Abstract
Therapeutic and industrial applications of pluripotent stem cells and their derivatives require large cell quantities generated in defined conditions. To this end, we have translated single cell-inoculated suspension cultures of human pluripotent stem cells (hPSCs; including human induced pluripotent stem cells [hiPS] and human embryonic stem cells [hESC]) to stirred tank bioreactors. These systems that are widely used in biopharmaceutical industry allow straightforward scale up and detailed online monitoring of key process parameters. To ensure minimum medium consumption, but in parallel functional integration of all probes mandatory for process monitoring, that is, for pO2 and pH, experiments were performed in 100 mL culture volume in a “mini reactor platform” consisting of four independently controlled vessels. By establishing defined parameters for tightly controlled cell inoculation and aggregate formation up to 2×108 hiPSCs/100 mL were generated in a single process run in 7 days. Expression of pluripotency markers and ability of cells to differentiate into derivates of all three germ layers in vitro was maintained, underlining practical utility of this new process. The presented data provide key steps toward scalable mass expansion of human iPS and ES cells thereby enabling translation of stem cell research to (pre)clinical application in relevant large animal models and valuable in vitro assays for drug development and validation as well.
Introduction
Human pluripotent stem cells (hPSCs; including human induced pluripotent stem cells (hiPS) and human embryonic stem cells (hESC)) and their progenies are considered excellent research tools to elucidate cellular mechanisms of “stemness” and differentiation, and to investigate molecular disease pathways as well. Induction of pluripotency in somatic cells further stimulated consideration of such cells for cellular therapies.1,2 Estimations suggest that billions of cells per single patient will be required to replace substantial, irreversible cell loss induced by metabolic, inflammatory, or other disorders, such as neurodegeneration, cardiovascular disease, or diabetes.3,4 More immediately, equivalent cell numbers are mandatory to establish and optimize preclinical efficiency studies in physiologically relevant large animal models such as pigs, dogs, or primates.5,6 Both applications, in vitro assays and novel regenerative therapies, will require large cell numbers that cannot be produced by traditional two-dimensional (2D) culture as adherent colonies on mitotically inactivated feeder cells or other supportive substrates.7–10
In the field of vaccines and recombinant protein production, cultivation of mammalian cell lines in several 100–1,000 L dimensions has been thoroughly established in suspension culture bioreactors.11 Given this knowhow, suspension culture (“3D cultivation”) is the method of choice to generate stem cells and their progenies at a scale that deems feasible for their envisioned, high cell number demanding applications.
Initial reports aiming at adapting matrix-attached hESC cultivation to suspension culture focused on microcarriers.12–14 These spherical particles are kept in suspension by stirring or by other mixing techniques and provide an enlarged attachment surface in a relatively small reactor volume due to their high surface area to volume ratio. Microcarriers, which exist in a “plethora” of shapes and sizes, have been previously used in conventional cell culture for production of vaccines, recombinant proteins, or other mammalian cell-derived products.15,16 Despite published proof-of-concept for hPSC cultivation on microcarrieres12,13 critical assessment of these reports reveals a number of issues. Particularly, the tendency of undifferentiated hPSCs to preferentially stick to each other rather than to thoroughly prescreened types of microcarriers might induce additional levels of culture heterogeneity.12,13 This includes only partial and uncontrolled cell-substrate versus cell-cell attachment and subsequently bold heterogeneity of cell-particle and cell-cell clusters sizes that might further increase in stirred, dynamic systems. The approach would also require potentially cumbersome removal of microcarriers from clinical-grade cell preparations prior to clinical application.
Recently, we and others have demonstrated expansion of undifferentiated human ES and iPS cells as cell-only-aggregates in suspension culture.17–20
While the group of Itskovitz-Eldor has established culture conditions based on aggregate-passaging in an interleukin-supplemented medium,17 we have shown highly reproducible suspension cultures of several human ESC, human iPSC, and a cynomolgus monkey ESC line applying other conditions.19–21 Key features of the technology include (i) a fully defined serum-free culture media22 (ii) the use of a Rho-associated coiled-coil kinase (ROCK) inhibitor (RI)23 enabling defined, single cell-based culture inoculation, and (iii) significant long-term expansion of pluripotent hES/hiPS cells in scalable suspension culture independent of any extracellular matrices or scaffolds. In contrast to previously reported feeder-free culture systems,24 our technology does not require preadaptation (i.e., preselection) of cells prior to initiation of expansion culture.
Initial adaptation to dynamic culture was also tested employing stirred spinners or rotated Erlenmeyer flasks.19,20 Notably, robust expansion rates observed in static suspension were found to be reduced at dynamic conditions. Another issue is some degree of heterogeneity regarding the size of aggregates formed in suspension culture that depends on numerous parameters, in particular the inoculation density, the media and the applied culture platform, and the stirring device. Despite these limits we found that the method is extremely robust regarding reproducebility of growth kinetics, maintenance of stem cells pluripotency, and karyotype stability.19,20
In the present article we leapt ahead to translate the culture method to an impeller stirred tank bioreactor system in 100 mL culture scale. Stirred tank reactors have a relatively simple basic design that is broadly applied in the biopharmaceutical industry. Such systems have not only been scaled to >1,000 L dimensions for mammalian cell culture but any intermediate reactor volume exists and even disposable systems were developed in recent years.25–27 Thus, once a culture process is successfully established in low scale, relative straightforward scale up deems feasible including translation to a good manufacturing practice (GMP)-conform production process. Here, we provide strategies and well-defined parameters for tightly controlled aggregate formation from single hPSCs. Robust and highly reproducible greater than fourfold hPSC expansion was achieved in serum-free culture conditions readily yielding 2×108 pluripotent cells in a 100 mL single process run. By showing that the cells also maintained their pluripotency marker expression and differentiation potential the study presents a substantial step toward the envisioned, well controlled mass expansion of hPSCs, particularly considering the relative universal and easy-to-scale reactor setup utilized.
Material and Methods
Parallel bioreactor system
For these studies the following stem cell cultivation system was utilized: cellferm® pro Parallel Bioreactor System (DASGIP AG). This system consists of four stem cell culture vessels (DS0200TPSS), various monitoring and control opportunities and the Software DASGIP control. Several stirrer types (DASGIP AG) were designed and tested; see Supplementary Figure S1(Supplementary Data are available online at www.liebertonline.com/tec) for details on culture vessel dimensions and stirrer design.
Analyzing mixing characteristics of stirrer designs
Cytodex 1 microcarrier with an average particle size of 190 μm and a density of 1.03 g/mL (GE-Healthcare) were prepared according to the manufacturer's instructions; 0.02 g/mL were stained with Coomassie blue. For impeller testing, 1:20 dilution of stained microcarriers in 100 mL phosphate-buffered saline (PBS; without Ca2+/Mg2+) was transferred to a reactor vessel. After 10 min stirring with the respective device at 20, 30, 40, 50, or 60 rpm respectively, photographs were immediately taken from “top-down” from the headspace of the vessel (using a standard digital camera at daylight illumination optimized for good contrast) to capture distribution patterns of suspended carriers.
Human iPSCs
Experiments were performed using hiPSCs generated from cord blood-derived endothelial cells, particularly the line hCBiPSC2.1 Prior to suspension cultures hiPSCs were maintained using standard feeder layer depended culture conditions as previously described.1
Suspension cultures of human iPSCs
To initiate suspension cultures, hiPSC colonies were detached from a mitotic inactivated fibroblast feeder layer by collagenase IV (0.2%; Invitrogen) treatment and washed once with PBS without Ca2+/Mg2+ following a published protocol.19 In brief, for single cell preparation, colonies were resuspended in collagenase B solution (1 mg/mL; Roche) and incubated for 15 min at 37° by gently shaking until a single cell solution was achieved. Cells were counted and suspended in mTeSR™ 1 (Stem Cell Technologies) supplemented with the ROCK inhibitor Y-27623 (10 μM; synthesized as previously described28). For passaging cells from suspension culture, aggregates were harvested by centrifugation, washed with PBS without Ca2+/Mg2+, and dissociated by collagenase B treatment. Viable cell numbers were determined by trypan blue staining.
Static suspension cultures were performed on suspension culture six-well dishes (Greiner) as previously described {Olmer, 2010 #59}.
Bioreactor cultures
Process setup and feeding
Culture vessels were assembled and sterilized. Each vessel was equipped with probes for pO2 (220 mm; Broadley James) and pH (220 mm; Mettler Toledo). pH probes were calibrated by two point calibration. For pO2 probe calibration, vessels were filled with mTeSR 1 (100 mL) and aerated with air and 5% CO2 by headspace gassing under process conditions (stirring, 37°C) for at least 10 h. After stable values were reached a slope calibration was performed. For culture inoculation 25 mL of a single cell suspension at the respective cell concentration was added. From day 3, the entire media were replaced daily excluding any cell loss. A sampling volume of 3.5 mL culture per day (see below) was designedly not replaced to prevent culture dilution; this strategy resulted in subsequent culture volume reduction from 125 to 100 mL in a 7 day process.
Sampling, aggregate analysis, and statistics
Samples were taken daily (without interruption of stirring) via a luer-lock equipped sample port. To account for the clearance volume, 1.5 mL were discarded before 2 mL of the culture were collected in a 15 mL tube; after centrifugation supernatants were stored at −20°C for glucose, amino acids, and lactate analysis. Cells/aggregates were placed in a culture dish and three independent light microscopic images were captured from each sample at respective magnification (Axiovert A1; Zeiss) followed by image J (1.43 μ) processing for aggregate diameter analysis. Results were further analyzed by GraphPad Prism 5 to determine aggregate size distribution. Mean diameters represent the arithmetic average of 400 to 700 single aggregates.
For cell counting and flow cytometry, aggregates were washed with PBS without Ca2+/Mg2+ and dissociated by enzymatic treatment with collagenase B.
Results are reported as mean±SEM. Values with p<0.05 were considered statistically significant. One way analysis of variance and Bonferroni multiple comparison tests were performed with GraphPad Prism5.
Flow cytometry
Single cell suspensions were prepared and aliquots were incubated with a primary antibody against TRA 1-60 (1:100; mouse IgM, Abcam) and corresponding isotype control for 30 min at 4°C. After washing, cells were incubated with the corresponding secondary antibody Cy™3-labeled donkey anti-mouse IgM (1:200; Jackson Immunoresearch Laboratories) for 30 min at 4°C. Cells were analyzed using a FACSCalibur analyzer (BD Bioscience) and data were processed using WinMDI 2.9 software.
Real-time polymerase chain reaction
Total RNA was prepared using RNeasy Kit (Macherey-Nagel, Düren, Germany) and reverse transcribed with RevertAid™ H Minus (Fermentas, Thermo Scientific) using random primers according to the manufacturer's instructions. Reverse transcriptase-polymerase chain reaction (RT-PCR) and quantitative real time PCR (qPCR) were performed as described.1 Sequences and specifications of primers are shown in Supplementary Tables S1 and S2. Expression levels of target genes were normalized to β-actin transcript levels, respectively. Data are shown as mean±SEM of normalized gene expression levels from three experiments.
Glucose, lactate, and amino acids analysis
Glucose and lactate levels were determined in the supernatant using YSI 2700 Select™ Biochemistry Analyzer (YSI Incorporated Life Sciences) and concentrations of amino acids (alanine, arginine, asparagine, aspartic acid, glutamic acid, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, tyrosine, serine, and valine) were determined by high-performance liquid chromatography.
In vitro differentiation
Aggregates from day 7 cultures were plated onto 0.1% gelatine coated dishes in differentiation media (80% IMDM, Invitrogen; 20% fetal calf serum, HyClone;1 mM L-glutamine, 0.1 mM β-mercaptoethanol, and 1% nonessential amino acid stock, all Invitrogen) and cultured for 3 weeks (applying media change every 3–4 days) before immunocytology was performed to assess lineage differentiation.
Generation of cryosections
Aggregates were harvested, washed once with PBS without Ca2+/Mg2+, embedded in Tissue-Tek® (Sakura Finetek), and stored at −80°C. Subsequently 10μm cryosections were generated using a microm cryostar HM 560 (Thermo Scientific) cryotome.
Immunocytological staining
Immunostainings were performed as described.29 In brief, cells and cryosections were fixed with 4% paraformaldehyde for 20 and 5 min, respectively at room temperature and subsequently blocked with 5% donkey serum and 0.25% Triton X-100 (Sigma-Aldrich) diluted in Tris-buffered saline for 20 min at room temperature. Cells and cryosections were incubated for 1 h at room temperature with respective primary antibodies diluted in PBS without Ca2+/Mg2+ with 1% bovine serum albumin (1:300 anti-α-Fetoprotein, mouse IgG1; R&D Systems; 1:400 anti-β-Tubulin mouse IgG2a, Upstate; 1:100 anti-Troponin T mouse IgG2a, Thermo scientific; 1:100 anti-Oct4 mouse IgG2b, Santa Cruz Biotechnology Inc.; 1:100 anti-SSEA3 mouse IgM clone MC-631, Developmental Studies Hybridoma Bank; 1:50 anti-Ki-67 mouse IgG1, Dako Denmark) and then rinsed thrice with PBS without Ca2+/Mg2+. After incubation with secondary antibody (donkey anti-mouse IgG Cy3-labeled, Jackson Immunoresearch Laboratories; 1:100 in PBS, 1% bovine serum albumin) for 30 min at room temperature cells and cryosections were rinsed, counterstained with DAPI (Sigma-Aldrich), and analyzed with camera equipped Axio Observer A1 fluorescencemicroscope (Zeiss).
Terminal deoxynucleotidyl transferase dUTP nick end labelling assay
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay (in situ cell death detection kit, fluorescein ROCHE) for detection of DNA fragmentation indicative of apoptosis was performed according to the manufacturer's instructions. In brief, cryosections of aggregates were fixed with 4% paraformaldehyde, permeabilized with 0.1% sodium citrate, 0.1% Triton X, stained with TUNEL reaction mixture, counterstained with DAPI (Sigma-Aldrich,), and analyzed with camera equipped Axio Observer A1 fluorescencemicroscope (Zeiss).
Results
Adapting a stirred bioreactor system for the expansion of pluripotent human stem cells
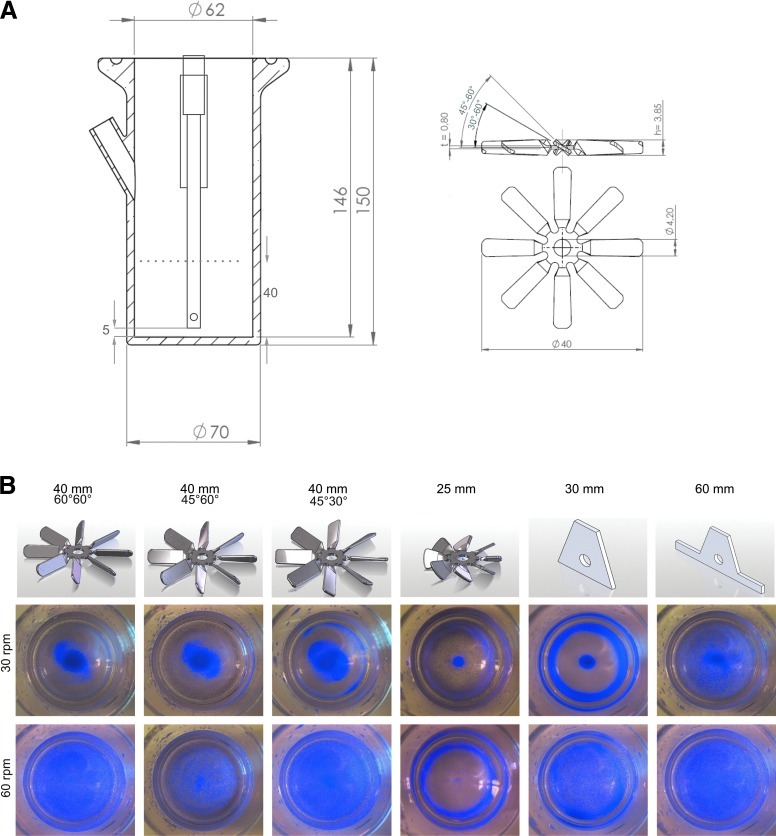
A parallel bioreactor system consisting of 4 independently controlled vessels with a maximal working volume of 250 mL each was applied. To reduce medium throughput but ensure functional integration of all probes (i.e., for monitoring pO2 and pH) experiments were performed at 100 mL culture volume; further reduction to 75 mL was found to be inapplicable for consistent processes operation (data not shown). A key property of established stirred cultures is the homogeneous distribution of all components in the reactor vessel avoiding local cell agglomeration or attachment. Triangular Teflon stirrer with diameters of 40 mm and 60 mm and blade impellers with diameters of 25 mm and 40 mm were tested (Fig. 1). For the 40 mm blade impeller three different designs were developed as defined by respective variations of blade angles, that is, 45° (centre) 60° (edge), 45° (centre) 30° (edge), and 60° (both; Fig. 1). To identify the stirrer-dependent “just suspended speed” (JSS; 30), Coomassie-stained microcarriers were utilized as aggregate surrogates, which represented cell aggregate-like sedimentation features (data not shown). Agitating at 30 revolutions per minute (rpm) did not lead to homogeneous carrier distribution (Fig. 1 upper row); local accumulation was found in the center and at the borders of the vessel (see distribution pattern in Fig. 1 and Supplementary Fig. S2). Stepwise, increasing the agitation speed to 60 rpm resulted in a more homogenous distribution of microcarriers applying the 40 mm impellers and the 60 mm stirring bar (Fig. 1 lower row and Supplementary Fig. S2), whereas the other stirring devices failed to achieve homogenous mixing even at higher rotational speeds tested (Fig. 1 and data not shown).
FIG. 1.
Reactor vessel and pitched blade impeller(s) design and geometry. Impact of stirrer design and stirring speed on microcarrier distribution. All dimensions of reactor and impeller geometry are shown in mm, t=thickness, h=height. The liquid level at 125 mL culture volume is indicated as a dashed line. Impellers were fixed to the bottom of the axis thereby reducing the free space from 5 mm to 1.2 mm relative to the impeller tips (A). To evaluate suitable stirring conditions Coomassie-stained microcarrier were used as cell aggregate surrogate. Design and dimension of tested stirring devices is depicted in the upper row. To equilibrate microcarrier distribution in 100 mL buffer volume in the reactor vessel 10 min stirring at 30 or 60 rpm was performed, respectively. Immediately thereafter, photographs were taken from the top to the vessel bottom to capture pattern of carrier distribution. Homogenous distribution was obtained with all 40 mm impellers and the 60 mm straight stirring bar at a rotational speed of 60 rpm (B). Color images available online at www.liebertonline.com/tec
Based on these observations the 40 mm blade impellers and the 60 mm Teflon stirrer deemed suitable for cell culture experiments. However, due to a more universal applicability of blade impellers in stirred tank reactors (regarding future scale up) only these stirring devices were tested in cell culture experiments.
Controlling aggregate formation by impeller design
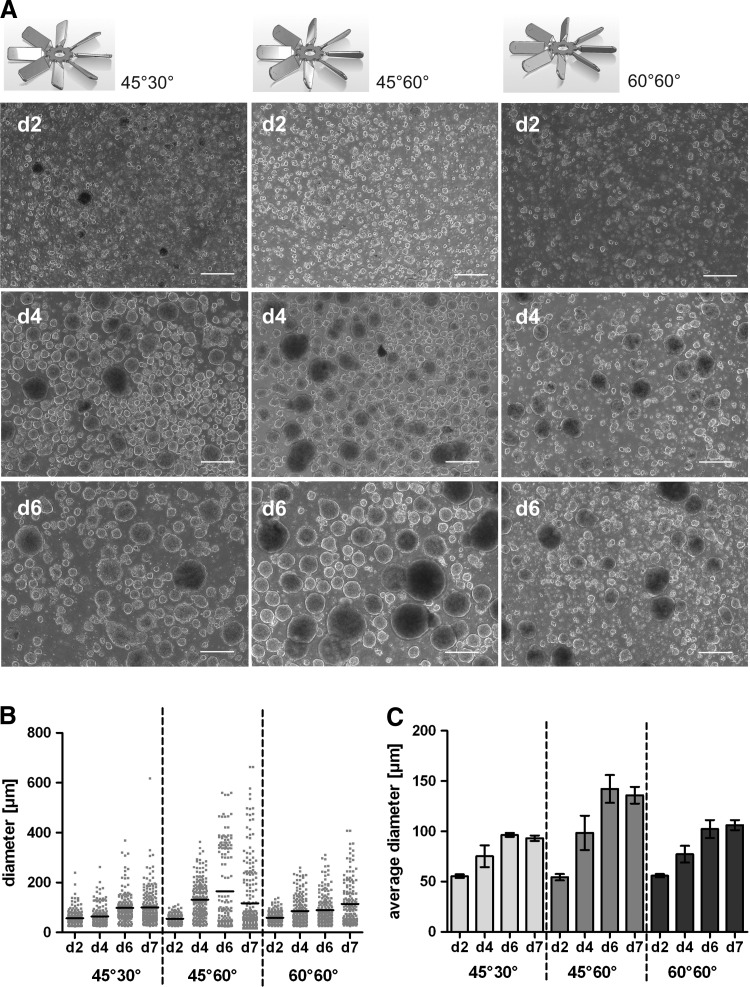
Expansion cultures of undifferentiated hiPSCs were inoculated as single cell suspensions (4–5×105 single cells/mL, d 0) in mTeSR supplemented with 10 μM RI in a total volume of 125 mL and stirred at 60 rpm. To evaluate the impact of the three 40 mm impeller design variations, images of forming cell aggregates were harvested at day 2, 4, 6, and 7 post inoculation (Fig. 2A) and aggregate diameter distribution and the mean were determined (Fig. 2B, C). On day 2, small cell aggregates were obtained with no remarkable differences between the respective impeller designs. On day 4, a mean diameter of 98 μm was found in cultures stirred with the 45°60° impeller (mean diameters are represented by black lines in Fig. 2B) compared to 75 μm and 77 μm for the 45°30° and 60°60° stirrers, respectively; on day 7, mean diameters were increased to 125 μm (45°60°), 93 μm (45°30°), and 106 μm (60°60°). Thorough analysis revealed that a substantially higher aggregate heterogeneity accounted for the higher mean diameter calculated in 45°60° impeller stirred cultures, as compared to the (desired) relatively narrow aggregate size distribution range in 60°60° and 45°30° impeller stirred cultures, respectively (Fig. 2B), but statistical analysis revealed no significance of this aspect. Notably, stagnation of the average aggregate size increase was found when comparing day 6 and 7 results (Fig. 2C, for all three impeller types) although the increase in cell numbers was retained (Fig. 3).
FIG. 2.
Aggregate formation and size distribution depending on impeller designs. Single cell-inoculated cultures (5×105 cells/mL; day 0) were stirred at 60 rpm with respective impeller designs displayed in the upper row. On day 2, 4, and 6 aggregates were assessed by light microscopy as exemplary shown in (A; scale bars=200 μm); applying image J and GraphPad prism software, three independent images were analyzed from each individual run at all time points measured. Impeller design-dependent distribution of aggregate diameters on days 2, 4, 6, and 7 representing a typical run is shown in (B) whereby black bars indicate calculated mean of aggregate size, respectively. In (C) the impeller- and time-dependent average of aggregate diameter is shown whereby each column represents 2–3 independent reactor runs.
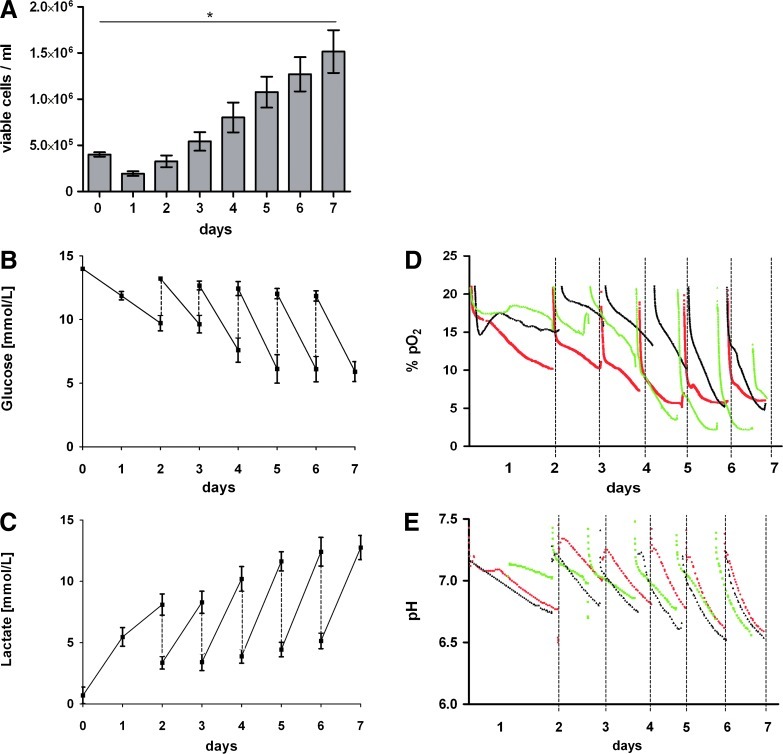
FIG. 3.
Growth kinetics and metabolic activity of human induced pluripotent stem cells (hiPSCs) in stirred suspension culture. Cells were seeded at 4–5×105 cells/mL (day 0) and cell numbers were determined daily. Up to 5.5-fold increase in cell numbers was achieved in individual runs over 7 days resulting in 2.4×106 cells/mL. Columns in (A) represents n=8 independent experiments. Culture media were replaced daily from day 3 onward (dashed lines) resulting in respective glucose and lactate concentration patters shown in (B) and (C) respectively (n=5 independent reactor runs). (E) To ensure clarity of illustration, oxygen tension (D) and pH levels (E) are exemplary depicted of three independent reactor runs only. pH levels were 7.4–7.3 on day 0 and after each medium change but dropped to 6.5 at the end of a 24 h feeding interval on days 5–7. Color images available online at www.liebertonline.com/tec
Growth kinetic revealed highly robust and reproducible greater than fourfold expansion of human iPS cells
A significant increase in cell numbers of up to 5.5-fold has been achieved over 7 days in stirred suspension cultures whereby greater than fourfold expansion of inoculated cells was found on average (n=8). Since expansion rates were found to be equivalent for all impeller type variations, Figure 3A summarizes growth kinetics of eight independent experiments including results of all three impeller designs applied. Metabolic activity was monitored by determining glucose, lactate, and amino acids concentrations and pH and pO2 levels (Fig. 3B–E), Supplementary Fig. S3). Initially, after the first 2 days without media change, about 40% of glucose was depleted (Fig. 3A). Performing daily media replacement from day 3 onward, no more than 50% of glucose consumption was measured. The expected lactate accumulation did not exceed concentration of 13 mM. As shown in Figure 3, increasing cell numbers over time resulted in a maximum pH drop to 6.5 (compared to 7.4 in fresh media) despite daily culture media replacement. Amino acid analysis revealed, besides glutamine, only moderate consumption of serine, asparagine, arginine, histidine, glycine, leucine, and isoleucine and some accumulation of alanine, aspartate, and glutamate (Supplementary Fig. S3); no particular amino acid was completely depleted from the media in reflection to our feeding strategy. To achieve a substantial cell mass expansion and to test feasibility of sequential passaging in our bioreactor setting, reseeding of harvested cells after the first expansion in stirred culture was performed. Growth kinetic observed in the following expansion process was comparable to the first reactor run (Supplementary Fig. S3). Harvested cells from both passages showed equivalent expression of the pluripotency-associated surface marker TRA 1-60 and monitoring of pH and pO2 levels confirmed similar metabolic activity in these subsequent processes (Supplementary Fig. S3).
Cells from stirred cultures retain their pluripotency and differentiation capacity in vitro
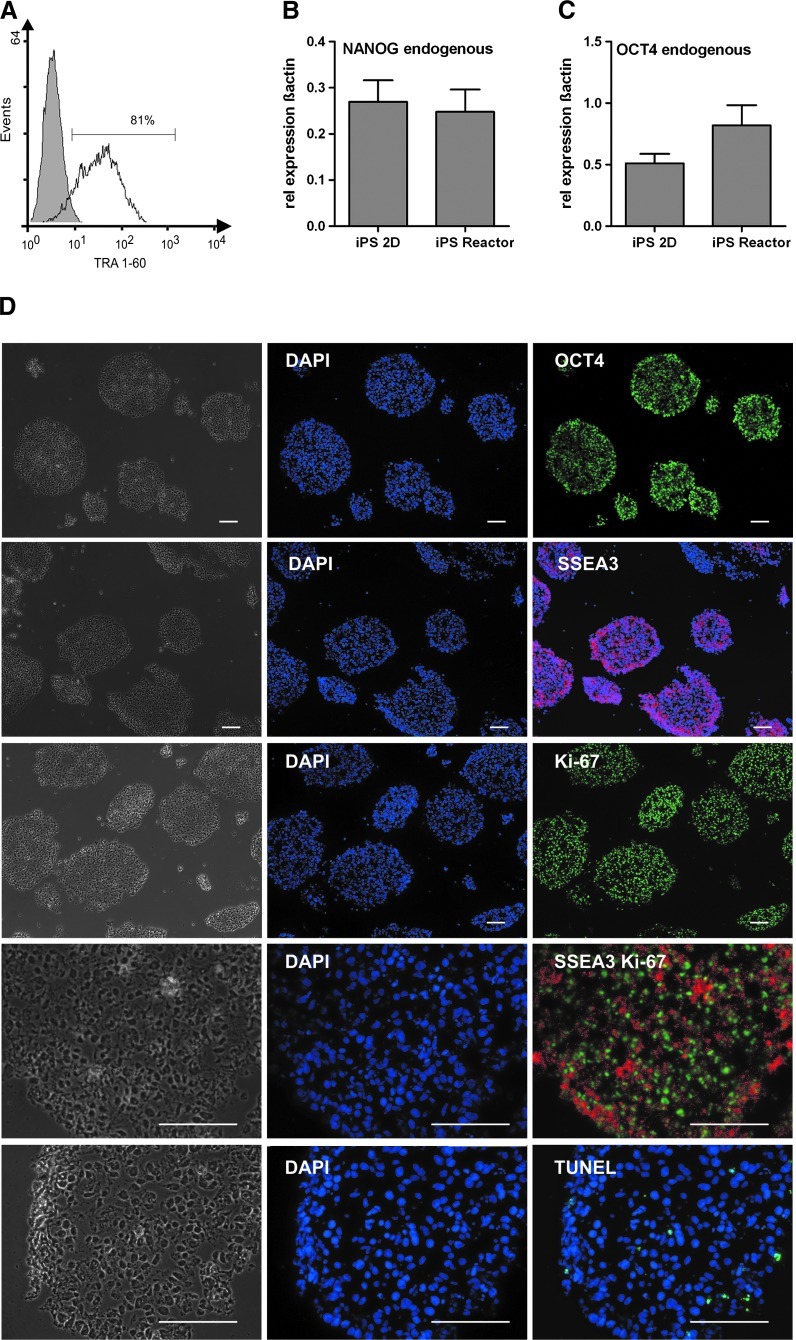
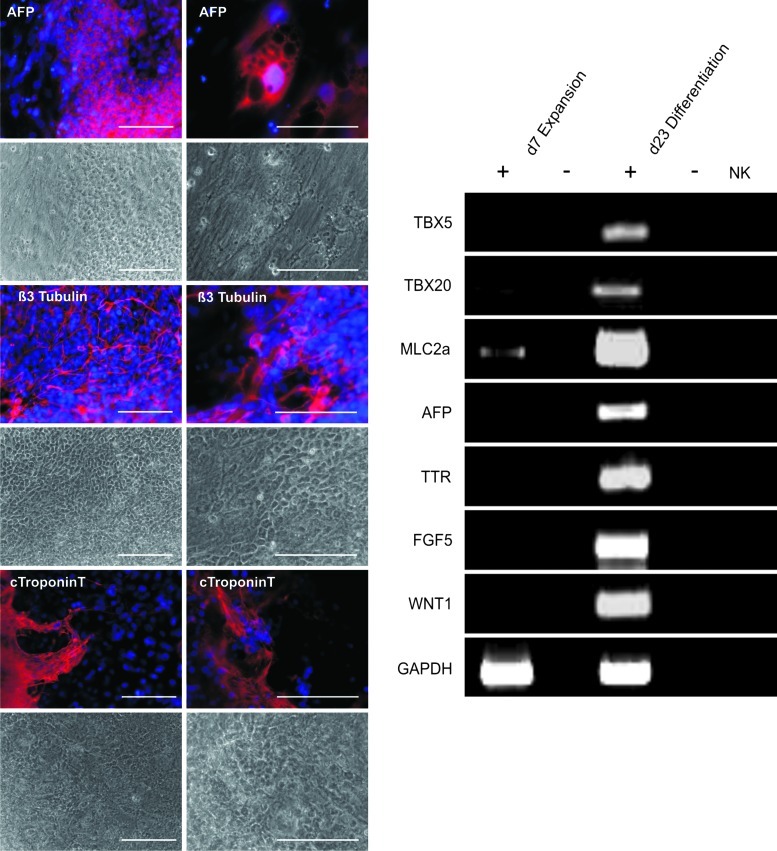
Indicative of maintenance of pluripotency of bioreactor-derived cells harvested on day 7 was demonstrated by high TRA 1-60 positivity via flow cytometry (Fig. 4A) and OCT4 and NANOG expression levels (qRT-PCR) that were equivalent or higher compared with conservative feeder-based control cultures (Fig. 4B, C). Homogenous staining for the pluripotency-associated markers SSEA3 and OCT4 on cryosections of day 7 aggregates (Fig. 4D) further supports the flow cytometry and qRT-PCR data. Moreover, the extensive staining for Ki-67 on sections underlines the highly proliferative state of cells in aggregates, even on day 7 of culture (Fig. 4D). Detection of apoptosis by the TUNEL assay showed no obvious local accumulation of positive cells neither at the border nor inside of the bioreactor-derived aggregates and the level of positive cells was comparable to static controls (Fig. 4D and Supplementary Fig. S4). By directly plating day 7-derived cell aggregates onto culture dishes spontaneous differentiation was assessed. After 23 days of differentiation, cells were stained for markers of all three germ layers. As exemplarily shown for positive staining specific to alpha-fetoprotein (AFP; endoderm), cardiac TroponinT (mesoderm), and ß3 Tubulin (ectoderm) expected multilineage differentiation was found. These data were supported by RT-PCR analysis demonstrating expression of additional differentiation markers including TBX5, TBX20, MLC2a (mesoderm), AFP, TTR (endoderm), FGF5, and WNT1 (ectoderm) in hiPSCs expanded in stirred bioreactor cultures (Fig. 5).
FIG. 4.
Pluripotency markers expression of bioreactor-expanded hiPSCs. Flow cytometry revealed that the majority of cells expressed pluripotency-associated surface marker TRA 1-60 (A; isotype control shown in gray). Quantitative real time analysis for transcription factors NANOG and OCT4 showed no significant deviation in expression levels of reactor-expanded cells compared to conventional 2D feeder based cultures (B–C). Primers were defined to detect endogenous variant of the factor to exclude false positive results in induced pluripotent stem cells (see Methods for details). Immunofluorescence of cryosectioned aggregates revealed positive staining for the pluripotency-associated markers SSEA3 and OCT4 and the proliferation-associated marker Ki-67. Some degree of apoptosis was indicated by TUNEL assay positive cells within the aggregates. Respective isotype/negative controls confirmed specificity of stainings (data not shown). Scale bars=100 μm (D).
FIG. 5.
Bioreactor-expanded hiPSCs differentiate into derivatives of all three germ layers. Immunofluorescence analysis (upper row; phase contrast microscopy of respective spots below) of day 7 bioreactor-derived hiPSC aggregates induced for spontaneous differentiation revealed expression of marker proteins representative of all three germ layers (positive staining in red); alpha-fetoprotein (AFP, endoderm), cTroponinT (mesoderm), and ß3 Tubulin (ectoderm). Nuclei were stained with DAPI (blue). Respective isotype controls confirmed specificity of stainings (data not shown). Scale bars=100 μm. Upregulation of ectodermal, endodermal, and mesodermal markers was also confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) on undifferentiated cells (day 7 aggregates) and cells after 23 day of differentiation. To exclude false-positive results due to DNA contamination, RT (-) controls were included.
Discussion
Initiating cultures with single cells, in contrast to semi-dissociated colonies or clumps, is a preferred mode of process inoculation to ensure fully defined starting conditions and subsequently improved process control and reproducibility.
In the previous work focusing mainly on static conditions we have established single cell- inoculated suspension culture of hPSCs in defined media (mTeSR) supplemented with a ROCK inhibitor to ensure single cell survival.19,21 Inoculation at relative low density, that is 5×104 cells/mL, enabled cell aggregation in a highly robust and reproducible manner and resulted in substantial approximately sixfold hPSC expansion in 4 days. At static conditions, however, single cells rapidly collect by gravity at the bottom of a (low attachment) dish. Due to surface tension effects, cells further locally accumulate at the center of a dish resulting in cell–cell contact-dependent aggregate formation even at relative low inoculation density.21 On the downside, such static conditions often enhance uncontrolled mass aggregation of cells into heterogeneous, relative large clumps as described for mouse ESCs31,32 and to some extent for hPSCs as well.21
In the current study, inoculation of stirred tanks with 5×104 hiPSCs/mL in preliminary experiments led to irreproducible results; aggregate formation was either heterogeneous or failed completely (data not shown). This might suggest that in impeller stirred cultures, particularly at relative low inoculation density, the likelihood of cell aggregation is diminished and unpredictable due to a number of potential reasons. These include: (i) homogeneous cell distribution in stirred suspension (3D) avoids local cell accumulation (in contrast to “pseudo 3D” in static suspension culture) thereby diminishing the likelihood of cell proximity, (ii) hydrodynamic forces interfere with the formation of stable cell–cell contacts mandatory for successful aggregation, and finally, (iii) hydrodynamic stress might be particularly detrimental to freshly dissociated single hPSCs, which further lowers the number of remaining vital cells that have the potential to form aggregates and to proliferate.
In another study aimed at comparing static versus dynamic conditions, hESCs were single cell inoculated at 5×105 cells/mL in mTeSR.20 Efficient cell aggregate formation in suspension was achieved whereby cell line-dependent expansion rates at static conditions were 2.5–3-fold. Translation to rotated Petri dishes supported formation of more homogeneous aggregates but expansion rates notably dropped to 1.5–2-fold20 suggesting relatively high susceptibility of hPSCs to mechanical forces (i.e., cells rolling over the surface of rotated dishes). However, regarding the inoculation density, Serra and coworkers also suggest a concentration of 4.5×105 cells/mL to ensure homogenous distribution of human PSC in suspension culture, whereby microcarrier-dependent cultivation was notably tested.33
Applying “lessons learned” from these previous attempts and from failed preliminary reactor runs at low inoculation density (i.e., 5×104 cells/mL) as well, we have established 5×105 single hPSC/mL being a useful starting point for impeller stirred cultures in the applied setup. Despite hydrodynamic and physical forces a greater than fourfold hPSC expansion was achieved on average in a large number of stirred culture processes. This is substantially higher compared to 1.5–2-fold expansion rates observed in rotated Petri dishes 20 applying equivalent culture conditions (i.e., same inoculation density, culture media, feeding strategy, and culture duration), which underscores utility of the process developed in our current work.
In addition to the inoculation density, another key factor is the choice of agitation conditions, that is, the impeller type and rotational speed, as it has to fulfill a number of potentially conflicting goals in our process. These include: (i) homogeneous distribution of all culture components (i.e., cells, media, and gas), (ii) enabling aggregation of cells, (iii) controlling aggregate dimension and homogeneity, (iv) maintaining aggregates in complete suspension, and finally, (v) limiting mechanical constraints on cells/aggregates. As a starting point we have built on our experience with mouse ESC suspension culture. An axial 8-blade pitched impeller was previously designed and successfully applied to control single cell-inoculated mESC aggregate formation in a stirred 2 L tank reactor.34,35 Here, we have downscaled dimensions of this impeller aiming at geometric similarity of the reactor to impeller relation from the 2 L reactor35 to our current 250 mL vessel. For all three derivatives of the original impeller design JSS was 60 rpm when tested with cell-free microcarriers (utilized as aggregate surrogates to provide a fast but meaningful initial assessment of stirrer features). Importantly, applying this rotational speed, aggregate formation from single cell-inoculated hPSCs was enabled. Moreover, a desired relative narrower range of aggregate size distribution was achieved, with slight but nonsignificant variation between design alternatives. When optimizing the stirring speed for mESC aggregation, 65 rpm was found being optimal regarding several parameters such as the cell expansion rate, aggregate size, and homogeneity of aggregate size distribution35 (note that 35, 65, 80, and 125 rpm were tested in that study). At 65 rpm in the 2 L culture the average aggregates size for mESC was ∼250 μm at day 7 post inoculation. Utilizing the downscaled impeller design at 60 rpm in our present study (i.e., in 100 mL culture volume) led to an average hPSCs aggregate size of 100–150 μm on day 7. This suggests that geometric downscaling resulted in the formation of an equivalent aggregate size range for mouse and human PSCs despite substantial species differences and composition of culture media as well.35–38 Although mechanical constraints were not quantified (because calculation of key parameters such as the “impeller power number” and the related factor “power per volume” requires very accurate power readings39 in watt [W], for which the applied bioreactor systems are not specified) these observations indicate equivalent (hydrodynamic and mechanical) conditions in both reactor systems, which suggest that straightforward 20-fold scale up of the established process from 100 mL to the 2 L dimension deems feasible; experimental confirmation, however, will be essential. Below the line, combining distinct inoculation and agitation conditions, a highly reproducible process yielding 2×108 pluripotent human stem cells in 100 mL culture volume (∼2×106 cell/mL on day 7) was established, whereby ∼670 mL media are consumed. This represents a substantial step toward the envisioned, well controlled mass expansion of hPSCs, particularly considering the defined culture media and relative universal and easy-to-scale tank reactor setup applied. Importantly, in line with previous studies,13,19,40 the majority of bioreactor-expanded cells remained pluripotent. The minor proportion of cells that was negative for SSEA3 or TRA 1-60 specific staining might reflects some background level of differentiation that is typically also detected in feeder layer-dependent cultures and is therefore not specific to our novel suspension culture conditions. Homogenous staining patterns even in aggregates with diameters larger than 200 μm were observed for the pluripotency-associated markers and the proliferation-associated Ki-67-specific staining. These findings strongly suggest that the aggregate-dependent culture did not interfere with the maintenance of cell pluripotency and proliferation. Further, cells that were expanded under these culture conditions were able to differentiate into cells from all three germ layers.
However, growth kinetic observed in our system showed a more linear increase in cell numbers rather than an exponential cell growth that is expected under optimal conditions. This observation suggests inhibitory, limiting, and/or detrimental effects that might be dependent on a number of factors. Analysis of amino acids and glucose consumption suggested no limits of these nutrients in consequence to the daily media exchange strategy. On the other hand, from culture day 4 onward, a relative substantial pH drop to 6,5 was observed accompanied with some level of lactate accumulation and reduction in dissolved oxygen, despite the moderate cell concentration at that culture stage. In line with other studies this suggests an elevated metabolic activity of cells cultured at dynamic conditions as compared to static controls.41 On aggregate cross-sections some homogeneously distributed TUNEL assay-positive cells were observed. These findings apparently suggest some degree of culture-dependent apoptosis that might explain the rather linear but not exponential increase in cell numbers (shown in Fig. 3). On the other hand, prominent local cell death in the center of aggregates, which might have indicated lack of nutrition, was not indicated by the TUNEL assay. Interestingly, comparison to control aggregates from static suspension culture also revealed no apparent difference in the distribution pattern and incidence of TUNEL assay-positive cells on respective sections. This might also argue against an increase of shear stress-induced apoptosis in stirred cultures.
Regarding the impact of oxygen, recent studies showed that culturing hPSCs or mESCs under reduced oxygen (2%–5%, sometimes referred to as physiological oxygen concentration) resulted in reduced cell proliferation.33,42 Thus, since in our study oxygen levels dropped to 5%–7% pO2, especially from day 5 onward, some degree of growth retardation cannot be excluded.33,43 However, controversial data were presented by other investigators,44 suggesting that this aspect might vary in different systems requiring more in detail investigation in future studies. Another more local aspect of oxygen limitation (and other soluble gases, nutrients, and metabolites as well) relates to diffusion gradients that might arise in spherical structures of > 300 μm diameter (recently reviewed by Kinney et al.45). In spinner flasks stirred by a bulb-shaped device large hES cell aggregates of > 500 μm diameter were formed whereby diffusion limits might explain the low expansion rates of ∼1.5–2-fold per passage and some degree of cell differentiation observed in that study.20 In contrast, in the present work, aggregate size did not exceed 200 μm that makes diffusion limits unlikely and supports usability of the process for hPSC expansion.
Considering published data it seems unlikely that the maximal lactate concentration of 13 mM found by us had a profound impact on cell proliferation.40,46 An evaluation by Chen et al.40 showed no remarkable influence on human ES cell growth rates at 11 mM lactate concentration (1 g/L); only supplementation of 22 mM lactate (2 g/L) or more induced substantial growth retardation. But no differential assessment of the lactate concentration and the interlinked change in pH values was performed by Chen and coworkers.40 This makes it difficult to assign such effects directly to the concentration of the metabolic product or, more indirectly, to the induced pH drop. Our preliminary experiments on controlling the pH by addition of base were not conclusive (data not shown). Although in depth analysis of this factor is required, a general transition from batch feeding to continuous media exchange, which has been shown to have substantial impact on hPSC culture and differentiation control,33,34,47 is probably a more meaningful amelioration in future studies.
Finally, mechanical and hydrodynamic forces, despite the initiated impeller design optimization, might have interfered with exponential cell growth in our process vis-a-vis above discussion on the observed hPSC sensitivity to mechanical stress. Trials with limited stirring speed < 60 rpm were performed. But local cell agglomeration at the vessel bottom was observed shortly after inoculation when stirring at lower speed, making further experiments pointless (data not shown). Further testing of alternative axial impeller designs such as the TTP or the Elephant Ear impeller, which have been shown to produce the smallest mechanical constraints at their just-suspended speed compared to five other models,30 is expedient. In addition, supplementation of cytoprotective additives such as pluronic F68, methylcellulose, or polyethylene glycol that have been shown to lower cell sensitivity to hydrodynamic stresses48,49 might be worthwhile testing.
Conclusion
The successful transfer of single cell-inoculated suspension cultures of hPSCs to fully controlled, stirred bioreactors offers the opportunity of detailed online monitoring, evaluation, and optimization of complex, multifactorial culture parameters. The achieved yield of 2×108 pluripotent cells in a single process run represents new dimensions in cell number production that are now sufficient for preclinical studies in physiological relevant animal models such as pigs. The process is characterized by a defined culture media and high reproducibility of individual runs that are prerequisites for clinical and industrial translation of hPSC-technology. Further, the linear scalability of the applied stirred tank reactors facilitates easy scale up to much higher culture volumes without extensive process adaptations.
Supplementary Material
Acknowledgments
This work was supported by the initiatives: (1) BIOSCENT [European Union; European Atomic Energy Community] Seventh Framework Program ([FP7/2007–2013] [FP7/2007–2011]) under grant agreement no. [214539], (2) Autologous Heart Tissue for Myocardial Repair (01GN0958), and (3) BIO-DISC (0315493) of the Federal Ministry of Education and Research (BMBF).
The authors are grateful to Martina Weiss and Maria Ensthaler for providing technical assistance.
Author Contributions
Ruth Olmer: Collection and assembly of data, data analysis and interpretation, and article writing
Andreas Lange: Collection of data
Sebastian Selzer: Provision of study material
Cornelia Kasper: Collection of data
Axel Haverich: Conception and design
Ulrich Martin: Conception and design, article writing, data analysis and interpretation, and financial support
Robert Zweigerdt: Conception and design, article writing, and data analysis and interpretation
Disclosure Statement
No competing financial interests exist.
References
- 1.Haase A. Olmer R. Schwanke K. Wunderlich S. Merkert S. Hess C., et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Seissler J. Schott M. Generation of insulin-producing beta cells from stem cells—perspectives for cell therapy in type 1 diabetes. Horm Metab Res. 2008;40:155. doi: 10.1055/s-2007-1022553. [DOI] [PubMed] [Google Scholar]
- 4.Jing D. Parikh A. Canty J. M., Jr. Tzanakakis E.S. Stem cells for heart cell therapies. Tissue Eng Part B Rev. 2008;14:393. doi: 10.1089/ten.teb.2008.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gandolfi F. Vanelli A. Pennarossa G. Rahaman M. Acocella F. Brevini T.A. Large animal models for cardiac stem cell therapies. Theriogenology. 2011;75:1416. doi: 10.1016/j.theriogenology.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Zweigerdt R. Large Scale Production of Stem Cells and Their Derivatives. Adv Biochem Eng Biotechnol. 2009;114:201. doi: 10.1007/10_2008_27. [DOI] [PubMed] [Google Scholar]
- 7.Amit M. Carpenter M.K. Inokuma M.S. Chiu C.P. Harris C.P. Waknitz M.A., et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 8.Amit M. Itskovitz-Eldor J. Feeder-free culture of human embryonic stem cells. Methods Enzymol. 2006;420:37. doi: 10.1016/S0076-6879(06)20003-X. [DOI] [PubMed] [Google Scholar]
- 9.Stojkovic P. Lako M. Przyborski S. Stewart R. Armstrong L. Evans J., et al. Human-serum matrix supports undifferentiated growth of human embryonic stem cells. Stem Cells. 2005;23:895. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- 10.Stojkovic P. Lako M. Stewart R. Przyborski S. Armstrong L. Evans J., et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- 11.Nienow A.W. Reactor engineering in large scale animal cell culture. Cytotechnology. 2006;50:9. doi: 10.1007/s10616-006-9005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kehoe D.E. Jing D. Lock L.T. Tzanakakis E.M. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng Part A. 2009;16:405. doi: 10.1089/ten.tea.2009.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh S.K. Chen A.K. Mok Y. Chen X. Lim U.M. Chin A., et al. Long-term microcarrier suspension cultures of human embryonic stem cells. Stem Cell Res. 2009;2:219. doi: 10.1016/j.scr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Phillips B.W. Horne R. Lay T.S. Rust W.L. Teck T.T. Crook J.M. Attachment and growth of human embryonic stem cells on microcarriers. J Biotechnol. 2008;138:24. doi: 10.1016/j.jbiotec.2008.07.1997. [DOI] [PubMed] [Google Scholar]
- 15.Souza M.C. Freire M.S. Schulze E.A. Gaspar L.P. Castilho L.R. Production of yellow fever virus in microcarrier-based Vero cell cultures. Vaccine. 2009;27:6420. doi: 10.1016/j.vaccine.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Genzel Y. Olmer R.M. Schafer B. Reichl U. Wave microcarrier cultivation of MDCK cells for influenza virus production in serum containing and serum-free media. Vaccine. 2006;24:6074. doi: 10.1016/j.vaccine.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Amit M. Chebath J. Margulets V. Laevsky I. Miropolsky Y. Shariki K., et al. Suspension culture of undifferentiated human embryonic and induced pluripotent stem cells. Stem Cell Rev. 2010;6:248. doi: 10.1007/s12015-010-9149-y. [DOI] [PubMed] [Google Scholar]
- 18.Krawetz R. Taiani J.T. Liu S. Meng G. Li X. Kallos M.S., et al. Large-Scale expansion of pluripotent human embryonic stem cells in stirred suspension bioreactors. Tissue Eng Part C Methods. 2010;16:573. doi: 10.1089/ten.TEC.2009.0228. [DOI] [PubMed] [Google Scholar]
- 19.Olmer R. Haase A. Merkert S. Cui W. Palecek J. Ran C., et al. Long term expansion of undifferentiated human iPS and ES cells in suspension culture using a defined medium. Stem Cell Res. 2010;5:51. doi: 10.1016/j.scr.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Singh H. Mok P. Balakrishnan T. Rahmat S.N. Zweigerdt R. Up-scaling single cell-inoculated suspension culture of human embryonic stem cells. Stem Cell Res. 2010;4:165. doi: 10.1016/j.scr.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Zweigerdt R. Olmer R. Singh H. Haverich A. Martin U. Scalable expansion of human pluripotent stem cells in suspension culture. Nat Protoc. 2011;6:689. doi: 10.1038/nprot.2011.318. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig T.E. Bergendahl V. Levenstein M.E. Yu J. Probasco M.D. Thomson J.A. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637. doi: 10.1038/nmeth902. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T., et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 24.Bigdeli N. Andersson M. Strehl R. Emanuelsson K. Kilmare E. Hyllner J., et al. Adaptation of human embryonic stem cells to feeder-free and matrix-free culture conditions directly on plastic surfaces. J Biotechnol. 2008;133:146. doi: 10.1016/j.jbiotec.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Eibl R. Kaiser S. Lombriser R. Eibl D. Disposable bioreactors: the current state-of-the-art and recommended applications in biotechnology. Appl Microbiol Biotechnol. 2010;86:41. doi: 10.1007/s00253-009-2422-9. [DOI] [PubMed] [Google Scholar]
- 26.Kalmbach A. Bordas R. Oncul A.A. Thevenin D. Genzel Y. Reichl U. Experimental characterization of flow conditions in 2-, 20-L bioreactors with wave-induced motion. Biotechnol Prog. 2011;27:402. doi: 10.1002/btpr.516. [DOI] [PubMed] [Google Scholar]
- 27.Xing Z. Kenty B.M. Li Z.J. Lee S.S. Scale-up analysis for a CHO cell culture process in large-scale bioreactors. Biotechnol Bioeng. 2009;103:733. doi: 10.1002/bit.22287. [DOI] [PubMed] [Google Scholar]
- 28.Palecek J. Zweigerdt R. Olmer R. Martin U. Kirschning A. Drager G. A practical synthesis of Rho-Kinase inhibitor Y-27632 and fluoro derivatives and their evaluation in human pluripotent stem cells. Org Biomol Chem. 2011;9:5503. doi: 10.1039/c1ob05332a. [DOI] [PubMed] [Google Scholar]
- 29.Mauritz C. Schwanke K. Reppel M. Neef S. Katsirntaki K. Maier L.S., et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008;118:507. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 30.Marie-Laure Collignon. Angélique Delafosse. Michel Crine. Toye Dominique. Axial impeller selection for anchorage dependent animal cell culture in stirred bioreactors: methodology based on the impeller comparison at just-suspended speed of rotation. Chem Eng Sci. 2010;65:5929. [Google Scholar]
- 31.Zandstra P.W. Bauwens C. Yin T. Liu Q. Schiller H. Zweigerdt R., et al. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]
- 32.Zweigerdt R. Burg M. Willbold E. Abts H. Ruediger M. Generation of confluent cardiomyocyte monolayers derived from embryonic stem cells in suspension: a cell source for new therapies and screening strategies. Cytotherapy. 2003;5:399. doi: 10.1080/14653240310003062. [DOI] [PubMed] [Google Scholar]
- 33.Serra M. Brito C. Sousa M.F. Jensen J. Tostoes R. Clemente J., et al. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J Biotechnol. 2010;148:208. doi: 10.1016/j.jbiotec.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Niebruegge S. Nehring A. Bar H. Schroeder M. Zweigerdt R. Lehmann J. Cardiomyocyte production in mass suspension culture: embryonic stem cells as a source for great amounts of functional cardiomyocytes. Tissue Eng Part A. 2008;14:1591. doi: 10.1089/ten.tea.2007.0247. [DOI] [PubMed] [Google Scholar]
- 35.Schroeder M. Niebruegge S. Werner A. Willbold E. Burg M. Ruediger M., et al. Differentiation and lineage selection of mouse embryonic stem cells in a stirred bench scale bioreactor with automated process control. Biotechnol Bioeng. 2005;92:920. doi: 10.1002/bit.20668. [DOI] [PubMed] [Google Scholar]
- 36.Kuijk E.W. Chuva de Sousa Lopes S.M. Geijsen N. Macklon N. Roelen B.A. The different shades of mammalian pluripotent stem cells. Hum Reprod Update. 2011;17:254. doi: 10.1093/humupd/dmq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnerch A. Cerdan C. Bhatia M. Distinguishing between mouse and human pluripotent stem cell regulation: the best laid plans of mice and men. Stem Cells. 2010;28:419. doi: 10.1002/stem.298. [DOI] [PubMed] [Google Scholar]
- 38.Sato N. Sanjuan I.M. Heke M. Uchida M. Naef F. Brivanlou A.H. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404. doi: 10.1016/s0012-1606(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 39.Singh H. Hutmacher D.W. Bioreactor studies and computational fluid dynamics. Adv Biochem Eng Biotechnol. 2009;112:231. doi: 10.1007/978-3-540-69357-4_10. [DOI] [PubMed] [Google Scholar]
- 40.Chen X. Chen A. Woo T.L. Choo A.B. Reuveny S. Oh S.K. Investigations into the metabolism of two-dimensional colony, suspended microcarrier cultures of human embryonic stem cells in serum-free media. Stem Cells Dev. 2010;19:1781. doi: 10.1089/scd.2010.0077. [DOI] [PubMed] [Google Scholar]
- 41.Collins P.C. Miller W.M. Papoutsakis E.T. Stirred culture of peripheral and cord blood hematopoietic cells offers advantages over traditional static systems for clinically relevant applications. Biotechnol Bioeng. 1998;59:534. doi: 10.1002/(sici)1097-0290(19980905)59:5<534::aid-bit2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes A.M. Fernandes T.G. Diogo M.M. da Silva C.L. Henrique D. Cabral J.M. Mouse embryonic stem cell expansion in a microcarrier-based stirred culture system. J Biotechnol. 2007;132:227. doi: 10.1016/j.jbiotec.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes T.G. Diogo M.M. Fernandes-Platzgummer A. da Silva C.L. Cabral J.M. Different stages of pluripotency determine distinct patterns of proliferation, metabolism, and lineage commitment of embryonic stem cells under hypoxia. Stem Cell Res. 2010;5:76. doi: 10.1016/j.scr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Niebruegge S. Bauwens C.L. Peerani R. Thavandiran N. Masse S. Sevaptisidis E., et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 45.Kinney M.A. Sargent C.Y. McDevitt T.C. The multiparametric effects of hydrodynamic environments on stem cell culture. Tissue Eng Part B Rev. 2011;17:249. doi: 10.1089/ten.teb.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ozturk S.S. Riley M.R. Palsson B.O. Effects of ammonia and lactate on hybridoma growth, metabolism, and antibody production. Biotechnol Bioeng. 1992;39:418. doi: 10.1002/bit.260390408. [DOI] [PubMed] [Google Scholar]
- 47.Fong W.J. Tan H.L. Choo A. Oh S.K. Perfusion cultures of human embryonic stem cells. Bioprocess Biosyst Eng. 2005;27:381. doi: 10.1007/s00449-005-0421-5. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z. al-Rubeai M. Thomas C.R. Effect of Pluronic F-68 on the mechanical properties of mammalian cells. Enzyme Microb Technol. 1992;14:980. doi: 10.1016/0141-0229(92)90081-x. [DOI] [PubMed] [Google Scholar]
- 49.Wu J. Mechanisms of animal cell damage associated with gas bubbles and cell protection by medium additives. J Biotechnol. 1995;43:81. doi: 10.1016/0168-1656(95)00133-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.