Abstract
Background
The impact of age on prognosis in advanced stage non-small cell lung cancer (NSCLC) may differ by sex.
Patients and methods
Eligible patients (N = 1590) from E1594, a 4-arm platinum-based chemotherapy trial, and E4599 (carboplatin/paclitaxel ± bevacizumab) chemotherapy arm were divided into male and female cohorts and separated into age groups of <60 or ≥60 years old. Eligible E4599 patients (N = 850) were similarly separated by age and sex and by treatment (±bevacizumab). Survival was calculated separately for each cohort.
Results
The median survival time (MST) for women ≥60 years old treated with chemotherapy alone on E1594 and E4599 was 11.6 months versus 9.0 months for women <60 (p = 0.03). MST was 7.4 and 8.3 months for men ≥60 and <60 years old respectively (NS). In E4599 the age <60 by bevacizumab treatment interaction was statistically significant (p = 0.03) for women (younger had greater benefit), with no age effect in men.
Conclusions
In this unplanned, exploratory subgroup analysis of advanced stage NSCLC ECOG trials, women ≥60 years old treated with chemotherapy live longer than men and younger women. In contrast, bevacizumab survival benefit was more pronounced in men of any age and in younger women on E4599.
Keywords: Age/sex interactions, Bevacizumab, Chemotherapy, Sex/gender differences, NSCLC, Prognostic factors
1. Introduction
Differential survival by sex in lung cancer, the leading cause of cancer death in both men and women, was first reported in the 1970s and since then, analyses of all stages have shown women with non-small cell lung cancer (NSCLC) living longer than men with the disease [1]. Multiple population based and institutional cohort studies have reported relative risks of death for men of 1.15–1.20 compared to women with NSCLC [2,3].
Retrospective analysis of cooperative group trials in advanced stage NSCLC in the 1970s and 1980s also showed improved survival for women compared to men [4,5]. More recently, we analyzed survival by sex on E1594, a large phase III study of nearly 1200 patients, which found no survival difference between 4 different chemotherapy doublets for the first line treatment of advanced stage NSCLC. We were thus able to pool all the arms together to determine if a sex effect on survival existed in this similarly staged and treated advanced NSCLC population. No known prognostic factor differences were found between the male and female cohorts and response rates were identical; yet the median survival time for women was significantly longer at 9.2 months (95% confidence interval (CI) 8.1–10.4 months) versus 7.3 months for men (95% CI 6.8–8.0 months); p = 0.004 log-rank test [6].
The Southwest Oncology Group (SWOG) pooled data from 6 recent trials including a total of 1334 patients with advanced stage NSCLC and also looked at sex differences in survival, but additionally looked at age/sex interactions [7]. A multivariate analysis reported that older women (>60 years old) had a survival advantage compared to younger women. Assuming that age is a surrogate for menopausal status, differences in hormonal levels, particular estrogen and progesterone, could be a potential explanation for these survival differences [7]. Detailed information on menopausal status and other aspects of female hormone exposure, and estrogen and progesterone levels are lacking from the SWOG and ECOG databases, so this assumption can only be hypothesis generating, but is worth exploring nonetheless.
Based on these data we sought to determine whether an age/sex survival interaction was evident in ECOG trials of chemotherapy in advanced stage NSCLC. We also sought to determine if an age/sex interaction was apparent for patients with NSCLC being treated with bevacizumab.
2. Patients and methods
E1594 enrolled 1207 patients (of whom 1157 were eligible) with untreated stage IIIB (with malignant effusion) or IV NSCLC, and randomized them to one of four different chemotherapy treatment arms [8]. The reference regimen, arm-A, consisted of paclitaxel and cisplatin. Arm B, C and D consisted of cisplatin/gemcitabine, cisplatin/docetaxel and carboplatin/paclitaxel respectively. E4599 enrolled 850 eligible patients with previously untreated advanced stage (IIIB with malignant effusion or stage IV) NSCLC with nonsquamous histology and no evidence of brain metastases and randomized them to carboplatin/paclitaxel (as given on E1594) with or without the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab [9]. All patients on both trials gave written informed consent [8,9].
For the first analysis of chemotherapy alone, eligible patients from both E1594 and the control arm of E4599 (N = 1590) were divided into male and female cohorts and separated into age groups of <60 or ≥60 years old, with a secondary analysis separately evaluating those <45 years old. For the bevacizumab analysis, eligible patients from E4599 (N = 850) were divided into male and female cohorts by treatment (with and without bevacizumab) and segregated into age groups of <60 or ≥60 years old (with a separate look at those <45 years old). In all analyses, survival was calculated separately for each cohort using the method of Kaplan-Meier and was compared using log rank tests. Known prognostic factors such as performance status, weight loss, and stage were also compared for each sex/age cohort using two-sided Fisher's exact tests.
Overall survival time was calculated from the date of registration to date of death from any cause; patients who were alive were censored at the date last known alive. Progression free survival (PFS) was calculated from the date of registration to date of progression or death without documented progression; patients who were alive and progression-free were censored at the date of last known follow-up. Response was evaluated by standard ECOG response criteria [8,9]. No adjustments have been made for multiple comparisons. All p-values are two-sided and confidence limits are at the 95% level.
3. Results
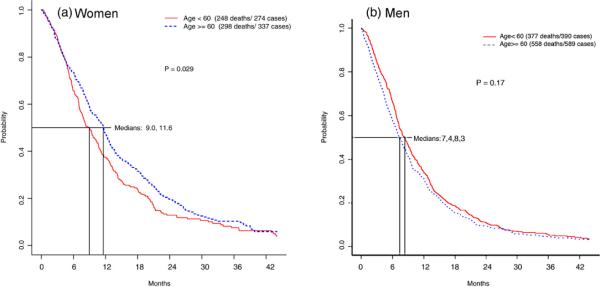
The chemotherapy alone analysis included 1590 eligible patients; 1157 from E1594 and 433 from the chemotherapy only arm of E4599. This group included 611 (38%) women and 979 (62%) men; of whom 274 (45%) and 390 (40%) of women and men respectively were <60 years old (Table 1). A higher proportion of those ≥60 were male compared to those <60 years old (p = 0.05), but there were no other statistically significant differences in known prognostic factors between the 4 sex/age cohorts (Table 1). A statistically significant difference in survival, however, was found favoring older women receiving chemotherapy compared to younger women and men of any age (Table 2 and Fig. 1). The median survival time (MST) for women ≥60 years old was 11.6 months (95% confidence interval (CI) 10.2–12.7 months), versus 9.0 months (95% CI 7.7–10.4 months) for women <60 (log rank p = 0.03). Men overall had lower survival than women, but no difference was seen for older men (MST of 7.4 months; 95% CI 6.7–8.1 months) versus men <60 years old (MST of 8.3 months, 95% CI 7.3–9.4 months) (log rank p = 0.17) (Table 2 and Fig. 1). No difference in survival was observed for women <60 years old compared to men <60 years old (p = 0.15).
Table 1.
Demographics of chemotherapy only patients on E1594 and E4599 (N = 1590) divided into age/sex cohorts using age 60 as a cut-point.
| Total | Age <60 years | Age ≥60 years | |
|---|---|---|---|
| 1590 (100%) | 664 (41.8%) | 926 (58.2%) | |
| Female | 611(38.4%) | 274(41.3%) | 337(36.4%) |
| Male | 879 (61.6%) | 390 (58.7%) | 589 (63.6%) |
| PS 0 | 522 (32.8%) | 232 (35%) | 290 (31.4%) |
| PS1 | 1000 (62.9%) | 408 (61.6%) | 592 (64%) |
| PS2 | 65 (4.1%) | 22 (3.3%) | 43 (4.6%) |
| Stage IIIB | 205 (12.9%) | 87 (13.1%) | 118 (12.8%) |
| Stage IV | 1382 (86.9%) | 576 (86.9%) | 806 (87.2%) |
| Wt loss >10% | 182(11.4%) | 79(11.9%) | 103(11.1%) |
PS, performance status on the Eastern Cooperative Oncology Group scale; wt, weight.
Table 2.
Median survival time by sex and age with chemotherapy using age 60 as a cut-point (N = 1590).
| Sex | Age in years |
p (age) | |
|---|---|---|---|
| <60 years | ≥60 years | ||
| Women 95% CI | 9.0 months (7.7–10.4 mo) N = 274 | 11.6 months (10.2 – 12.7 mo) N = 337 | 0.029 |
| Men 95% CI | 8.3 months (7.3–9.4 mo) N = 390 | 7.4 months (6.7–8.1 mo) N = 589 | NS (0.17) |
| p (sex) | NS | <0.0001 | |
Fig. 1.
Overall survival (OS) in months by Kaplan-Meier analysis for female and male cohorts, divided into age cohorts (using age 60 as a cut-point) with advanced NSCLC on ECOG 1594 and the control arm (chemotherapy alone) of E4599 (n = 1590). (a) OS for women using age 60 as a cut-point. (b) OS for men using age 60 as a cut-point.
Progression free survival (PFS) was also better for women ≥60 years old (4.7 months; 95% CI 4.1–5.3 months) versus 3.8 months (95% CI 3.1–4.3 months) for younger women (log rank p = 0.009). Again no difference was seen for PFS for men of different ages; 3.4 months (95% CI 3.0–3.8 months) for those ≥60 years old versus 4.0 months (95% CI 3.4–4.4 months) for younger men (log rank p = 0.5). Response rates in patients with measurable disease (N = 1532) were similar in all 4 age/sex groups at 19%, 15%, 18%, and 19% for women ≥ 60 years old, women <60 years old; respectively.
The age trend was even more striking, though of borderline statistical significance, when women were divided into those clearly pre-menopausal (<45 years old), clearly post-menopausal (≥60 years old) and those of unclear menopausal status based on age (45–59 years old). The youngest women (<45 years old, n = 37) had the worst survival at 7.0 months (95% CI 5.9–9.6 months), whereas women age 60 years and over (n = 337) had the best survival at 11.6 months (95% CI 10.2–12.7 months) (log rank p = 0.05 for equality of the 3 age groups). Survival for women aged 45–59 years old was 9.5 months (95% CI 7.9–10.8 months). There was no statistically significant difference in survival between women and men <45 years old (7.0 months (95% CI 5.9–9.6 months) versus 8.7 months (95% CI 6.1–13.8 months), respectively), whereas the difference in survival between men and women ≥60 years old was noteworthy and sta statistically significant at 7.4 months (95% CI 6.7–8.1 months) versus 11.6 months (95% CI 10.2–12.7 months), respectively (p < 0.001).
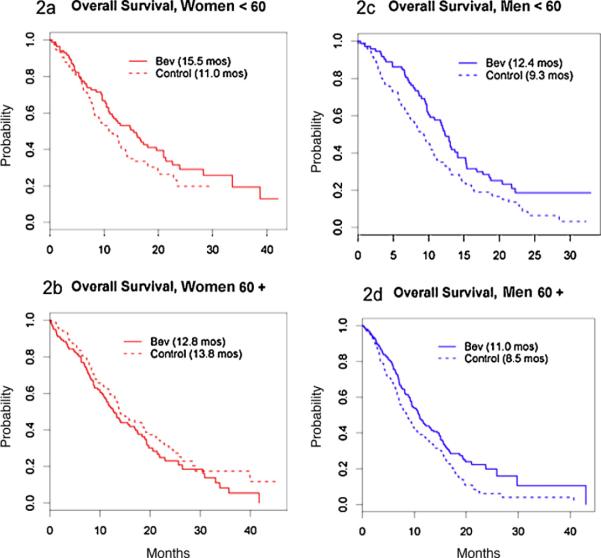
We also evaluated survival differences between men and women receiving carboplatin/paclitaxel with or without bevacizumab on E4599. This analysis included 850 eligible patients with 387 (46%) women and 463 (54%) men, of whom 160 (41%) and 168 (36%), respectively, were <60 years old. There were no significant associations between known prognostic factors (PS, weight loss, and stage) and sex or age (Table 3). As predicted, older women fared better than younger women on the chemotherapy alone arm. However, no survival difference was observed in women ≥60 years old with the addition of bevacizumab. MST was 12.8 months (95% CI 10.8–17.0 months) for women ≥60 years old receiving bevacizumab versus 13.8 months (95% CI 12.4–19.6 months) for those treated with chemotherapy alone (p = 0.2) (Table 4 and Fig. 2). In women <60 years old, the MST was 15.5 months (95% CI 11.0–21.0 months) versus 11.0 months (95% CI 8.2–14.2 months) with and without bevacizumab (p = 0.12), trending in favor of benefit with the bevacizumab (Table 4 and Fig. 2). For women <45 years old (N = 19) the MST was 16.8 months (95% CI 10.4 months-Inf) versus 5.8 months (95% CI 2.8 months-Inf) with and without bevacizumab (p = 0.07), just missing statistical significance despite the small numbers. In a Cox model predicting overall survival, the overall p-value for the age <60 years old by treatment interaction was statistically significant among women (p = 0.03) after adjusting for age and treatment. The age by treatment interaction among women remained statistically significant (p = 0.01) in a multivariable Cox model adjusting for age (<60 years old versus at least 60 years old) (p = 0.05), treatment (p = 0.28), stage (IIIB/IV versus recurrent) (p = 0.27), performance status (0 versus 1) (p = 0.01) and presence of liver metastases (p = 0.01).
Table 3.
Demographics on E4599 (N = 850) by treatment, divided into age/sex cohorts using age 60 as a cut-point.
| <60 yo | <60 yo | ≥60 yo | ≥60 yo | |
|---|---|---|---|---|
|
|
||||
| PC (n = 170) | PC + Bev (n = 158) | PC (n = 263) | PC + Bev (n = 259) | |
| Female | 75 (44%) | 85 (54%) | 105 (40%) | 122 (47%) |
| Male | 95 (56%) | 73 (46%) | 158 (60%) | 137 (53%) |
| PS 0 | 69 (41%) | 70 (45%) | 101 (39%) | 97 (38%) |
| PS1 | 99 (59%) | 86 (55%) | 161 (61%) | 161 (62%) |
| Stage IIIB | 26 (15%) | 18 (11%) | 29 (11%) | 32 (12%) |
| Stage IV | 144 (85%) | 140 (89%) | 234 (89%) | 227 (88%) |
| Wt loss ≥5% | 44 (26%) | 46 (29%) | 77 (29%) | 71 (27%) |
| Wt loss <5% | 126 (74%) | 112 (71%) | 186 (71%) | 188 (73%) |
PC, paclitaxel/carboplatin; Bev, Bevacizumab; 60 yo, 60 years old; PS, performance status; wt loss weight loss.
Table 4.
Median survival time in months (mo) by sex and age with and without bevacizumab on E4599 (N = 850) using age 60 as a cut-point.
| Age |
p value-age | ||
|---|---|---|---|
| <60 years | ≥60 years | ||
| Women − Bev | 11.0 mo (8.2–14.2) N = 75 | 13.8 mo (12.4–19.6) N = 105 | 0.11 |
| Women + Bev | 15.5 mo (11.0–21.0) N = 85 | 12.8 mo (10.8–17.0) N = 122 | 0.18 |
| Men − Bev | 9.3 mo (7.3–10.8) N = 95 | 8.5 mo (7.1–10.3) N = 158 | 0.85 |
| Men + Bev | 12.4 mo (10.2–15.2) N = 73 | 11.0 mo (9.3–14.4) N = 137 | 0.59 |
| p value-sex/treatment | 0.0006 | <0.0001 | |
Mo, months; Bev, bevacizumab.
Fig. 2.
Overall survival (OS) in months by Kaplan-Meier analysis for female and male cohorts, divided into age cohorts (using age 60 as a cut-point) with advanced NSCLC on E4599 (n = 850) ± bevacizumab (bev). (a) OS for women <60 yo ± bevacizumab. (b) OS for women ≥60 yo ± bevacizumab. (c) OS for men <60 yo ± bevacizumab. (d) OS for men ≥60 yo ± bevacizumab. Bev, bevacizumab.
Overall survival was improved in men in both age groups with the addition of bevacizumab. MST improved from 9.3 months (95% CI 7.3–10.8 months) for younger men treated with chemotherapy alone to 12.4 months (95% CI 10.2–15.2 months) for those treated with bevacizumab and MST improved from 8.5 months (95% CI 7.1–10.3 months) to 11.0 months (95% CI 9.3–14.4 months) in men at least 60 years old with the addition of bevacizumab (Table 4 and Fig. 2).
The age by treatment interaction among women was not a statistically significant predictor of progression free survival (p = 0.5). Men and women of all ages had a statistically significant improvement in PFS with the addition of bevacizumab (data not shown). Bevacizumab significantly improved response rates in all 4 age/sex groups as follows; in women ≥60 years old from 15% to 40%; in women <60 years old from 8% to 36%; in men ≥60 years old from 15% to 20% and in men <60 years old from 11% to 30%.
4. Discussion
Multiple studies have demonstrated a survival advantage for women with NSCLC compared to men, regardless of treatment. An analysis of pooled SWOG advanced stage NSCLC data found an age/sex differential with superior survival for women at least 60 years of age compared to younger women and men of any age [7]. We find similar results in our analysis of pooled ECOG data including 1590 patients with advanced stage NSCLC treated with first line chemotherapy. Women 60 years of age and older have a substantial survival benefit compared to younger women (MST 11.6 months versus 9 months for younger women) and men of any age (MST of 7.4 months for men at least 60 years old and 8.3 months for men <60 years old). Men had no differential survival by age. Known prognostic factors (performance status, weight loss, stage) cannot account for these differences, nor can differential response rates. Previous analysis have not shown age to be an independent predictor of survival for patients with NSCLC, likely due to a preponderance of men on NSCLC trials [4,5,10].
The superior survival of the older women on these trials with chemotherapy alone masks the poor outcome with chemotherapy alone for the small number of women in the youngest cohort <45 years old. The MST in this group of 7.0 months is nearly identical to that of men ≥60 years old (7.4 months) and significantly worse than that of women ≥60 (11.6 months). Because of small numbers of younger patients, those outcomes may have been hidden in previous analyses that grouped all women together.
Direct measurement of estrogen and progesterone levels will be necessary to confirm the hypothesis that high levels of female sex hormones in younger women may be part of the explanation for these results; for now, we can only base our conclusions on age as a surrogate marker of menopausal status. The age of menopause is variable and the median age varies in the literature, and we have therefore taken a very conservative approach in assuming that menopause prior to age 45 or after age 60 is very unusual. Clearly using age as a surrogate for menopause has many limitations, but further data looking at menopausal status, age of menarche, oral contraceptive/menopausal hormone therapy use and actual estrogen and progesterone levels are not available in the SWOG or ECOG databases.
The links of female sex hormones to lung cancer are multifaceted. Estrogen receptor-β (ERβ) has been documented in the majority of tested NSCLC tumors, suggesting a potential role of estrogen in NSCLC [11–13]. In addition, murine xenograft models have been reported in which NSCLC xenografts proliferate and grow with ERβ activation [12]. On a clinical level, one study reported that the continued use of female sex hormones after diagnosis of lung cancer worsened outcome [14], and in the randomized Women's Health Initiative (WHI) trial, the use of combined estrogen and progestin, though not estrogen alone, significantly increased lung cancer mortality in postmenopausal women [15,16]. Estrogen may also have an effect on angiogenesis, as illustrated by the identification of estrogen response elements in vascular endothelial growth factor (VEGF) [17,18].
Female sex hormones are unlikely to be the only explanation for these results. It does not explain why older, presumably post-menopausal women have improved survival compared to men of any age. Potentially, women may be more susceptible than men to chemotherapy due to decreased DNA repair capacity [19–22]. However, this would not explain the sex/age interactions we see. In fact, a recent analysis revealed a survival benefit for elderly women versus elderly men with early stage NSCLC, regardless of therapy, thus arguing against differential chemotherapy sensitivity as a key explanation of the sex differences in survival with NSCLC [23]. Further research is necessary to understand this phenomenon.
Our results also show a differential survival benefit from bevacizumab by age in women, but not men. The survival benefit of bevacizumab was seen for men of all ages on E4599 in this analysis, though an earlier subset analysis focusing on the elderly had found a decreased benefit in those over 70 years of age [24]. Other analyses of sex differences on E4599 demonstrated no survival benefit with bevacizumab for women despite significant response rate and progression free survival improvements; without a clear explanation such as imbalances in second line therapy [9,25]. The differential survival benefit of bevacizumab for women <60 years old (MST of 15.5 months with bevacizumab compared to 11 months without) compared to women at least 60 years old (12.8 months with bevacizumab compared to 13.8 months without) reaches statistical significance in our analysis (p < 0.03 in a Cox model for overall survival looking at the age/treatment interaction in women adjusted for age and treatment). The youngest cohort of women with NSCLC treated on E4599 fared better than any other cohort with a 15.5 months median survival, though lack of statistical significance preclude any definitive conclusions. The known interactions between the estrogen pathway and VEGF may play a role in this difference, though extensive further research is necessary to explore this hypothesis. Response rates and PFS were improved with bevacizumab in all age/sex cohorts.
VEGF gene transcription is known to be regulated by estrogen receptors alpha and beta [17,18,26], and in vitro studies have demonstrated VEGF induction by estrogen [27]. The correlation between VEGF and estrogen levels, however, is complex. In pre-menopausal women, VEGF levels vary considerably during the menstrual cycle in an inverse relationship with progesterone [28,29]. Menopausal hormone therapy in post-menopausal women was associated with higher VEGF levels in one analysis [30]; yet in two other studies VEGF levels dropped with extended estrogen therapy (over 6 months) [31,32]. On a tissue specific level, investigators using a micro-dialysis technique have looked at correlations between estradiol and VEGF in both normal and cancerous breast tissue with no clear pattern emerging [33,34]. Therefore, though the estrogen and VEGF pathways directly interact, how that interaction might explain our differential results in pre and post-menopausal women remains elusive.
On E4599, higher VEGF levels were correlated with increased likelihood of response to bevacizumab, but did not correlate with a survival benefit, nor were they prognostic for overall survival [35]. VEGF levels were not associated with sex, though a detailed age/sex/VEGF level analysis has not been performed due to small number of samples. So, while estrogen is known to up-regulate VEGF levels, and pre-menopausal women have the greatest benefit with bevacizumab in our analysis, it is unlikely that there is a straightforward explanation such that higher VEGF levels in premenopausal women, related to higher estrogen levels, increases response to bevacizumab. Nor would this explain the bevacizumab benefit seen in men, or the lack of survival benefit observed in older women.
Further biologic understanding of the sex differences in NSCLC will hopefully come from the ongoing Southwest Oncology Group (SWOG) trial, S0424, an Intergroup study investigating molecular epidemiology of NSCLC in smoking and non-smoking men and women which includes analyses of hormonal and reproductive factors. Unfortunately, this study will not provide information on treatment effects, nor on any interactions with anti-angiogenic agents. It will be important to also explore age/sex/treatment interactions with bevacizumab in other malignancies where it has efficacy.
Though further analysis including larger numbers of patients will be needed to confirm our results, this unplanned subgroup analysis supports a survival benefit of bevacizumab in all eligible male patients regardless of age, and in younger women with advanced NSCLC who are otherwise eligible to receive the drug. Given the unplanned, retrospective nature of our analysis, it is premature to state that older women do not benefit from bevacizumab. Caution must be used when considering bevacizumab for all patients over 70 years of age, given a retrospective analysis of E4599 which indicated increased toxicity in this patient population [24]. All age/sex cohorts benefited in terms of response and PFS with the addition of bevacizumab in our analysis of E4599. This analysis is an unplanned, post hoc, subgroup analysis and as such can only be hypothesis generating, but further exploration of the interaction of estrogen and progesterone levels and VEGF pathway inhibition in lung cancer and other malignancies is warranted based on our findings.
Acknowledgments
Previously presented in part at the 12th IASLC World Congress on Lung Cancer in Seoul, Korea September, 2006 and at the ASTRO/IASLC/ASCO 2008 Chicago Multidisciplinary Symposium in Thoracic Oncology November, 2008.
Funding This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, M.D., Chair) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA16116, CA21076, CA15488, CA27252, CA49957, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflicts of interest H.A. Wakelee has served on advisory panels for Genentech/Roche but has received no compensation and has received institutional research support from Genentech/Roche.
J.R. Brahmer served on an advisory panel and received an honorarium from Genentech/Roche.
J.H. Schiller has served as a consultant to Genentech/Roche and has received institutional research support from Genentech/Roche.
C.J. Langer has been compensated for serving on advisory boards and on a Speaker's Bureau for Genentech/Roche and has received institutional research support from Genentech/Roche.
A.B. Sandler has been compensated for serving on advisory boards and on a Speaker's Bureau for Genentech/Roche and has received institutional research support from Genentech/Roche. Additionally he has received money for legal work with Genentech/Roche.
The following authors have no conflicts to declare: S.E. Dahlberg, M.C. Perry, C.P. Belani, and D.H. Johnson.
References
- [1].Edmonson JH, Lagakos SW, Selawry OS, Perlia CP, Bennett JM, Muggia FM, et al. Cyclophosphamide and CCNU in the treatment of inoperable small cell carcinoma and adenocarcinoma of the lung. Cancer Treat Rep. 1976;60:925–32. [PubMed] [Google Scholar]
- [2].Radzikowska E, Glaz P, Roszkowski K. Lung cancer in women: age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–93. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- [3].Visbal AL, Williams BA, Nichols FC, 3rd, Marks RS, Jett JR, Aubry MC, et al. Gender differences in non-small-cell lung cancer survival: an analysis of 4618 patients diagnosed between 1997 and 2002. Ann Thorac Surg. 2004;78:209–15. doi: 10.1016/j.athoracsur.2003.11.021. discussion 215. [DOI] [PubMed] [Google Scholar]
- [4].Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: an Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4:702–9. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- [5].Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- [6].Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, et al. Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group Trial 1594. J Thorac Oncol. 2006;1:441–6. [PubMed] [Google Scholar]
- [7].Albain KS, Unger J, Gotay CC, Davies AM, Edelman M, Herbst RS, et al. Toxicity and survival by sex in patients with advanced non-small cell lung carcinoma (NSCLC) on modern Southwest Oncology Group (SWOG) trials. J Clin Oncol. 2007;25:396s. [Abstr#7549] [Google Scholar]
- [8].Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- [9].Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- [10].Langer CJ, Vangel M, Schiller J, Harrington DP, Sandler A, Belani CP, et al. Age-specific subanalysis of ECOG 1594: fit elderly patients (70–80 yrs) with NSCLC do as well as younger pts (<70) Proc Am Soc Clin Oncol. 2003;22:639. [Abstract#2571] [Google Scholar]
- [11].Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- [12].Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–50. [PubMed] [Google Scholar]
- [13].Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–9. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- [14].Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- [15].Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women's Health Initiative trial): a post hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–51. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, et al. Lung cancer among postmenopausal women treated with estrogen alone in the women's health initiative randomized trial. J Natl Cancer Inst. 2010;102:1413–21. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest. 1993;91:2235–43. doi: 10.1172/JCI116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res. 2000;60:3183–90. [PubMed] [Google Scholar]
- [19].Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, et al. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92:1764–72. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- [20].Spitz MR, Wei Q, Dong Q, Amos CI, Wu X. Genetic susceptibility to lung cancer: the role of DNA damage and repair. Cancer Epidemiol Biomarkers Prev. 2003;12:689–98. [PubMed] [Google Scholar]
- [21].Ryberg D, Hewer A, Phillips DH, Haugen A. Different susceptibility to smokinginduced DNA damage among male and female lung cancer patients. Cancer Res. 1994;54:5801–3. [PubMed] [Google Scholar]
- [22].Mollerup S, Ryberg D, Hewer A, Phillips DH, Haugen A. Sex differences in lung CYP1A1 expression and DNA adduct levels among lung cancer patients. Cancer Res. 1999;59:3317–20. [PubMed] [Google Scholar]
- [23].Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25:1705–12. doi: 10.1200/JCO.2006.08.1455. [DOI] [PubMed] [Google Scholar]
- [24].Ramalingam SS, Dahlberg SE, Langer CJ, Gray R, Belani CP, Brahmer JR, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol. 2008;26:60–5. doi: 10.1200/JCO.2007.13.1144. [DOI] [PubMed] [Google Scholar]
- [25].Brahmer JR, Gray R, Schiller JH, Perry M, Sandler A, Johnson DH. ECOG 4599 phase III trial of carboplatin and paclitaxel ± bevacizumab: subset analysis of survival by gender. J Clin Oncol. 2006;24:373s. [Abstract #7036] [Google Scholar]
- [26].Mueller MD, Vigne JL, Minchenko A, Lebovic DI, Leitman DC, Taylor RN. Regulation of vascular endothelial growth factor (VEGF) gene transcription by estrogen receptors alpha and beta. Proc Natl Acad Sci USA. 2000;97:10972–7. doi: 10.1073/pnas.200377097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Seo KH, Lee HS, Jung B, Ko HM, Choi JH, Park SJ, et al. Estrogen enhances angiogenesis through a pathway involving platelet-activating factor-mediated nuclear factor-kappaB activation. Cancer Res. 2004;64:6482–8. doi: 10.1158/0008-5472.CAN-03-2774. [DOI] [PubMed] [Google Scholar]
- [28].Heer K, Kumar H, Speirs V, Greenman J, Drew PJ, Fox JN, et al. Vascular endothelial growth factor in premenopausal women - indicator of the best time for breast cancer surgery? Br J Cancer. 1998;78:1203–7. doi: 10.1038/bjc.1998.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kusumanto YH, Hospers GA, Sluiter WJ, Dam WA, Meijer C, Mulder NH. Circulating vascular endothelial growth factor during the normal menstrual cycle. Anticancer Res. 2004;24:4237–41. [PubMed] [Google Scholar]
- [30].Agrawal R, Prelevic G, Conway GS, Payne NN, Ginsburg J, Jacobs HS. Serum vascular endothelial growth factor concentrations in postmenopausal women: the effect of hormone replacement therapy. Fertil Steril. 2000;73:56–60. doi: 10.1016/s0015-0282(99)00476-8. [DOI] [PubMed] [Google Scholar]
- [31].Akkad A, Al-Azzawi F. Changes in serum vascular endothelial growth factor following initiation of estrogen replacement after hysterectomy and oophorectomy. Acta Obstet Gynecol Scand. 2001;80:554–8. [PubMed] [Google Scholar]
- [32].Sumino H, Nakamura T, Ichikawa S, Kanda T, Sakamaki T, Sato K, et al. Serum level of vascular endothelial growth factor is decreased by hormone replacement therapy in postmenopausal women without hypercholesterolemia. Atherosclerosis. 2000;148:189–95. doi: 10.1016/s0021-9150(99)00262-2. [DOI] [PubMed] [Google Scholar]
- [33].Garvin S, Dabrosin C. In vivo measurement of tumor estradiol and vascular endothelial growth factor in breast cancer patients. BMC Cancer. 2008;8:73. doi: 10.1186/1471-2407-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dabrosin C. Positive correlation between estradiol and vascular endothelial growth factor but not fibroblast growth factor-2 in normal human breast tissue in vivo. Clin Cancer Res. 2005;11:8036–41. doi: 10.1158/1078-0432.CCR-05-0977. [DOI] [PubMed] [Google Scholar]
- [35].Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab - an Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–12. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]