups (Figure 3).
Figure 3.
Mean (± SE) blood glutathione peroxidase activity in adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); * the mean of horses receiving supplementary Se is significantly different from the mean of the control group (P < 0.05).
Lymphocyte blastogenesis
Lymphocyte blastogenesis in response to 2 concentrations of the mitogen ConA was not significantly affected by Se supplementation or time. Stimulation indices (± SE) are reported in Table I. There was no significant difference between the groups for the results of the medium control (0 ConA).
Table I.
Lymphocytes blastogenesis (mean ± SE) in response to 2 concentrations of Con A at study start, 1 and 3 mo after dietary assignment, in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Study start | 1 month | 3 months | |
|---|---|---|---|
| Con A 5 μg/mL | |||
| No Se | 28.2 (3.0) | 12.2 (1.8) | 42.0 (7.5) |
| Inorganic Se | 35.4 (7.9) | 15.6 (3.3) | 38.2 (8.9) |
| Organic Se | 33.4 (8.6) | 10.6 (2.6) | 47.4 (21.0) |
| Con A 2 μg/mL | |||
| No Se | 59.6 (8.3) | 67.2 (12.2) | 70.6 (13.0) |
| Inorganic Se | 78.2 (22.2) | 80.4 (17.6) | 91.2 (26.6) |
| Organic Se | 84.2 (20.7) | 67.4 (7.0) | 79.0 (13.9) |
Neutrophil phagocytosis
Phagocytosis of fluorescent beads, measured using flow cytometry, was also not significantly affected by Se treatment or time. The results (% phagocytosis) for the 3 groups are reported in Table II.
Table II.
Mean (± SE) neutrophil phagocytic activity (%) at study start, 1 and 3 mo after dietary assignment, in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Study start | 1 month | 3 months | |
|---|---|---|---|
| Phagocytosis (%) | |||
| No Se | 5.8 (2.6) | 15.6 (2.2) | 20.8 (1.8) |
| Inorganic Se | 4.2 (1.4) | 16.8 (2.8) | 19.8 (3.6) |
| Organic Se | 5.8 (1.7) | 14.4 (3.9) | 32 (8.3) |
Rabies antibody production in response to vaccination
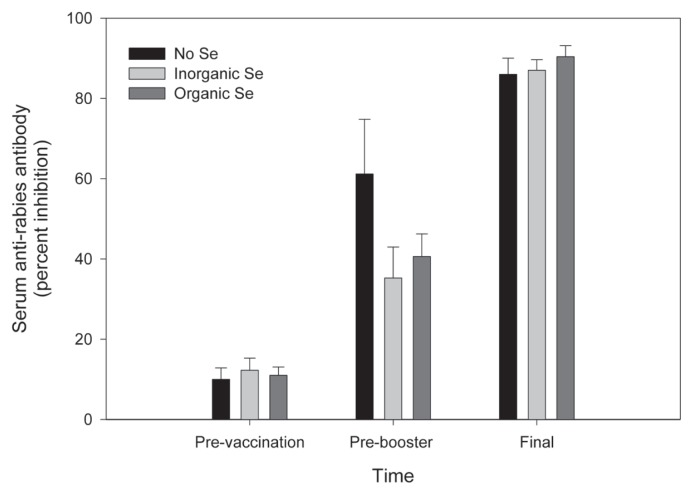
Rabies antibody production, measured by percent inhibition using a competitive ELISA, was not affected by Se supplementation. All but one horse had baseline values < 20% inhibition, indicative of immunologic naivety to rabies virus. One horse had a baseline value of 45% inhibition, indicative of previous immunization against rabies. This horse was removed from the statistical analysis of rabies antibody production data. Seroconversion (increase from < 20% to > 20% inhibition) occurred in all previously naïve animals after initial vaccination, and percent inhibition increased in all animals after the booster vaccination. Percent inhibition (± SE) after initial vaccination was 61.2 (13.6), 35.25 (7.7), and 40.6 (5.6) for the control, inorganic, and organic groups, respectively. Percent inhibition after booster vaccination was 86 (4), 87 (2.7), and 90.4 (2.7) for the control, inorganic, and organic groups, respectively (Figure 4).
Figure 4.
Percent inhibition of serum anti-rabies antibodies prior to vaccination, prior to booster vaccination (8 wk later), and at the end of the study (4 wk after booster) in adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM).
Cytokine gene expression measured by RT-PCR
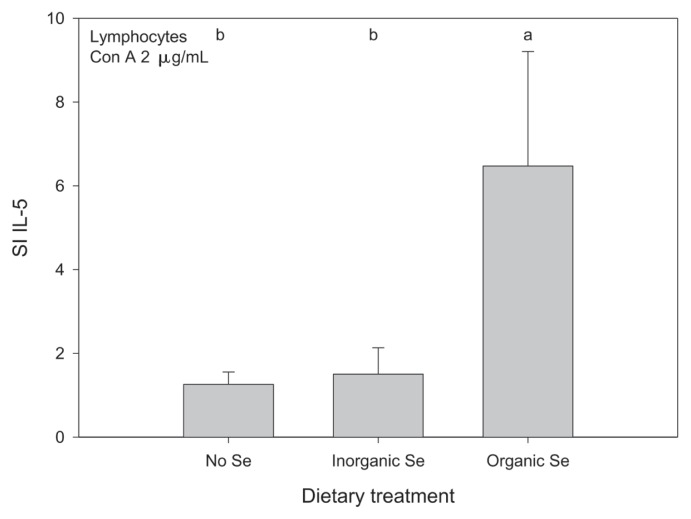
Horses receiving organic Se had a higher mean SI for relative expression of IL-5 by lymphocytes compared with horses receiving inorganic Se or no supplementary Se (P < 0.05); there was no significant time by treatment interaction so the Se treatment main effect, based on the relative gene expression at 1 and 3 mo, is presented (Figure 5). There was no significant difference between the groups when comparing the results of the media control. Gene expression of the remaining lymphocyte cytokines (IFNγ, IL-2, IL-10, TNFα) was not significantly altered by Se supplementation. The lymphocyte gene expression data are reported in Tables IIIa and IIIb.
Figure 5.
Mean stimulation index (SI) for expression of IL-5 in lymphocytes from adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); a,b means with different superscripts are significantly different (P < 0.05); data were log10-transformed before statistical analysis to normalize their distribution.
Table IIIa.
Relative cytokine gene expression of stimulated lymphocytes (mean RQ ± SE) 1 and 3 mo after dietary assignment in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Cytokine | No selenium | Inorganic selenium | Organic selenium |
|---|---|---|---|
| 1 month | |||
| IFNγ | 161.68 (22.08) | 185.24 (68.97) | 249.74 (100.74) |
| IL-2 | 5.89 (1.78) | 5.46 (1.52) | 6.28 (1.78) |
| IL-5 | 85.55 (9.08) | 222.32 (81.45) | 493.75 (275.81) |
| IL-10 | 98.64 (18.37) | 139.98 (37.70) | 148.05 (27.52) |
| TNFα | 17.05 (4.26) | 20.66 (7.25) | 23.61 (3.87) |
| 3 months | |||
| IFNγ | 75.02 (28.80) | 87.17 (35.72) | 88.02 (41.20) |
| IL-2 | 3.29 (0.82) | 5.78 (2.98) | 3.29 (1.01) |
| IL-5 | 32.02 (11.69) | 64.77 (54.40) | 67.48 (22.06) |
| IL-10 | 66.87 (15.25) | 145.40 (58.33) | 68.75 (19.97) |
| TNFα | 10.02 (1.77) | 13.34 (4.06) | 10.58 (3.36) |
Table IIIb.
Relative cytokine gene expression of stimulated lymphocytes (mean RQ ± SE) 1 and 3 mo after dietary assignment in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Cytokine | No selenium | Inorganic selenium | Organic selenium |
|---|---|---|---|
| 1 month | |||
| IFNγ | 161.68 (22.08) | 185.24 (68.97) | 249.74 (100.74) |
| IL-2 | 5.89 (1.78) | 5.46 (1.52) | 6.28 (1.78) |
| IL-5 | 85.55 (9.08) | 222.32 (81.45) | 493.75 (275.81) |
| IL-10 | 98.64 (18.37) | 139.98 (37.70) | 148.05 (27.52) |
| TNFα | 17.05 (4.26) | 20.66 (7.25) | 23.61 (3.87) |
| 3 months | |||
| IFNγ | 75.02 (28.80) | 87.17 (35.72) | 88.02 (41.20) |
| IL-2 | 3.29 (0.82) | 5.78 (2.98) | 3.29 (1.01) |
| IL-5 | 32.02 (11.69) | 64.77 (54.40) | 67.48 (22.06) |
| IL-10 | 66.87 (15.25) | 145.40 (58.33) | 68.75 (19.97) |
| TNFα | 10.02 (1.77) | 13.34 (4.06) | 10.58 (3.36) |
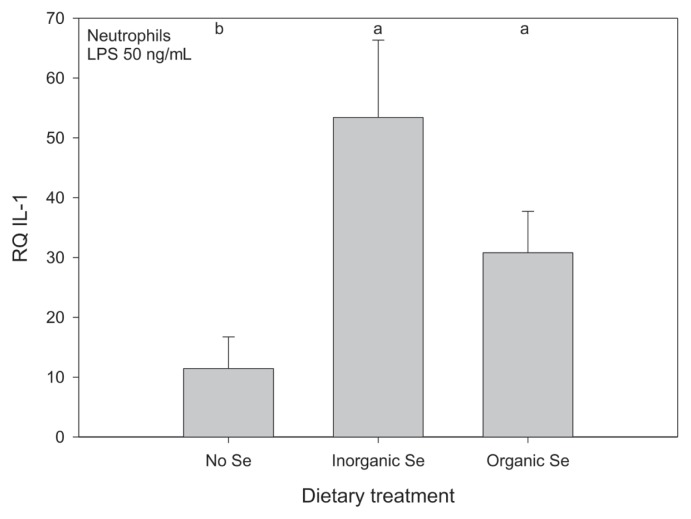
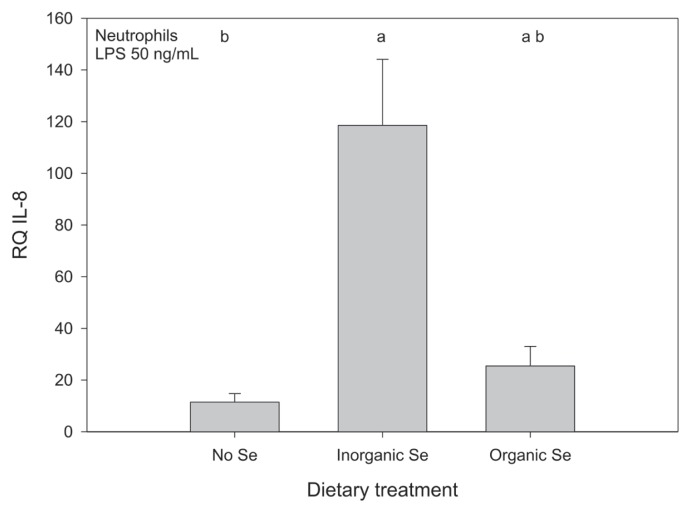
Relative gene expression of the neutrophil cytokines was affected by Se supplementation, but the effect of Se source depended on the cytokine measured. Horses receiving Se, either organic or inorganic, had higher mean relative expression of IL-1 in neutrophils compared with horses receiving no supplementary Se but the effect of source of Se was not significant (Figure 6). Horses receiving inorganic Se had significantly higher mean relative expression of IL-8 in stimulated neutrophils compared with horses receiving no supplementary Se, but mean expression for horses receiving organic Se was not significantly different from horses receiving either inorganic or no Se (Figure 7). For the remaining cytokines (IL-6, IL-12, TNFα) measured in the neutrophil samples a usable signal could also not be detected with RT-RCR. The neutrophil gene expression data is reported in Tables IVa and IVb.
Figure 6.
Mean relative quantification of IL-1 expression in stimulated neutrophils from adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); a,b means with different superscripts are significantly different (P < 0.05); data were log10-transformed before statistical analysis to normalize their distribution.
Figure 7.
Mean relative quantification of IL-8 expression in stimulated neutrophils from adult horses (n = 5 per group) receiving supplementary inorganic Se (0.3 ppm DM, as sodium selenite), organic Se (0.3 ppm DM, as Se yeast), or receiving no supplementary Se (< 0.05 ppm DM); a,b means with different superscripts are significantly different (P < 0.05); data were log10-transformed before statistical analysis to normalize their distribution.
Table IVa.
Cytokine gene expression of stimulated neutrophils (mean RQ ± SE) 1 and 3 mo after dietary assignment in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Cytokine | No selenium | Inorganic selenium | Organic selenium |
|---|---|---|---|
| 1 month | |||
| IL-1 | 6.025 (0) | 87.162 (6.281) | 40.288 (3.182) |
| IL-8 | 14.961 (0) | 178.782 (13.158) | 32.444 (14.749) |
| 3 months | |||
| IL-1 | 14.118 (7.926) | 28.065 (8.145) | 21.300 (9.808) |
| IL-8 | 10.272 (4.384) | 78.434 (9.996) | 18.424 (4.628) |
Table IVb.
Stimulation indices of relative neutrophil cytokine gene expression (mean RQ ± SE) 1 and 3 mo after dietary assignment in adult horses (n = 5 per group) receiving supplementary inorganic selenium (0.3 ppm DM, as sodium selenite), organic selenium (0.3 ppm DM, as Se yeast), or receiving no supplementary selenium (< 0.05 ppm DM)
| Cytokine | No selenium | Inorganic selenium | Organic selenium |
|---|---|---|---|
| 1 month | |||
| IL-1 | 0.596 (0) | 4.702 (0) | 0.639 (0.427) |
| IL-8 | 3.918 (0) | 2.307 (2.143) | 0.415 (0.282) |
| 3 months | |||
| IL-1 | 1.481 (0.956) | 0.768 (0.1258) | 1.122 (0.808) |
| IL-8 | 0.563 (0.281) | 1.840 (0.737) | 1.219 (0.610) |
Discussion
Effect of Se supplementation and source on blood Se parameters and glutathione peroxidase activity
The results of the present study are in general agreement with Calamari et al (16), who found that Se yeast was more effective than sodium selenite at increasing total Se concentration in the blood of horses, but was no more effective at increasing glutathione peroxidase-1 activity over a 16-week experimental period. While the bioavailability of inorganic versus organic Se has not been extensively studied in horses, this area of research has received a great deal of attention in other domestic animal species. Mahan et al (13) demonstrated that supplementation of pigs with either inorganic or organic Se resulted in significant differences in Se retention, tissue Se concentration, serum Se concentration, with organic Se supplementation being more effective overall. Studies in cattle also have shown that organic Se is more effective than inorganic Se at raising the concentration of Se in whole blood and serum (14,27). Our study shows that in horses, Se supplementation of Se-deficient horses will increase plasma and red blood cell Se, with organic Se supplementation resulting in higher Se levels; with no difference observed in blood glutathione peroxidase activity between inorganic and organic Se.
Both plasma and red blood cell Se concentrations were measured in this study as they reflect the amount of Se contained in 2 distinct Se pools (28): red blood cell Se is almost entirely composed of Se in the form of selenocysteine, as part of the enzyme glutahione peroxidase. As such, changes in red blood cell Se in response to supplementation can be taken as a reflection of availability of Se for incorporation into selenoproteins. Red blood cell Se takes longer to respond to Se supplementation because the selenocysteine is incorporated into red blood cell glutathione peroxidase only at the time of erythropoesis (29). In contrast, Se in plasma reflects short-term changes in Se intake (20). This is likely a result of incorporation of Se non-specifically into plasma proteins, but it would take a large or carefully designed study to demonstrate this, as protein is a small component of plasma. The glutathione peroxidase findings are likely a result of the fact that erythrocyte glutathione peroxidase activity is not linearly related to Se intake and tends to plateau at higher intakes of Se (30).
Effect of Se supplementation and source on antibody production
Selenium deficiency has been shown to affect both innate and adaptive immunity in domestic animals and humans, including neutrophil and macrophage function, lymphocyte function, and antibody production (6,31). To date, there are only 2 published studies in adult horses and ponies investigating the relationship between Se status of the animal and immune response, both examining humoral responses. These studies compared the feeding of an inorganic Se supplement to unsupplemented controls. One study showed an increase in antibody response after vaccination against influenza in horses after supplementation with vitamin E and Se compared with no supplementation or supplementation with Se alone (4). The other study, in ponies, found a higher concentration of immunoglobulins after antigen challenge with sheep red blood cells in ponies supplemented with Se compared to unsupplemented ponies (5).
Results from a recent study in cattle indicated that the effect of Se supplementation on antibody titers may be influenced by the amount of Se supplemented, and that excessive doses of Se may lead to immune suppression (32). In the present study, no difference was seen in the humoral immune response to rabies vaccination between the control and Se-supplemented groups. These findings are similar to findings in cattle by Reis et al (32), who found that supplementation of Se-deficient 12-month old calves with different doses of Se, or no Se, did not affect their rabies antibody titers post-vaccination. However, the amount of Se supplemented did affect the persistence of titers. Only calves receiving 3 to 6 mg Se daily had antibody titers considered to be protective against rabies for the entire study period of 120 d. As the horses in the present study received a booster vaccine, the duration of protective titers after only one vaccination could not be evaluated, but could be subject to future research as it has been shown that rabies antibody titers decline to low titers within 6 mo in Se-marginal horses that only receive one dose of vaccine (33).
Effect of Se supplementation and source on lymphocyte function
The effect of Se status on cellular immune function has mainly been studied by investigating in vitro blastogenesis of lymphocytes after mitogen stimulation. Research in other species indicates that Se and vitamin E deficiency lead to a decrease in lymphocyte proliferation in response to some mitogens (phytohemagglutinin, ConA, pokeweed) (34). In the present study, no effect of Se supplementation on lymphocyte proliferation could be observed. This may be due, in part, to the relatively small number of animals in each group, as this biological assay can be quite variable, both between and within animals. Additionally, the incubation time of the lymphocytes with the mitogen or the choice of mitogen may play a role.
Roy et al (35) found a significant difference in human lymphocyte blastogenesis only after 72 h of incubation with phytohemagglutinin when comparing Se-deficient and Se-supplemented individuals. In a more recent study, Calamari et al (36) found greater lymphocyte counts in the blood of horses supplemented with Se yeast (0.3 mg/kg DM) compared with horses supplemented with sodium selenite (0.3 mg/kg DM); however, lymphocyte proliferation was not studied.
Cells of the innate and adaptive immune system mediate their effects through the production and release of different cytokines. The effects of Se status on cytokine gene expression in immune cells have not been well-investigated in domestic animal species, but deserve consideration as a mechanism through which Se could influence the immune response. Recent research has shown that Se can attenuate pro-inflammatory gene expression in macrophages of mice, and therefore has an important role as an anti-inflammatory agent in those cells (8). Furthermore, Se status can modulate the activity of NF-κB in murine monocytes and macrophages (37,38), which may be an explanation as to how Se status could also alter cytokine gene expression.
A recent study in mice investigated the difference in cytokine profile of lymphocytes in Se-deficient mice compared to mice of adequate Se status after infection with Cryptosporidium parvum (39). Results from that study showed that lymphocytes from mice with an adequate Se status produced higher levels of IFNγ, IL-2, and IL-4 throughout the course of infection compared to Se-deficient mice.
In the present study, lymphocytes from horses receiving organic Se increased expression of IL-5 about 6-fold when stimulated with 2 μg/mL of ConA compared to unstimulated lymphocytes from the same horses. However, in horses receiving inorganic or no Se supplementation, IL-5 expression was not different in stimulated lymphocytes compared with unstimulated lymphocytes. Interleukin-5 is secreted by T-helper 2 lymphocytes and is involved in the later stages of B-lymphocyte activation during which it contributes, together with IL-6, to the differentiation of B-lymphocytes into antibody-secreting plasma cells (40). Interleukin-5 has been shown, in mice, to promote IgA secretion in plasma cells that have already undergone isotype switching (40). However, clonal expansion of B-lymphocytes, which precedes differentiation, requires the presence of IL-4, another T-helper 2 derived cytokine (40). Interleukin-4 expression was not measured in this study, but should be considered in future studies.
Effect of Se supplementation and source on neutrophil function
Horses receiving either organic or inorganic Se had higher mean relative expression of IL-1 by neutrophils compared with horses receiving no supplementary Se. In this case, source of Se was not important. In contrast, horses receiving inorganic Se had the highest mean relative expression of IL-8 by stimulated neutrophils. Interleukin-1 is produced by a number of cells, including neutrophils, and as a pro-inflammatory cytokine, it is an important mediator of inflammation. In neutrophils, IL-1 can induce chemotaxis and activate oxidative metabolism. Interleukin-8, also known as CXCL8, is a chemotactic factor, which plays an important role in the recruitment of leukocytes to the site of infection as well as the activation of neutrophils, including respiratory burst and the release of lysosomal content (40). Based on these results, there is evidence that the Se status of the host influences neutrophil function in adult horses. The effect of Se status and dietary Se source on molecular events such as intracellular signaling are ongoing areas of research; the data herein suggest that there may be an effect on neutrophil function in horses. This finding definitely warrants further investigation, especially regarding the biologically significance of these findings.
The effect of Se status on neutrophil function has been extensively studied in cattle, particularly dairy cows. One study demonstrated that dietary supplementation of cattle with Se led to a more rapid neutrophil influx into milk following intramammary bacterial challenge as well as increased intracellular killing of ingested bacteria by neutrophils compared with cattle not receiving supplementary Se (41). Another study in postparturient Holstein cows showed that higher blood Se concentrations resulted in enhanced neutrophil function, measured as increased neutrophil adhesion to nylon fibers and superoxide production (42). In the study by Cebra et al (42), all cows had blood Se concentrations within the reference range determined to prevent deficiency, but the cows with Se concentrations above the reference range had increased neutrophil function compared to cows within the reference range. This finding indicates that the Se requirements for prevention of classical deficiency syndromes and optimal immune function may differ, at least in regards to neutrophil function.
Based on previous research findings, which have demonstrated that Se can alter the activity of the transcription factor NF-κB (37,38), one may speculate that cytokine gene expression can be influenced by Se through a similar mechanism. If differences in Se status can affect the activity of NF-κB one may speculate further that inorganic and organic Se supplementation can have different effects on cytokine gene expression because different Se sources result in a difference in Se status. According to results from a study in humans, Se supplementation (100 μg of Se as sodium selenite daily) can result in increased expression of genes involved in protein biosynthesis and gene translation in lymphocytes of healthy individuals (43). The authors hypothesized that this upregulation may result in increased selenoprotein synthesis leading to an increase in lymphocyte activity. There is evidence that Se can affect gene expression on a transcriptional and translational level, and these mechanisms may be involved in the changes seen in relative cytokine gene expression after Se supplementation of horses.
In summary, Se supplementation of adult horses with inorganic or organic Se led to an increase in measures of Se status in blood, with horses receiving organic Se having the highest concentrations of Se in plasma and red blood cells. Dietary Se content and source appear to influence immune function in horses, elaborated as increased lymphocyte expression of IL-5 and neutrophil expression of IL-1 for the organic Se group and IL-1 and IL-8 neutrophil expression in the inorganic Se group. These results suggest potential mechanisms by which Se supplementation might influence immune function in horses.
Acknowledgments
The authors thank Dr. Amanda Adams for her help with the gene expression and acknowledge Judy Sheppard for her help with the lymphocyte blastogenesis and Alejandra Betancourt for her assistance with the PCR analysis.
References
- 1.Schomburg L, Schweizer U, Koehrle J. Selenium and selenoproteins in mammals: Extraordinary, essential, enigmatic. Cell Mol Life Sci. 2004;61:1988–1995. doi: 10.1007/s00018-004-4114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muth OH, Oldfield JE, Remmert LF, Schubert JR. Effects of selenium and vitamin E on white muscle disease. Science. 1958;128:1090. doi: 10.1126/science.128.3331.1090. [DOI] [PubMed] [Google Scholar]
- 3.Lofstedt J. White muscle disease of foals. Vet Clin North Am Equine Pract. 1997;13:169–185. doi: 10.1016/s0749-0739(17)30262-6. [DOI] [PubMed] [Google Scholar]
- 4.Baalsrud KJ, Overnes G. Influence of vitamin E and selenium supplement on antibody production in horses. Equine Vet J. 1986;18:472–474. doi: 10.1111/j.2042-3306.1986.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 5.Knight DA, Tyznik WJ. The effect of dietary selenium on humoral immunocompetence of ponies. J Anim Sci. 1990;68:1311–1317. doi: 10.2527/1990.6851311x. [DOI] [PubMed] [Google Scholar]
- 6.Finch JM, Turner RJ. Effects of selenium and vitamin E on the immune responses of domestic animals. Res Vet Sci. 1996;60:97–106. doi: 10.1016/s0034-5288(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 7.Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr. 2003;133:1457S–1459S. doi: 10.1093/jn/133.5.1457S. [DOI] [PubMed] [Google Scholar]
- 8.Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K. Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res. 2008;52:1316–1323. doi: 10.1002/mnfr.200700346. [DOI] [PubMed] [Google Scholar]
- 9.Kubota J, Allaway WH, LaVere D. Selenium in crops in the United States in relation to selenium-responsive diseases of animals. J Agric Food Chem. 1967;15:448–453. [Google Scholar]
- 10.Wichtel JJ, Keefe GP, Van Leeuwen JA, Spangler E, McNiven MA, Ogilvie TH. The selenium status of dairy herds in Prince Edward Island. Can Vet J. 2004;45:124–132. [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council; National Research Council, editor. Nutrient Requirements of Horses. 6th ed. Washington DC: National Academies Press; 2007. Minerals; pp. 94–97. [Google Scholar]
- 12.Pagan JD, Karnezos P, Kennedy MAP, Currier T, Hoekstra KE. Effect of selenium source on selenium digestibility and retention in exercised Thoroughbreds. Proc of Equine Nutrition and Physiology Society. 1999:135–140. [Google Scholar]
- 13.Mahan DC, Cline TR, Richert B. Effects of dietary levels of selenium-enriched yeast and sodium selenite as selenium sources fed to growing-finishing pigs on performance, tissue selenium, serum glutathione peroxidase activity, carcass characteristics, and loin quality. J Anim Sci. 1999;77(8):2172–2179. doi: 10.2527/1999.7782172x. [DOI] [PubMed] [Google Scholar]
- 14.Ortman K, Andersson R, Holst H. The influence of supplements of selenite, selenate and selenium yeast on the selenium status of dairy heifers. Acta Vet Scand. 1999;40:23–34. doi: 10.1186/BF03547038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson SM, Siciliano PD, Engle TE, Larson CK, Ward TL. Effect of selenium supplementation and source on the selenium status of horses. J Anim Sci. 2006;84:1742–1748. doi: 10.2527/jas.2005-413. [DOI] [PubMed] [Google Scholar]
- 16.Calamari L, Ferrari A, Bertin G. Effect of selenium source and dose on selenium status of mature horses. J Anim Sci. 2009;87:167–178. doi: 10.2527/jas.2007-0746. [DOI] [PubMed] [Google Scholar]
- 17.Watkinson JH. Semi-automated fluorimetric determination of nanogram quantities of selenium in biological material. Anal Chimica Acta. 1979;105:319–325. [Google Scholar]
- 18.National Research Council; The National Academies Press, editor. Nutrient Requirements of Horses. 6th ed. Washington DC: The National Academies Press; 2007. Nutrient requirements, feedstuff composition, and other tables; pp. 294–314. [Google Scholar]
- 19.Feuerstein M, Schlemmer G. Determination of selenium in human serum by GFAAS with traverse heated graphite atomizer and longitudinal Zeeman-Effect background correction. At Spectrosc. 1999;20:180–185. [Google Scholar]
- 20.Thompson KG, Fraser AJ, Harrop BM, Kirk JA. Glutathione peroxidase activity in bovine serum and erythrocytes in relation to selenium concentrations of blood, serum and liver. Res Vet Sci. 1980;28:321–324. [PubMed] [Google Scholar]
- 21.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 22.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Volume 1, Supplement. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Greene Publishing Associates and Wiley-Interscience; 1992. [Google Scholar]
- 23.Raidal SL, Bailey GD, Love DN. The flow cytometric evaluation of phagocytosis by equine peripheral blood neutrophils and pulmonary alveolar macrophages. Vet J. 1998;156:107–116. doi: 10.1016/s1090-0233(05)80036-x. [DOI] [PubMed] [Google Scholar]
- 24.Elmgren LD, Wandeler AI. Competitive ELISA for the detection of rabies virus neutralizing antibodies. In: Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory Technique in Rabies. 4th ed. Geneva, Switzerland: World Health Organization; 1996. pp. 200–208. [Google Scholar]
- 25.Adams AA, Breathnach CC, Katepalli MP, Kohler K, Horohov DW. Advanced age in horses affects divisional history of T cells and inflammatory cytokine production. Mech Ageing Dev. 2008;129:656–664. doi: 10.1016/j.mad.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[-delta delta C(T)] method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Ortman K, Pehrson B. Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J Anim Sci. 1999;77:3365–3370. doi: 10.2527/1999.77123365x. [DOI] [PubMed] [Google Scholar]
- 28.Waldner C, Campbell J, Jim GK, Guichon PT, Booker C. Comparison of 3 methods of selenium assessment in cattle. Can Vet J. 1998;39:225–231. [PMC free article] [PubMed] [Google Scholar]
- 29.Wright PL. Life span of ovine erythrocytes as estimated from selenium-75 kinetics. J Anim Sci. 1965;24:546–550. doi: 10.2527/jas1965.242546x. [DOI] [PubMed] [Google Scholar]
- 30.Blackmore DJ, Campbell C, Dant C, Holden JE, Kent JE. Selenium status of Thoroughbreds in the United Kingdom. Equine Vet J. 1982;14:139–143. doi: 10.1111/j.2042-3306.1982.tb02368.x. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res. 2008;52:1273–1280. doi: 10.1002/mnfr.200700330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reis LS, Chiacchio SB, Oba E, Pardo PE, Frazatti-Gallina NM. Selenium supplementation and rabies antibody titres in cattle. Vet Rec. 2008;163:343–344. doi: 10.1136/vr.163.11.343-b. [DOI] [PubMed] [Google Scholar]
- 33.Muirhead TL, McClure JT, Wichtel JJ, et al. The effect of age on serum antibody titers after rabies and influenza vaccination in healthy horses. J Vet Intern Med. 2008;22:654–661. doi: 10.1111/j.1939-1676.2008.0091.x. [DOI] [PubMed] [Google Scholar]
- 34.Lessard M, Yang WC, Elliott GS, et al. Suppressive effect of serum from pigs and dogs fed a diet deficient in vitamin E and selenium on lymphocyte proliferation. Vet Res. 1993;24:291–303. [PubMed] [Google Scholar]
- 35.Roy M, Kiremidjian-Schumacher L, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace Elem Res. 1994;41:103–114. doi: 10.1007/BF02917221. [DOI] [PubMed] [Google Scholar]
- 36.Calamari L, Abeni F, Bertin G. Metabolic and hematological profiles in mature horses supplemented with different selenium sources and doses. J Anim Sci. 2010;88:650–659. doi: 10.2527/jas.2009-1855. [DOI] [PubMed] [Google Scholar]
- 37.Zamamiri-Davis F, Lu Y, Thompson JT, et al. Nuclear factor-kappaB mediates over-expression of cyclooxygenase-2 during activation of RAW 264.7 macrophages in selenium deficiency. Free Radic Biol Med. 2002;32:890–897. doi: 10.1016/s0891-5849(02)00775-x. [DOI] [PubMed] [Google Scholar]
- 38.Youn HS, Lim HJ, Choi YJ, Lee JY, Lee MY, Ryu JH. Selenium suppresses the activation of transcription factor NF-kappa B and IRF3 induced by TLR3 or TLR4 agonists. Int Immunopharmacol. 2008;8:495–501. doi: 10.1016/j.intimp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang C, Wu Y, Qin J, Sun H, He H. Induced susceptibility of host is associated with an impaired antioxidant system following infection with Cryptosporidium parvum in Se-deficient mice. PLoS ONE. 2009;4:e4628. doi: 10.1371/journal.pone.0004628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology. 6th ed. New York, New York: Garland Science Publishing; 2005. [Google Scholar]
- 41.Hogan JS, Weiss WP, Smith KL. Role of vitamin E and selenium in host defense against mastitis. J Dairy Sci. 1993;76:2795–2803. doi: 10.3168/jds.S0022-0302(93)77618-3. [DOI] [PubMed] [Google Scholar]
- 42.Cebra CK, Heidel JR, Crisman RO, Stang BV. The relationship between endogenous cortisol, blood micronutrients, and neutrophil function in postparturient holstein cows. J Vet Intern Med. 2003;17:902–907. doi: 10.1111/j.1939-1676.2003.tb02531.x. [DOI] [PubMed] [Google Scholar]
- 43.Pagmantidis V, Meplan C, van Schothorst EM, Keijer J, Hesketh JE. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am J Clin Nutr. 2008;87:181–189. doi: 10.1093/ajcn/87.1.181. [DOI] [PubMed] [Google Scholar]