Abstract
Polyphenols found in foods and beverages are under intense scrutiny for their potential beneficial effects on human health. We examined the stability of two bioactive polyphenols, epigallocatechin-O-gallate (EGCg) and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), in a model digestive system at low oxygen tension with and without added digestive components and foods. Both compounds were stable at pH values of 5–6 and below, indicating gastric stability. Both compounds decomposed at pH 7.0. PGG was stabilized in a model system containing pepsin, pancreatin, bile and lipase, and/or baby food, but was not stabilized by dry cereal. EGCg was not stabilized by the addition of any biomolecule. The effects of polyphenols on human health should be evaluated in the context of their stability in the digestive tract with and without added digestive components and/or food.
Keywords: Polyphenol; digestion; tannin-protein interaction; epigallocatechin-O-gallate; 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose
1. Introduction
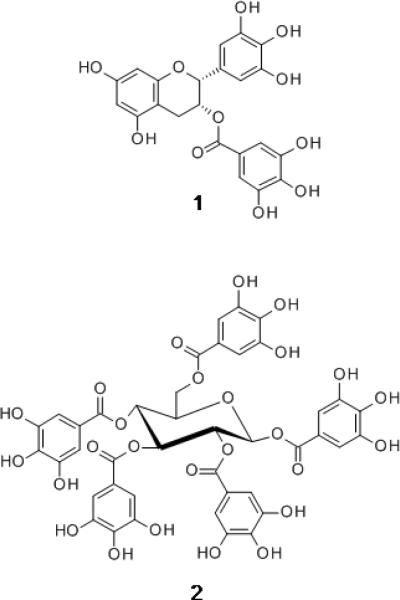
Polyphenol-rich foods may have beneficial effects against cardiovascular disease, various cancers and neurodegenerative diseases (de Pascual-Teresa, Moreno, & Garcia-Viguera, 2010; Neto, 2011; Scapagnini et al., 2011). Phenolic compounds are potent anti-oxidants and may be anti-inflammatory, but efforts to develop therapeutic agents are focused on polyphenols that have more selective bioactivities. Two polyphenolic compounds that have attracted attention are epigallocatechin-O-gallate (EGCg) and its derivatives, found in green and black tea (Wang & Ho, 2009), and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose (PGG), found in some herbal teas and traditional medicines (Zhang, Li, Kim, Hagerman, & Lu, 2009) (Fig. 1). EGCg inhibits metalloproteases, protein kinases, proteins involved in DNA replication, and tumor proteosomal activity (Dou, 2009). Modes of activity reported for PGG include anti-angiogenesis, inhibition of DNA synthesis, S and G1 phase arrest, and inhibition of apoptosis (Zhang et al., 2009). Epidemiological studies have demonstrated the beneficial effects of consuming EGCg-rich beverages such as tea (Cooper, 2012). Promising clinical trials have been conducted with EGCg (Larsen, Dashwood, & Bisson, 2010), while PGG shows positive effects against breast (Chai et al., 2010) and prostate cancer (Hu et al., 2008) in animal xenograft models.
Fig. 1.
Structural formulas for epigallocatechin-O-gallate, EGCg (1) and 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose, PGG (2).
EGCg and PGG are found in foods or traditional medicines, making them attractive candidates for oral administration. However, orally administered therapeutic agents must exhibit gastrointestinal stability and undergo efficient uptake. Solubility, permeability, stability, and metabolic interconversions may constrain the efficacy of dietary polyphenols, with different polyphenols exhibiting different bioavailabilities (Gao & Hu, 2010; Manach, Scalbert, Morand, Remesy, & Jimenez, 2004; Perez-Jimenez & Torres, 2011). Metabolic products including products of hydrolysis such as gallic acid from gallotannins, ellagic acid from ellagitannins, and ring fission products of flavan-3-ols from proanthocyanidins may be more bioavailable than the parent compounds (Gao & Hu, 2010; Nakamura, Tsuji, & Tonogai, 2003; Seeram, Lee, & Heber, 2004).
Few relevant uptake studies have been carried out with PGG, but the available evidence, summarized here, suggests it has limited bioavailability. Although PGG did reach micromolar levels in the serum after administration to mice by intraperitoneal (i.p.) injection, when PGG was administered by oral gavage, plasma levels remained below the limit of detection (sub-micromolar) (Li et al., 2011). In both experiments, unrealistically high doses (20 mg/kg i.p., 80 mg/kg orally) were used, reinforcing the conclusion that PGG is not bioavailable. Uptake of EGCg has been extensively examined in both human studies and animal models. When mice were fed EGCg at levels similar to typical human dietary ingestion (<1 mg/kg), nM levels of EGCg were achieved in the plasma (Dube, Nicolazzo, & Larson, 2011). Similar levels were achieved in several human studies in which uptake of EGCg from tea was assessed (Williamson, Dionisi, & Renouf, 2011). When EGCg was administered by i.p. injection at a similar level (2 mg/kg), much higher plasma levels (0.2 μM) were rapidly achieved (Long et al., 2001), suggesting that gastrointestinal conditions might adversely affect EGCg bioavailability.
The low bioavailability of dietary polyphenols may be due in part to their lability under conditions of the mammalian digestive tract. For example, EGCg undergoes oxidation and rearrangement reactions at neutral to basic pH and in the presence of dissolved oxygen (Neilson, Bomser, & Ferruzzi, 2007; Wang, Zhou, & Jiang, 2008). PGG is less susceptible to oxidation (Chen & Hagerman, 2005) but is somewhat susceptible to hydrolysis at physiological pH (Cai, Hagerman, Minto, & Bennick, 2006). Although both EGCg and PGG are attractive therapeutic agents because of their specific molecular mechanisms of action, and their natural occurrence in foods and traditional medicines, their probable low stability in gastrointestinal conditions could limit their utility. Improved stability could allow for more effective use of natural polyphenols as nutraceuticals and dietary supplements, or as additives to improve food safety (Perumalla & Hettiarachchy, 2011).
We propose that introducing PGG or EGCg to the digestive system in the presence of foods will improve the stability of these compounds. We test this hypothesis using an in vitro model of the digestive system that includes low oxygen tension, pH conditions, gastrointestinal enzymes/bile, and food components (Hur, Lim, Decker, & McClements, 2011).
2. Materials and methods
2.1. Chemicals
EGCg was supplied by Douglas Balentine (Lipton Tea Company, Englewood Cliffs, NJ, USA) and PGG was purified by methanolysis (Chen & Hagerman, 2004a) from commercial tannic acid from Fisher Scientific Company (Waltham, MA, USA). Identity and purity were confirmed using HPLC and mass spectrometry. Gerber® Natural Select commercial baby food (2nd foods Turkey & Rice Dinner) and General Mills Cheerios® were purchased from a local grocery store. Porcine bile extract, porcine pancreatin, type II porcine lipase, and porcine pepsin were purchased from Sigma Chemical Company (St. Louis, MO, USA). HPLC grade solvents and all other chemicals were from either Fisher Scientific Company or Sigma Chemical Company and were the best grade available.
2.2. In vitro digestion
EGCg and PGG were separately subjected to a two-stage in vitro digestion model mimicking the human digestive system (Garrett, Failla, & Sarama, 1999; Green, Murphy, Schulz, Watkins, & Ferruzzi, 2007; Walsh, Zhang, Vodovotz, Schwartz, & Failla, 2003). Six conditions were tested for each compound: pH changes, pH changes with digestive components, pH changes with Gerber® baby food, pH changes with Cheerios®, pH changes with digestive components and Gerber® baby food, and pH changes with digestive components and Cheerios®.
The polyphenol was added to 4.4 mL of simulated stomach fluid, comprised of 0.9% saline, 9.1 mM mandelic acid in 0.01 M HCl, pH 2. The polyphenol levels in the simulated stomach were 1.5 mg/mL (1.6 mM PGG, 3.3 mM EGCg). These levels are similar to the average total phenolic content of brewed tea (Astill, Birch, Dacombe, Humphrey, & Martin, 2001). PGG was also examined at 0.4 mg/mL (0.4 mM). An aliquot of 25 μL was immediately removed for HPLC analysis, and was used to establish the starting amount of phenol for that reaction (100%). The remaining solution was bubbled with nitrogen gas for 5 min before initiating digestion by adding 500 μL of 100 mM HCl or 40 μg/μL pepsin in 100 mM HCl, bringing the pH to 1.8 ± 0.1. A second aliquot of 25 μL was removed for analysis (0 h, pH 1.8) and the remaining solution was sealed and incubated while rotating at 37° C. After 1 h of incubation, another 25 μL aliquot was removed for analysis (1 h, pH 1.8). To the remaining solution, 2140 μL of 0.2 M NaHCO3 or of NaHCO3 containing porcine bile extract (2.4 μg/μL), porcine pancreatin (0.4 μg/μL) and type II porcine lipase (0.2 μg/μL) was added, achieving a final pH of 7.0 ± 0.1. The solution was again bubbled with nitrogen before sealing the sample, and incubating while rotating at 37° C for 2 h. After incubation, a final aliquot was removed for HPLC analysis (2 h, pH 7.0).
For the conditions that included the food sources, the food was suspended in the saline solution prior to the addition of the polyphenol. The Gerber® Turkey & Rice Dinner baby food was diluted five-fold with water and 150 μL of the suspension was added to the reaction mixture. Cheerios® were ground to a fine powder with a pestle and mortar and 6.5 ± 0.2 mg was added to the reaction mixture. For EGCg, additional experiments were performed in which the solutions were adjusted to pH 5.0 or pH 6.0 in the first step of the incubation. Samples were taken immediately (0 h, pH 5.0 or 0 h, pH 6.0) and after 1 h of incubation at 37° C after bubbling with nitrogen (1 h, pH 5.0 or 1 h, pH 6.0). Digestive components or food were not added in these experiments.
2.3 HPLC analysis
As each aliquot was removed for HPLC analysis, it was mixed with an equal volume of 1% (w/v) sodium lauryl sulfate in water to ensure recovery of all polyphenols including sorbed and insoluble materials. Each sample was centrifugally filtered through a 0.22 μm cellulose acetate membrane (Costar Spin-X® Centrifuge Tube Filter, Fisher Scientific) for 1 m at 7,200 × g and was immediately analyzed by HPLC, with injection into the acidic mobile phase of the HPLC within 5 min of collecting the sample.
The amount of EGCg and PGG in each sample was analyzed by HPLC using an Agilent 1050 system (Santa Clara, CA, USA) equipped with a diode array detector and controlled with Agilent ChemStation software (A.09.03). The system was equipped with two tandem 5 μm Hypersil ODS2 C18 cartridge columns (30×2.1 mm) with a guard column of the same material (Grace Davison, Deerfield IL, USA). Separation was achieved with a gradient of 0.13% trifluroacetic acid (TFA) in H2O (A) and 0.1% TFA in acetonitrile (B) at 0.5 mL/min in a 24 min program as follows: starting at 5% B, linear increase to reach 30% B at 11 min and then return to the initial conditions at 20 min followed by 4 min re-equilibration. The injection volume was 5 μl, and the eluent was monitored at 220–400 nm. Analyte and internal standard were analyzed by integrating the signal at 220 nm, which achieved maximal sensitivity with negligible background noise, using Agilent Chemstation A.09.03.
2.4 Data Analysis
Each condition was replicated three times and data were normalized based on the recovery of mandelic acid (Duval, 1962). Data were statistically analyzed using GraphPad Prism 4.03 (GraphPad Software, Inc., San Diego, CA).
3. Results and discussion
3.1 Gastrointestinal pH
The stability of EGCg and PGG in the mammalian digestive system was evaluated using solutions at pH 1.8 ± 0.1, similar to the stomach, and at pH 7.0 ± 0.1, similar to the duodenum, under reduced oxygen concentration (Kararli, 1995). Both EGCg and PGG were stable at pH 1.8 but in the absence of food or digestive components, 90% of the EGCg was lost and 80% of the PGG was lost during the 2 h incubation at pH 7.0 (Table 1). In the absence of added biomolecules, these two polyphenols were not stable at pH 7.0 even when oxygen concentrations were very low.
Table 1.
% Recovery of EGCg and PGG in Model Digestive Systems
| % of Initial Polyphenola |
|||||||
|---|---|---|---|---|---|---|---|
| Compound | Time Point | pH Changes | pH Changes with Cheerios | pH Changes with baby food | Digestive Components | Digestive Components with baby food | Digestive Components with Cheerios |
| EGCg | 0 h pH 1.8 | 100.1 (9.8) | 103.5 (3.7) | 110.6 (8.3) | 103.2 (3.4) | 98.4 (2.6) | 105.2 (1.4) |
| EGCg | 1 h pH 1.8 | 97.3 (6.4) | 102.7 (4.0) | 99.2 (9.3) | 104.7 (4.2) | 100.6 (1.4) | 103.1 (2.7) |
| EGCg | 2 h pH 7.0 | 11.8 (4.4) | 13.9 (3.1) | 20.7 (8.0) | 18.7 (4.1) | 8.4 (0.6) | 9.2 (1.5) |
| PGG | 0 h pH 1.8 | 107.9 (7.0) | 103.1 (0.7) | 92.8 (2.7) | 104.1 (6.1) | 106.4 (4.6) | 97.6 (5.6) |
| PGG | 1 h pH 1.8 | 99.1 (6.5) | 101.8 (1.3) | 96.7 (2.6) | 104.2 (7.6) | 106.7 (3.1) | 119.2 (9.9) |
| PGG | 2 h pH 7.0 | 17.7 (4.5) | 30.9 (1.9) | 49.5 (6.6)* | 62.8 (7.4)** | 51.6 (1.9)** | 73.2 (5.5)** |
Values are the mean for n=3 (SEM). Student's t-test was used to compare conditions at each time point.
P<0.05
P<0.01 compared to other values in the same row.
Although the pH of the stomach during fasting or ingestion of beverages is about 1.8–2.0 (Nagita et al., 1996), during food digestion the pH is dynamic, with values reaching pH 5.4–6.2 immediately after a meal but falling back to less than pH 2 within 3 h (Tyssandier et al., 2003). Normal salivary pH ranges from 6.4–8.0 (Giudicelli, Freslon, & Richer, 1979). To evaluate how a broader range of salivary and gastric pH values affects polyphenol stability, we adjusted the starting pH of the model digestive system to pH 5.0 or pH 6.0. We found that EGCg was stable at pH 5, with no differences in recovery between pH 5.0 and pH 1.8 (data not shown). About 70% of the initial EGCg was recovered after 1 h incubation at pH 6.0, compared to only 12% at pH 7.0 (Table 1). The pH-dependence of EGCg stability that we observed is consistent with the reported half lives of more than 3 h and 1 h, respectively, for EGCg at pH 5 and 7.2 (Zhu, Zhang, Tsang, Huang, & Chen, 1997). PGG is stable for hours in solutions at pH 6.7 or lower (Cai et al., 2006; He, Shi, Yao, Luo, & Ma, 2001).
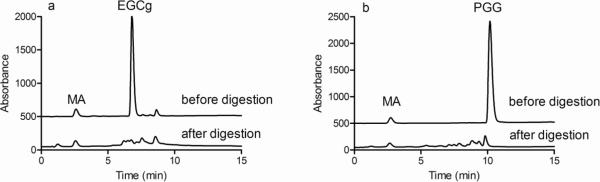
At pH 7.0, EGCg decomposed to products that were more highly retained than the starting material and were barely detectable with diode array detection (Fig. 2a) even at wavelengths as low as 205 nm (data not shown). These products were probably the homodimers that are formed by oxidative decomposition of EGCg (Neilson et al., 2007). The products of EGCg hydrolysis, gallic acid (1.3 min) and epigallocatechin (4.4 min), were not detected (Fig. 2a). At pH 7.0, PGG decomposition products included small amounts of compounds that co-eluted with pyrogallol (1.2 min) and gallic acid (1.3 min) or with lower galloyl esters of glucose such as tetragalloyl glucose and trigalloyl glucose (5.4–10 min) (Fig. 2b). However, most of the PGG was converted to products that were not detectable with our diode array HPLC, consistent with previous reports that PGG oxidation yields quinones that are difficult to separate and detect by HPLC (Chen & Hagerman, 2005).
Fig. 2.
Representative chromatograms of EGCg (a) and PGG (b) at the beginning and end of the simulated digestion without added biomolecules. The top trace in each chromatogram is the sample collected immediately after the polyphenol was mixed with the mandelic acid and the pH 1.8 HCl-saline solution. The bottom trace is the sample collected after the sample had been incubated for 2 h at pH 7.0. In each case, the sample was collected, mixed with an equal volume of 1% SDS, and immediately analyzed by reversed phase HPLC with detection at 220 nm, using a gradient of acetonitrile-water modified with 1% trifluoroacetic acid. MA, mandelic acid (internal standard).
3.2 Self-association of polyphenols
Some polyphenols are significantly self-associated in solution, and we speculated that aggregation might influence stability. We used self-association constants for PGG and EGCg at27 °C in solutions at pH 3.8 containing 10% organic solvent to estimate self-association in our system (Charlton et al., 2002). Based on Charlton's values, we estimated that 50% of the PGG was aggregated in our standard reaction mixtures (1.6 mM PGG), but that only 15% was aggregated when the PGG concentration was 0.4 mM. In the model digestion conditions PGG decomposition was independent of concentration over this concentration range (data not shown), leading us to conclude that self-association did not influence PGG stability. EGCg has a much lower self-association constant than PGG (Charlton et al., 2002), so we estimated that only about 10% of the EGCg was aggregated at our standard concentration of 3.3 mM EGCg.
3.3 Digestive biomolecules and food
To more completely simulate the mammalian digestive system, pepsin was added to the pH 1.8 samples, and pancreatin, lipase and bile were added to the pH 7.0 samples. In some samples, we added powdered Cheerios® cereal or Gerber® turkey-rice dinner baby food (Garrett et al., 1999). At each step of each reaction, the pH was adjusted after addition of the digestive components or foods to ensure that buffering effects of the biomolecules were not affecting the final pH.
Added biomolecules significantly stabilized PGG (Table 1). PGG was partially stabilized by addition of digestive components or by addition of baby food alone or in combination with digestive components, but PGG was not stabilized by addition of Cheerios® alone (Table 1). The highest recovery of PGG was achieved in samples containing Cheerios® plus the digestive components (Table 1). Addition of digestive components, food, or a combination of both did not stabilize EGCg at pH 7.0 (Table 1). At the end of the digestive process, only 10% of the EGCg was recovered for all treatments tested (Table 1), similar to the recovery of EGCg in an earlier study of artificial digestion of a mixture of green tea catechins (Green et al., 2007). In contrast to our results in which none of the added components stabilized EGCg, for the mixture of green tea catechins there was significant stabilization by added milk, ascorbic acid, or juice additives (Green et al., 2007). Further studies are needed to evaluate the different abilities of various foods and other additives to stabilize EGCg, and to establish the role of mixtures vs. pure phenolic compounds. For example, we have noted that EGCg is destabilized by methyl gallate (Min Li, Miami University, Oxford, OH personal communication).
We propose that stabilization of PGG by digestive components or foods may be a consequence of interaction between the polyphenol and protein or other biomolecules. PGG interacts strongly with protein in a pH-dependent fashion and readily forms highly cross-linked, insoluble complexes (Chen & Hagerman, 2004b; Hagerman, Rice, & Ritchard, 1998). The tendency of PGG to form multivalent complexes with protein could mask reactive moieties on the polyphenol, or could alter the polarity of the local microenvironment to change polyphenol reactivity. Earlier experiments that suggested that unoxidized EGCg binds protein relatively inefficiently (Hagerman, Dean, & Davies, 2003) have been confirmed by more recent experiments that show that EGCg does not readily form cross linked complexes with protein (data not shown). The limited ability of EGCg to interact with protein may explain why EGCg was not stabilized by added components in our model digestive system. In contrast, specific interactions between EGCg and specific thiols on proteins such as human serum albumin do provide stabilization of the phenolic under aerobic conditions (Bae et al., 2009).
Although added biomolecules stabilized PGG, protein-complexed polyphenol may have limited bioavailability. In Caco cell models, PGG transport is diminished when the salivary protein 1B4 is present, presumably because the PGG-protein complex is not efficiently transported (Cai et al., 2006). In human studies, foods or dietary proteins consumed simultaneously with tea catechins such as EGCg decrease bioavailability (Chow et al., 2005) (Egert et al. 2012). Formulation of modified foods such as edible films may further alter polyphenol stability during digestion (Lopez de Lacey et al., 2012).
Our digestive mixture included bile, which serves as an emulsifier during digestion, and which may form micelles in the gastrointestinal tract (Hur et al., 2011). We speculated that polyphenol interaction with lipids could provide stabilization in mixtures with the digestive components. PGG is much more hydrophobic than EGCg, and interacts more strongly with phospholipids than EGCg (Yu, Chu, Hagerman, & Lorigan, 2011), consistent with the stabilization by digestive components we noted for PGG but not for EGCg. Just as interactions with protein may stabilize PGG but decrease its bioavailability, there is some evidence that interactions with amphiphilic molecules might decrease PGG bioavailability. In an in vivo study, i.p. injection of PGG in 2% Tween 80-water vehicle gave much lower plasma levels than i.p. injection in 5% ethanol-saline (Li et al., 2011).
3.4 Conclusions
In our in vitro system, added biomolecules and/or food significantly stabilized PGG but had no effect on EGCg stability. The ability of digestive tract components to stabilize polyphenols is an important contribution to understanding the fate and effect of these bioactive molecules. Our data suggest that polyphenols will have different fates and effects depending on whether they are ingested as compounds, as constituents of beverages, or as constituents of foods. For supplements, fates will depend on carriers added to the supplement, and on whether the supplement is consumed with or without foods.
Highlights for Krook and Hagerman (2012)
Pentagalloyl glucose (PGG) & epigallocatechin gallate (EGCg) are food polyphenols
Polyphenols PGG & EGCg are stable to in vitro digestion at pH < 6
Polyphenols PGG & EGCg are destroyed during low oxygen, in vitro digestion at pH 7
Polyphenol PGG is protected from destruction during digestion by added biomolecules
Polyphenol EGCg is not protected during digestion by added biomolecules
Acknowledgements
Financial support was provided by the USDA Specific Cooperative Agreement 58-1932-5-534 and by NIDDK R15DK069285 to A.E.H. and by Miami University Dean's Scholar and Undergraduate Summer Scholars awards to M.A.K.
ABBREVIATIONS USED
- BSA
bovine serum albumin
- EGCg
epigallocatechin-O-gallate
- i.p.
intraperitoneal
- PGG
1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose)
- TFA
trifluroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Astill C, Birch MR, Dacombe C, Humphrey PG, Martin PT. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. Journal of Agricultural and Food Chemistry. 2001;49:5340–5347. doi: 10.1021/jf010759+. [DOI] [PubMed] [Google Scholar]
- Bae M-J, Ishii T, Minoda K, Ichikawa T, Mori T, Kamihira M, Nakayama T. Albumin stabilizes (−)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Molecular Nutrition & Food Research. 2009;53:709–715. doi: 10.1002/mnfr.200800274. [DOI] [PubMed] [Google Scholar]
- Cai K, Hagerman AE, Minto RE, Bennick A. Decreased polyphenol transport across cultured intestinal cells by a salivary proline-rich protein. Biochemical Pharmacology. 2006;71:1570–1580. doi: 10.1016/j.bcp.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Chai YB, Lee HJ, Shaik AA, Nkhata K, Xing CG, Zhang JH, Jeong SJ, Kim SH, Lu JX. Penta-O-galloyl-beta-D-glucose induces G(1) arrest and DNA replicative S-phase arrest independently of P21 cyclin-dependent kinase inhibitor 1A, P27 cyclin-dependent kinase inhibitor 1B and P53 in human breast cancer cells and is orally active against triple-negative xenograft growth. Breast Cancer Research. 2010;12:R67. doi: 10.1186/bcr2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton AJ, Baxter NJ, Khan ML, Moir AJG, Haslam E, Davies AP, Williamson MP. Polyphenol/peptide binding and precipitation. Journal of Agricultural and Food Chemistry. 2002;50:1593–1601. doi: 10.1021/jf010897z. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hagerman AE. Characterization of soluble non-covalent complexes between bovine serum albumin and beta-1,2,3,4,6-penta-O-galloyl-D-glucopyranose by MALDI-TOF MS. Journal of Agricultural and Food Chemistry. 2004a;52:4008–4011. doi: 10.1021/jf035536t. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hagerman AE. Quantitative examination of oxidized polyphenol-protein complexes. Journal of Agricultural and Food Chemistry. 2004b;52:6061–6067. doi: 10.1021/jf049602i. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hagerman AE. Reaction pH and protein affect the oxidation products of beta-pentagalloyl glucose. Free Radical Research. 2005;39:117–124. doi: 10.1080/10715760400013789. [DOI] [PubMed] [Google Scholar]
- Chow HHS, Hakim IA, Vining DR, Crowel JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clinical Cancer Research. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- Cooper R. Green tea and theanine: health benefits. International Journal of Food Sciences and Nutrition. 2012;63:90–97. doi: 10.3109/09637486.2011.629180. [DOI] [PubMed] [Google Scholar]
- de Pascual-Teresa S, Moreno DA, Garcia-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. International Journal of Molecular Sciences. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou QP. Molecular mechanisms of green tea polyphenols. Nutrition and Cancer. 2009;61:827–835. doi: 10.1080/01635580903285049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube A, Nicolazzo JA, Larson I. Assessment of plasma concentrations of (−)-epigallocatechin gallate in mice following administration of a dose reflecting consumption of a standard green tea beverage. Food Chemistry. 2011;128:7–13. doi: 10.1016/j.foodchem.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Duval C. Sur la stabilite termique des dtalons analytiques. X. Acide dl-mandelique. Mikrochimica Acta. 1962;1962:268–272. [Google Scholar]
- Egert S, Tereszczuk J, Wein S, Muller MJ, Frank J, Rimbach G, Wolffram S. Simultaneous ingestion of dietary protein reduces the bioavailability of galloylated catechins from green tea in humans. European Journal of Nutrition. 2012 doi: 10.1007/s00394-012-0330-8. Online First, DOI 10.1007/s00394-012-0330-8. [DOI] [PubMed] [Google Scholar]
- Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini-Reviews in Medicinal Chemistry. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DA, Failla ML, Sarama RJ. Development of an in vitro digestion method to assess carotenoid bioavailability from meals. Journal of Agricultural and Food Chemistry. 1999;47:4301–4309. doi: 10.1021/jf9903298. [DOI] [PubMed] [Google Scholar]
- Giudicelli JF, Freslon JL, Richer C. Acebutolol saliva excretion. British Journal of Clinical Pharmacology. 1979;8:373–375. doi: 10.1111/j.1365-2125.1979.tb04724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RJ, Murphy AS, Schulz B, Watkins BA, Ferruzzi MG. Common tea formulations modulate in vitro digestive recovery of green tea catechins. Molecular Nutrition & Food Research. 2007;51:1152–1162. doi: 10.1002/mnfr.200700086. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Dean RT, Davies MJ. Radical chemistry of epigallocatechin gallate and its relevance to protein damage. Archives of Biochemistry & Biophysics. 2003;414:115–120. doi: 10.1016/s0003-9861(03)00158-9. [DOI] [PubMed] [Google Scholar]
- Hagerman AE, Rice ME, Ritchard NT. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin16(4-->8)catechin (procyanidin) Journal of Agricultural and Food Chemistry. 1998;46:2590–2595. [Google Scholar]
- He Q, Shi B, Yao K, Luo Y, Ma ZH. Synthesis of gallotannins. Carbohydrate Research. 2001;335:245–250. doi: 10.1016/s0008-6215(01)00236-1. [DOI] [PubMed] [Google Scholar]
- Hu H, Lee H-J, Jiang C, Zhang J, Wang L, Zhao Y, Xiang Q, Lee E-O, Kim S-H, Lü J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Molecular Cancer Therapeutics. 2008;7:2681–2691. doi: 10.1158/1535-7163.MCT-08-0456. [DOI] [PubMed] [Google Scholar]
- Hur SJ, Lim BO, Decker EA, McClements DJ. In vitro human digestion models for food applications. Food Chemistry. 2011;125:1–12. [Google Scholar]
- Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory-animals. Biopharmaceutics & Drug Disposition. 1995;16:351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- Larsen CA, Dashwood RH, Bisson WH. Tea catechins as inhibitors of receptor tyrosine kinases: Mechanistic insights and human relevance. Pharmacological Research. 2010;62:457–464. doi: 10.1016/j.phrs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Shaik AA, Zhang JH, Nhkata K, Wang L, Zhang Y, Xing CG, Kim SH, Lu JX. Preparation of penta-O-galloyl-beta-D-glucose from tannic acid and plasma pharmacokinetic analyses by liquid-liquid extraction and reverse-phase HPLC. Journal of Pharmaceutical and Biomedical Analysis. 2011;54:545–550. doi: 10.1016/j.jpba.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Zhu YX, Cregor M, Tian FF, Coury L, Kissinger CB, Kissinger PT. Liquid chromatography with multi-channel electrochemical detection for the determination of epigallocatechin gallate in rat plasma utilizing an automated blood sampling device. Journal of Chromatography B. 2001;763:47–51. doi: 10.1016/s0378-4347(01)00365-6. [DOI] [PubMed] [Google Scholar]
- Lopez de Lacey AM, Gimenez B, Perez-Santın E, Faulks R, Mandalari G, Lopez-Caballero ME, Montero P. Bioaccessibility of green tea polyphenols incorporated into an edible agar film during simulated human digestion. Food Research International. 2012 (2012), doi: 10.1016/j.foodres.2012.04.024. [Google Scholar]
- Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. American Journal of Clinical Nutrition. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- Nagita A, Amemoto K, Yoden A, Aoki S, Sakaguchi M, Ashida K, Mino M. Diurnal variation in intragastric pH in children with and without peptic ulcers. Pediatric Research. 1996;40:528–532. doi: 10.1203/00006450-199610000-00003. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Tsuji S, Tonogai Y. Method for analysis of tannic acid and its metabolites in biological samples: Application to tannic acid metabolism in the rat. Journal of Agricultural and Food Chemistry. 2003;51:331–339. doi: 10.1021/jf020847+. [DOI] [PubMed] [Google Scholar]
- Neilson AP, Bomser JA, Ferruzzi MG. Catechin degradation with concurrent formation of homo- and heterocatechin dimers during in vitro digestion. Journal of Agricultural and Food Chemistry. 2007;55:8941–8949. doi: 10.1021/jf071645m. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberries: ripe for more cancer research? Journal of the Science of Food and Agriculture. 2011;91:2303–2307. doi: 10.1002/jsfa.4621. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez J, Torres JL. Analysis of nonextractable phenolic compounds in foods: The current state of the art. Journal of Agricultural and Food Chemistry. 2011;59:12713–12724. doi: 10.1021/jf203372w. [DOI] [PubMed] [Google Scholar]
- Perumalla AVS, Hettiarachchy NS. Green tea and grape seed extracts — Potential applications in food safety and quality. Food Research International. 2011;44:827–839. [Google Scholar]
- Scapagnini G, Sonya V, Nader AG, Calogero C, Zella D, Fabio G. Modulation of nrf2/are pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Molecular Neurobiology. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumptionof ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta. 2004;348:63–68. doi: 10.1016/j.cccn.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Tyssandier V, Reboul E, Dumas JF, Bougteloup-Demange C, Armand M, Marcand J, Sallas M, Borel P. Processing of vegetable-borne carotenoids in the human stomach and duodenum. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2003;284:G913–G923. doi: 10.1152/ajpgi.00410.2002. [DOI] [PubMed] [Google Scholar]
- Walsh KR, Zhang YC, Vodovotz Y, Schwartz SJ, Failla ML. Stability and bioaccessibility of isoflavones from soy bread during in vitro digestion. Journal of Agricultural and Food Chemistry. 2003;51:4603–4609. doi: 10.1021/jf0342627. [DOI] [PubMed] [Google Scholar]
- Wang R, Zhou WB, Jiang XH. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide temperature range. Journal of Agricultural and Food Chemistry. 2008;56:2694–2701. doi: 10.1021/jf0730338. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ho C-T. Polyphenolic chemistry of tea and coffee: A century of progress. Journal of Agricultural and Food Chemistry. 2009;57:8109–8114. doi: 10.1021/jf804025c. [DOI] [PubMed] [Google Scholar]
- Williamson G, Dionisi F, Renouf M. Flavanols from green tea and phenolic acids from coffee: Critical quantitative evaluation of the pharmacokinetic data in humans after consumption of single doses of beverages. Molecular Nutrition & Food Research. 2011;55:864–873. doi: 10.1002/mnfr.201000631. [DOI] [PubMed] [Google Scholar]
- Yu XT, Chu SD, Hagerman AE, Lorigan GA. Probing the interaction of polyphenols with lipid bilayers by solid-state nmr spectroscopy. Journal of Agricultural and Food Chemistry. 2011;59:6783–6789. doi: 10.1021/jf200200h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li L, Kim S-H, Hagerman AE, Lu J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharmaceutical Research. 2009;26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QY, Zhang A, Tsang D, Huang Y, Chen ZY. Stability of green tea catechins. Journal of Agricultural and Food Chemistry. 1997;45:4624–4628. [Google Scholar]