Abstract
Astrocyte signals can modulate arteriolar tone, contributing to regulation of cerebral blood flow, but specific intercellular communication mechanisms are unclear. Here we used isolated cerebral arteriole myocytes, astrocytes, and brain slices to investigate whether carbon monoxide (CO) generated by the enzyme heme oxygenase (HO) acts as an astrocyte-to-myocyte gasotransmitter in the brain. Glutamate stimulated CO production by astrocytes with intact HO-2, but not those genetically deficient in HO-2. Glutamate activated transient KCa currents and single KCa channels in myocytes that were in contact with astrocytes, but did not affect KCa channel activity in myocytes that were alone. Pre-treatment of astrocytes with chromium mesoporphyrin (CrMP), a HO inhibitor, or genetic ablation of HO-2 prevented glutamate-induced activation of myocyte transient KCa currents and KCa channels. Glutamate decreased arteriole myocyte intracellular Ca2+ concentration and dilated brain slice arterioles and this decrease and dilation were blocked by CrMP. Brain slice arteriole dilation to glutamate was also blocked by L-2-alpha aminoadipic acid, a selective astrocyte toxin, and paxilline, a KCa channel blocker. These data indicate that an astrocytic signal, notably HO-2 derived CO, is employed by glutamate to stimulate arteriole myocyte KCa channels and dilate cerebral arterioles. Our study explains the astrocyte and HO dependence of glutamatergic functional hyperemia observed in the newborn cerebrovascular circulation in vivo.
Keywords: newborn, cerebrovascular circulation, functional hyperemia, heme oxygenase
INTRODUCTION
Local cerebral arteriole smooth muscle tone is temporally and spatially coordinated to match blood flow to neuronal activity. This vascular response is termed functional hyperemia or neurovascular coupling1, 2. In the cerebrovascular circulation, signals to vascular smooth muscle derive from endothelium, nerves, astrocytes, or pericytes, which interact to form a neurovascular unit3. Astrocytes relay signals to neurons4 and other astrocytes5, 6. Parenchymal arterioles are ensheathed by astrocyte endfeet7, 8. Pial arterioles lay on a bed of astrocytes, the glia limitans, and are coated by astrocyte processes and endfeet9–11. Recent papers have reviewed astrocytes functioning as intermediaries between neurons and cerebral blood vessels to adjust cerebral blood flow to neuronal activity3, 7, 8. Potential astrocyte-derived vasoactive mediators of cerebrovascular responses include epoxyeicosatrienoic acids12, 13, K+ 14, 15 and ATP16 which appear to perform in concert8.
The principle excitatory cerebral neurotransmitter is glutamate that dilates cerebral arterioles to match blood flow to neural activity. Specific cells and mechanisms involved in coupling the glutamate release to arteriolar smooth muscle relaxation are uncertain. Astrocytes are uniquely positioned for sensing neuronal activity and regulating cerebral blood flow7, 8, 17–19. They possess both metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs). Glutamate stimulation of astrocytic mGluR and iGluR produces intracellular Ca2+ oscillations and localized and global elevations of astrocyte intracellular Ca2+ concentration ([Ca2+]i)20–24. In brain slices, glutamate elevates astrocyte [Ca2+]i but reduces adjacent vascular smooth muscle cell [Ca2+]i that produces vasodilation22, 25. However, the signaling mechanisms between astrocytes and vascular smooth muscle that result in this dilation to glutamate remain uncertain with eicosanoids17, 25, K+ 2, 14, and Ca2+ 24 among the mechanisms considered.
CO could be a glutamate-induced, diffusible messenger employed by astrocytes to signal for arteriole dilation. This hypothesis is rational because glutamatergic dilation of newborn pig cerebral arterioles, in vivo, is blocked by inhibition of HO that produces CO26, 27. Furthermore, astrocyte injury, in vivo, blocks piglet pial arteriolar dilation to glutamate9 making it reasonable to propose that astrocytes are the source of vasoregulatory CO involved in excitatory amino acid-induced cerebral vasodilation. CO dilates cerebral arterioles by activating smooth muscle cell large conductance Ca2+ activated potassium channels (KCa channels)28, 29. Therefore, we investigated whether astrocyte-derived CO increases smooth muscle cell KCa channel activity, providing a mechanism by which glutamate can cause arteriole dilation. Specifically, the present study tested the hypothesis that glutamate stimulates astrocyte production of CO that increases arteriole smooth muscle cell KCa currents, leading to a decrease in intracellular Ca2+ ([Ca2+]i) and vasodilation.
MATERIALS AND METHODS
Piglet cerebral arteriole smooth muscle cell isolation
Procedures were approved by the University of Tennessee Health Science Center Animal Care and Use Committee. Newborn pigs (1–3 days old, 1–2.5 kg) were anesthetized with ketamine hydrochloride (33 mg/kg im) and acepromazine (3.3 mg/kg im). The brain was removed and placed into ice-cold HEPES-buffered physiological saline solution (PSS). For electrophysiological studies, arterioles (50–200 µm in diameter) were dissected from the cerebral cortical surface. Individual vascular smooth muscle cells were enzymatically dissociated from cerebral arterioles using a procedure previously described30.
Astrocyte culture
Piglets were anesthetized as above, and adult HO-2+/+ and HO-2−/− mice were killed by peritoneal injection of sodium pentobarbital (130 mg/kg)(Mouse procedures were approved by New York Medical College Animal Care and Use Committee). Brains were removed and placed in ice-cold DMEM with antibiotic/antimycotic (100 U/ml penicillin, 100 mg/ml streptomycin, and 2.5 mg/ml amphotericin B). Astrocytes were isolated, grown, and identified as described previously9. Primary culture astrocytes were dislodged by trypsin-EDTA and either transferred to DMSO (10%)/FBS (90%) and frozen in liquid nitrogen for future use or directly plated on glass cover slips (4mm × 4mm) and grown to confluence. No differences were detectable in growth or phenotype between directly plated and frozen astrocytes. Astrocytes on cover slips were used for experiments with freshly isolated smooth muscle cells.
Astrocyte culture on Cytodex microcarrier beads
Confluent mouse HO-2+/+ and HO-2−/− astrocytes were dislodged with trypsin-EDTA and mixed with Cytodex microcarrier beads (175 µm diameter, denatured collagen chemically coupled to a matrix of cross-linked dextran, GE Healthcare), transferred into spinner flasks and stirred intermittently (20 rpm 30 min on and 3 h off) at 37 °C and 5% CO2 in air. After 12 h, the beads were stirred continuously (20 rpm) for 4–6 days before experiments. Cell density was approximately 106 cells/ml beads.
Patch-clamp electrophysiology
A glass coverslip with or without a confluent layer of astrocytes was placed in an experimental chamber. Freshly isolated cerebral arteriole smooth muscle cells were added to the chamber and allowed to settle for 10 min. Potassium currents were measured by using the whole cell perforated-patch-clamp configuration28. Transient KCa currents were measured at a steady membrane potential of −40 mV and calculated from ≥5 min of continuous gap-free data. At −40 mV, a transient KCa current was defined as the simultaneous opening of three KCa channels. Single KCa channel currents were recorded at a steady membrane potential of 0 mV in the presence of thapsigargin (100 nmol/L).
CO measurement
Following overnight incubation in L-glutamine and serum free DMEM, astrocytes grown on Cytodex microcarrier beads (1ml beads diluted to 1.7 ml with Krebs solution) were placed into amber vials with 13C18O (31CO, ISOTEC) as the internal standard. Following 1h incubation at 37°C, 100µl of head space gas was collected for CO detection by gas chromatography/mass spectrometry as described before31, 32.
Detection of HO-2 expression
Proteins from Laemmli buffer solubilized samples were separated by 12 % SDS PAGE, transferred to PVDF membranes and blocked with 10 % BSA in PBS-Tween 20 (0.1%). Immunoblotting was performed using the following antibodies: primary 1:2500 rabbit anti-mouse HO-2 antibodies (Stressgen), secondary 1:5000 goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology); primary 1:5000 mouse anti-mouse α-actin (Sigma Aldrich), secondary 1:10,000 goat anti-mouse IgG-HRP (Santa Cruz Biotechnology). The immunocomplexes were visualized with ECL Plus Western Blot detection reagent (GE Healthcare) and quantified by digital densitometry using Gel-Pro Analyzer software (Media Cybernetics).
Brain slice preparation, [Ca2+]i, and arteriolar diameter measurements
Newborn pig brains were removed and placed in cold (4–8°C) M199 with 10 mmol/L HEPES, and 10 mmol/L glucose, pH 7.37. Tangential slices were cut (200 µm thick) from the cerebral cortical surface and placed in M199. Diameters of cortical surface arterioles were measured in bicarbonate-buffered PSS (21% O2, 5% CO2) superfused (1.5 ml/min, 35°C) brain slices using an inverted microscope, CCD camera, and edge detection software (IonWizard, IonOptix, Milton, MA). [Ca2+]i was measured in cerebral arterioles of piglet brain slices using fura-2 with a method published previously33. The cerebral arteriolar wall in the brain slice was selected for data acquisition.
To selectively injure astrocytes, slices were incubated with L-2-alpha aminoadipic acid (L-AAA, 2 mmol/L). We9, and others10, 11, have shown previously that L-AAA treatment in vivo produces histological evidence of injury to the superficial glia limitans and loss of astrocyte-dependent cerebrovascular responses without altering responses in general. Control slices were incubated with D-AAA, the inactive isomer.
Chemicals
Cr (III) mesoporphyrin IX chloride (CrMP) was purchased from Frontier Scientific (Logan, Utah). Papain was purchased from Worthington Biochemical (Lakewood, NJ). All other chemicals were obtained from Sigma Chemical (St. Louis, MO) unless otherwise stated.
Statistical analysis
Values are means ± SEM. For comparisons among more than 2 groups, results were subjected to a one-way ANOVA for repeated measures with Tukey’s post hoc test to isolate differences between groups. Student t-tests were used for comparison between each two groups of paired or unpaired data, as appropriate. P < 0.05 was considered significant.
RESULTS
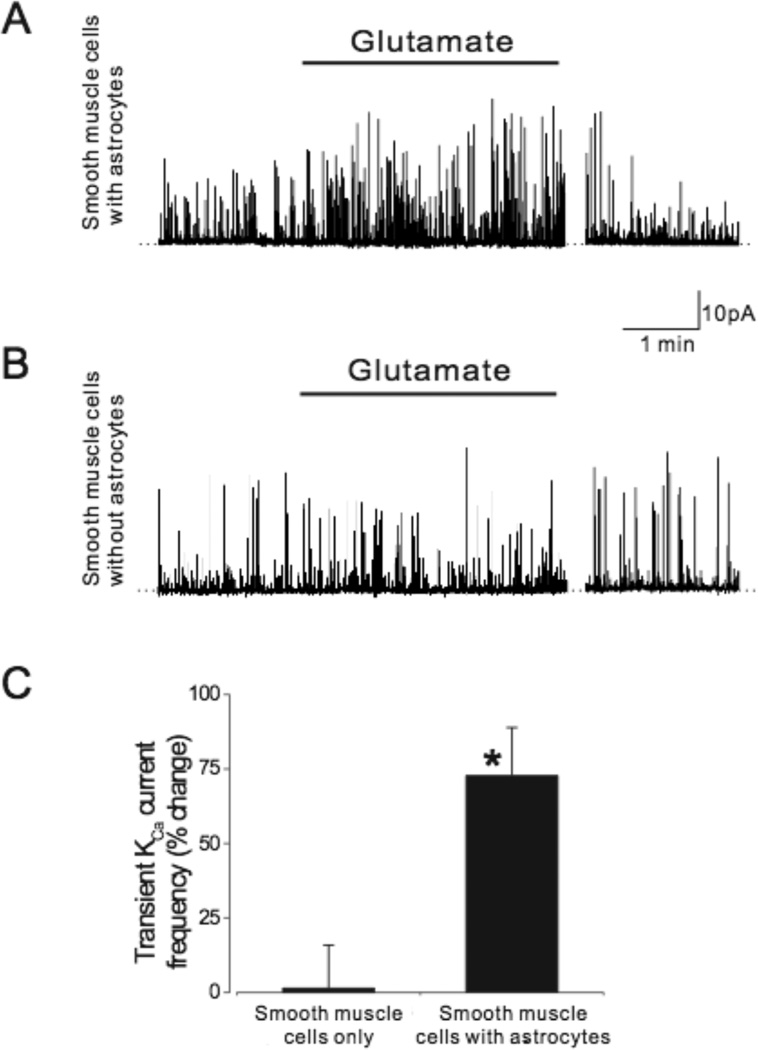
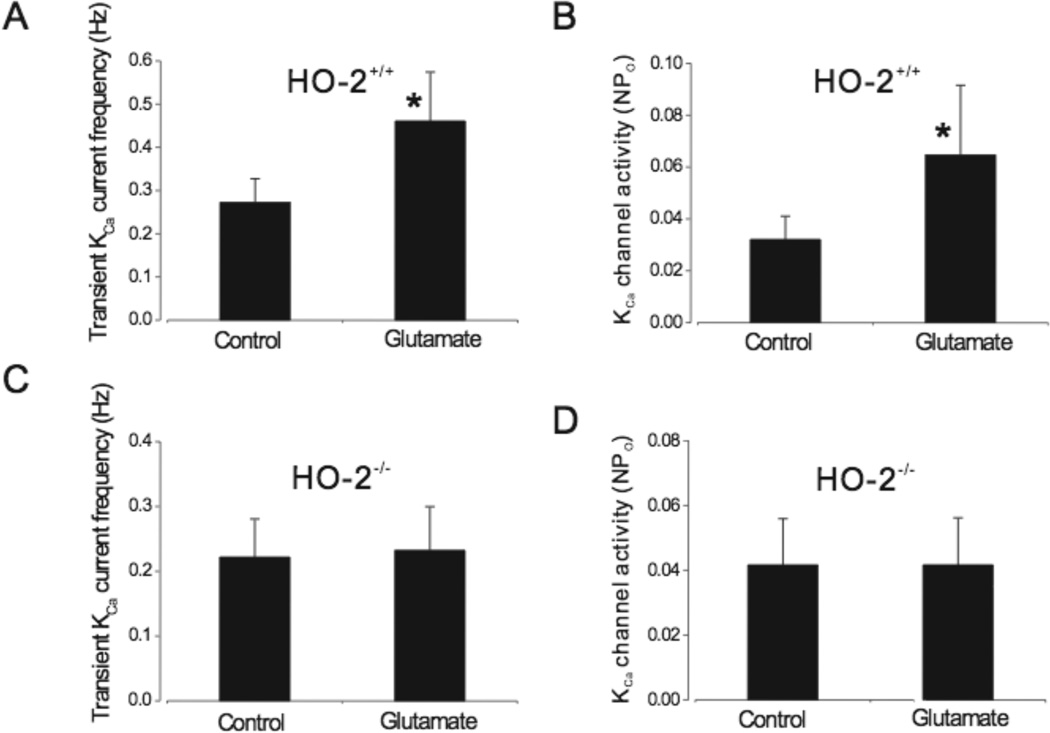
Cerebral arteriole smooth muscle cells were allowed to settle either on astrocytes or empty coverslips prior to measurement of whole cell currents. Using patch-clamp electrophysiology, transient KCa current frequency and KCa channel activity were recorded in smooth muscle cells. At −40mV, a physiological membrane potential, glutamate (20µmol/L) reversibly increased (~75%) transient KCa current frequency in cerebral arteriole smooth muscle cells that were in contact with astrocytes (Fig.1). Conversely, glutamate had no effect on the transient KCa current frequency of smooth muscle cells that were not in contact with astrocytes (Fig.1).
Fig. 1.
Glutamate (20µmol/L) elevates transient KCa current frequency in cerebrovascular smooth muscle cells in contact with astrocytes (A) but not in cerebrovascular smooth muscle cells alone (B). Summarized data are shown in C. n=7 and n=12 for smooth muscle cells alone and with astrocytes, respectively. *P<0.05 compared to zero.
Transient KCa current amplitudes are not normally distributed (failed Kolmogorov and Smirnov for a Gaussian distribution at P<0.0001). Therefore, transient KCa currents were divided into groups that are logical based on numbers of simultaneous channel openings (Table 1): small transient KCa currents (3–7 channel openings [1 channel = 2.8pA34]), midsized transient KCa currents (8–14 channel openings) and large transient KCa currents (15–30 channel openings). As can be seen in the table, the distribution is skewed toward smaller transient KCa currents, then medium sized transient KCa currents, and fewer large transient KCa currents. Glutamate increased the number of small (1.6 fold), medium (1.7 fold), and large (3.1 fold) transient outward currents in smooth muscle cells in contact with astrocytes, but did not change the number of outward currents of any amplitude in smooth muscle cells that were alone.
Table 1.
Effect of glutamate on piglet cerebral arteriole smooth muscle cell (SMC) transient KCa currents.
| Amplitude range | SMC with astrocytes | SMC alone | ||
|---|---|---|---|---|
| Control | Glutamate | Control | Glutamate | |
| 7–20pA | 70±4 | 110±6* | 61±6 | 67±4 |
| 21–40pA | 41±3 | 67±3* | 45±3 | 43±5 |
| >40pA | 6±1 | 19±2* | 4±1 | 3±1 |
Mean±SEM currents per cell in 10 min. N=7 cells with and without astrocytes.
P<0.05 compared to control.
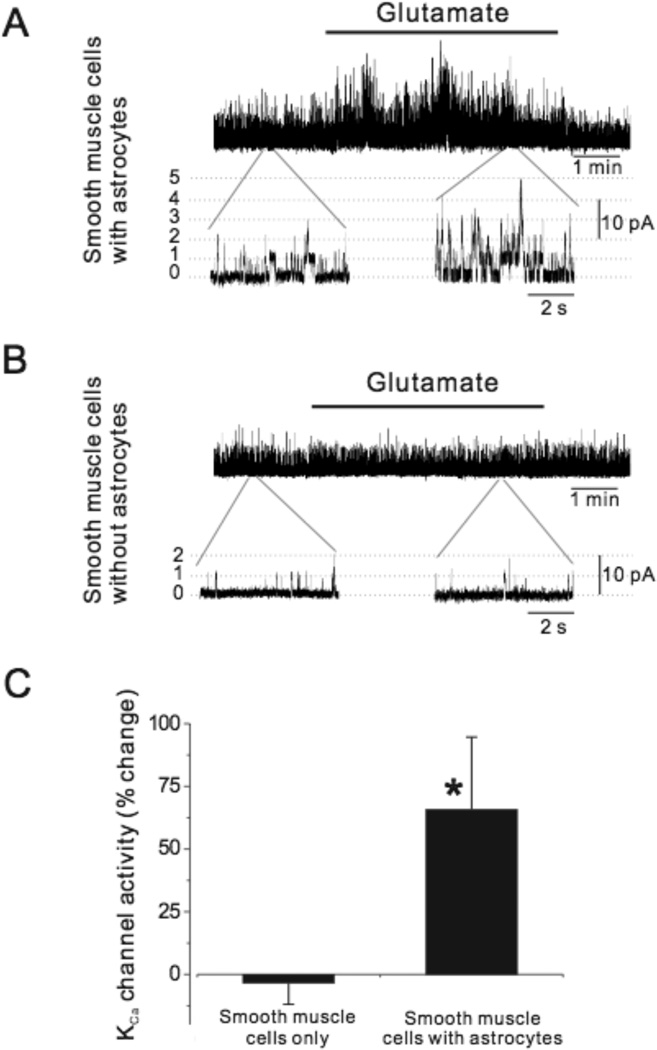
To investigate KCa channel regulation by glutamate, transient KCa currents were inhibited by depleting SR Ca2+ with thapsigargin (100nmol/L), a SR Ca2+ ATPase inhibitor that blocks Ca2+ sparks30. Glutamate increased (~65%) single KCa channel activity when smooth muscle cells were in contact with astrocytes (Fig.2). In contrast, glutamate did not change KCa channel activity in smooth muscle cells without astrocytes.
Fig. 2.
Glutamate (20µmol/L) activates KCa channels in cerebrovascular smooth muscle cells in contact with astrocytes (A) but not in cerebrovascular smooth muscle cells alone (B). Summarized data are shown in C. n=6 and n=13 for smooth muscle cells alone and with astrocytes, respectively. *P<0.05 compared to zero.
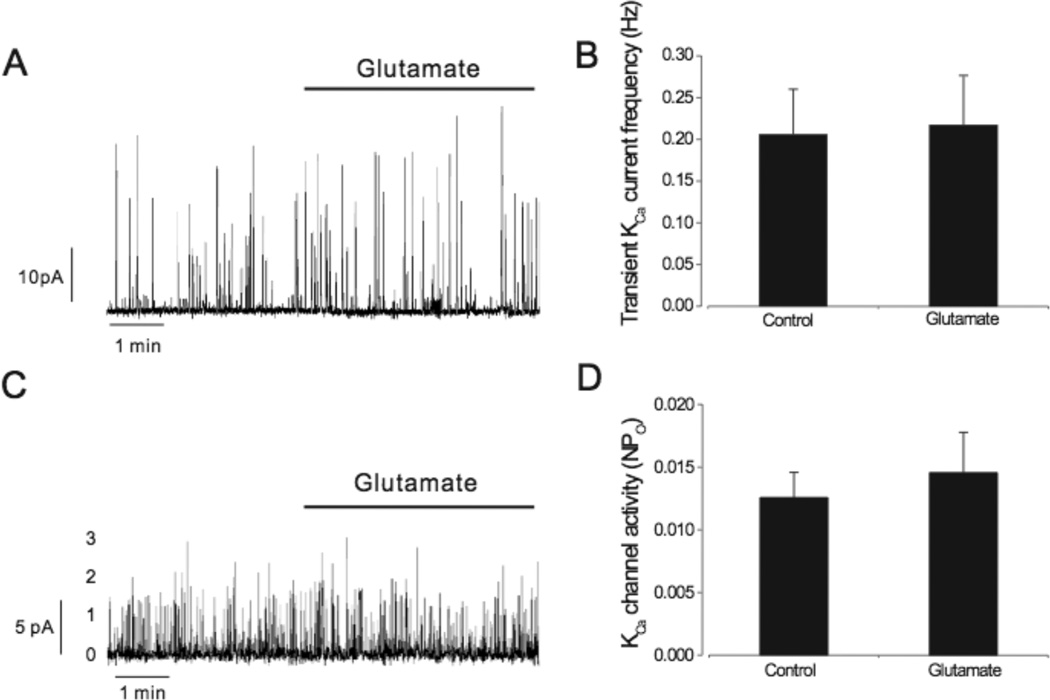
To investigate the contribution of astrocyte HO to the responses, astrocytes were pretreated with the HO inhibitor CrMP (20µmol/L, 1h). Coverslips with CrMP-treated astrocytes were rinsed and then added to the recording chamber to avoid exposure of smooth muscle cells to the HO inhibitor. Cerebral arteriole smooth muscle cells were allowed to settle on CrMP-treated astrocytes. In contrast to the effect of glutamate on vascular smooth muscle cells in contact with untreated astrocytes, where transient KCa current frequency increased ~75% and single KCa channel open probability increased ~65%, glutamate changed neither transient KCa current frequency nor KCa channel activity of smooth muscle cells in contact with CrMP-pretreated astrocytes (Fig. 3).
Fig. 3.
Chromium mesoporphyrin (20µmol/l)(CrMP) pretreatment of astrocytes blocks glutamate (20µmol/L) activation of transient KCa current frequency (A&B, n=8) and KCa channel activity (C&D, n=6) in smooth muscle cells in contact with astrocytes.
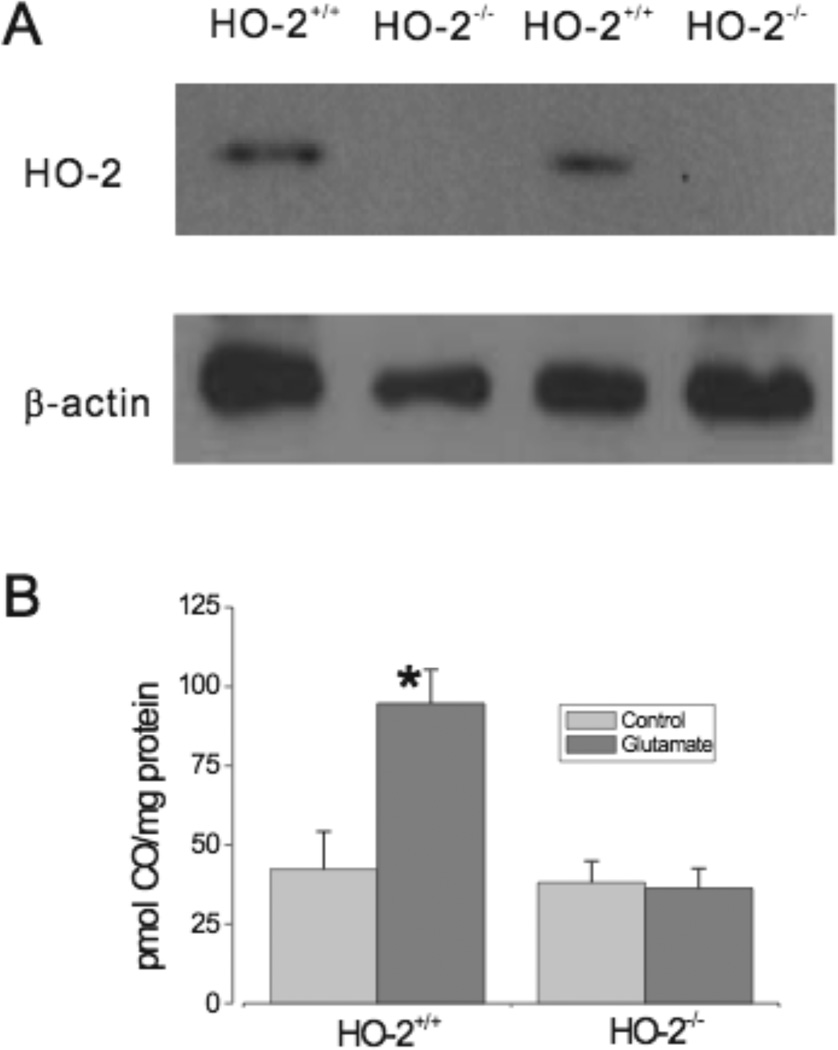
As an alternative approach for depression of astrocyte CO production, as well as for investigation of the HO subtype involved in glutamate-induced CO production, we used HO-2 knockout mice. Wild type (HO-2+/+) homogenates showed strong expression of HO-2, while there was no detectable HO-2 protein expression in HO-2 knockout (HO-2−/−) mice (Fig.4A). Glutamate more than doubled CO production by HO-2+/+ astrocytes (Fig. 4B). In contrast, glutamate had no effect on CO production by HO-2−/− astrocytes (Fig.4B).
Fig. 4.
A: HO-2 protein expression in HO-2+/+ and HO-2−/− mice. B: Glutamate (20µmol/L) stimulates CO production by HO-2+/+ mouse astrocytes but not by HO-2−/− mouse astrocytes (n=3 separate mouse brain preparations each for HO-2+/+ and HO-2−/− with 5 or 6 tubes for each preparation). *P<0.05 compared to basal production.
To examine the effect of HO-2 expression on the ability of astrocytes to activate KCa channels in cerebral arterial smooth muscle cells, piglet cerebral arteriole smooth muscle cells were allowed to settle on either HO-2+/+ or HO-2−/− mouse astrocytes. Transient KCa current frequency and KCa channel activity were recorded. As was the case when using piglet astrocytes, glutamate elevated transient KCa current frequency (2.3 fold) as well as KCa channel activity (2 fold) in piglet smooth muscle cells in contact with HO-2+/+ mouse astrocytes (Fig. 5). In contrast, glutamate did not alter transient KCa current frequency or KCa channel activity in piglet smooth muscle cells in contact with HO-2−/− astrocytes (Fig. 5).
Fig. 5.
Glutamate activates transient KCa current frequencies and single channel activity in piglet cerebral microvascular smooth muscle cells in contact with mouse HO-2+/+ (A& B, n=8) but not HO-2−/− (C& D, n=6) astrocytes. *P<0.05 compared to without glutamate.
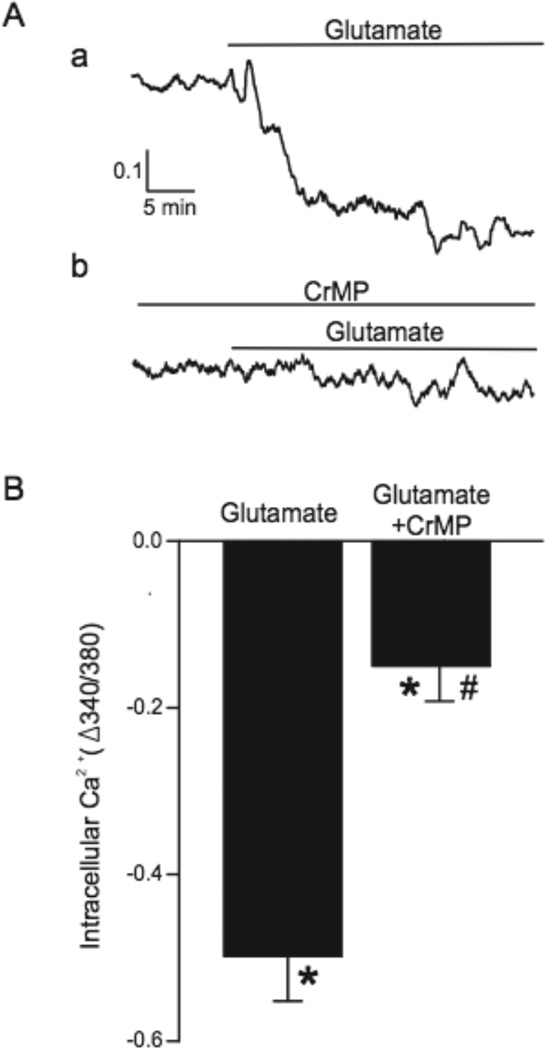
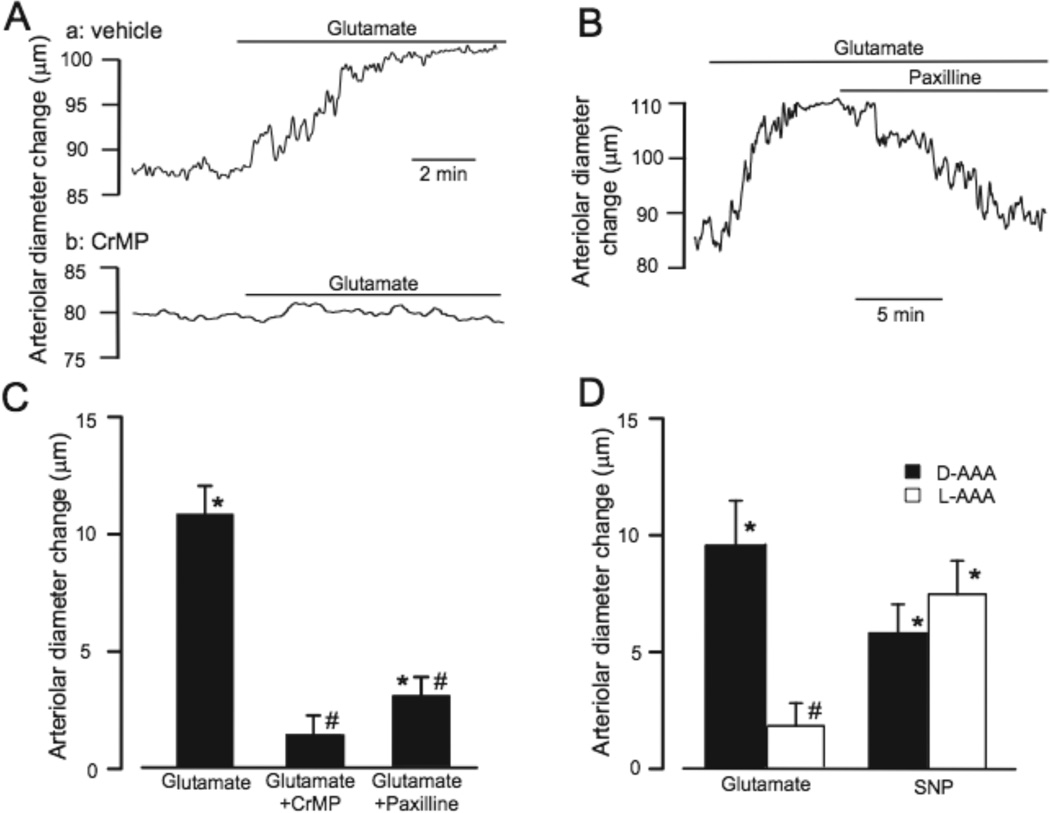
We used newborn pig cerebral cortex slices to examine contributions of HO and astrocytes to glutamate regulation of arteriolar [Ca2+]i and diameter in structurally intact brain. Glutamate (30µmol/L) decreased the fura-2 ratio (340/380 nm) in arterioles, indicating a decrease of [Ca2+]i (Fig. 6). Pretreatment of slices with CrMP markedly inhibited the effect of glutamate on [Ca2+]i. In addition, glutamate dilated piglet neocortical slice arterioles (from 111±10 to 123±11 µm) (Fig. 7). In contrast, glutamate did not alter the diameters of arterioles in brain slices pretreated with CrMP, suggesting CO generated by HO is required for glutamate-induced vasodilation. CrMP-treated slice arterioles constricted normally to the thromboxane receptor agonist U46619 (data not shown). Paxilline, a selective KCa channel blocker35, reversed glutamate-induced dilation, suggesting the dilation was mediated by KCa channel activation. Also, following treatment of brain slices with L-AAA (2mmol/L, 2h), a selective astrocyte toxin, arterioles did not dilate to glutamate (Fig. 7D). In contrast, arterioles within slices incubated with the inactive isomer, D-AAA, dilated similarly to glutamate as did arterioles in untreated slices. L-AAA did not depress overall arteriolar reactivity as indicated by similar dilations to sodium nitroprusside of arterioles in L-AAA (12%) and D-AAA (15%) treated slices. These data show that astrocytes, HO-2, and KCa channels are required for glutamate-induced cerebral arteriolar dilation in newborn pigs.
Fig. 6.
Glutamate (30 µmol/l) decreases intracellular Ca2+ in brain slice arteriole smooth muscle and this effect is blocked by chromium mesoporphyrin (CrMP, 20µmol/l). A. Representative traces of fura-2 fluorescence ratio in an arteriole of an untreated brain slice and a slice treated with CrMP. B. Collated data of brain slice arteriole responses to glutamate without and with CrMP (n=13 and 11, respectively). *P<0.05 compared to diameter prior to glutamate. # P<0.05 compared to without CrMP.
Fig. 7.
Chromium mesoporphyrin (CrMP, 20µmol/l), paxilline (20µmol/l), and L-2-alpha aminoadipic acid (L-AAA, 2 mmol/l) block glutamate (30µmol/L)-induced dilation of piglet cerebral cortical brain slice arterioles. A. Representative trace illustrating the effect of glutamate on the diameter of an arteriole in an untreated slice (a) and a slice treated with CrMP (b). B. Representative trace of glutamate-induced arteriolar dilation and the effect of paxilline on that dilation. C. Collated data of brain slice arteriole responses to glutamate without and with CrMP or paxilline (n=26, 11, and 11, respectively). D. Brain slice arteriolar diameter responses to glutamate and sodium nitroprusside (SNP, 140µmol/L) following treatment with D-AAA (n=10) or L-AAA (n=9). *P<0.05 compared to control diameter. # P<0.05 compared to without CrMP, paxilline, or L-AAA, as appropriate.
DISCUSSION
Major findings of the present study are that: 1) Glutamate activates transient KCa currents and KCa channel activity in cerebral arteriolar smooth muscle cells that are in contact with astrocytes but not in smooth muscle cells alone; 2) Glutamate increases CO production by intact astrocytes but not by genetically HO-2 deficient astrocytes; 3) Pharmacological inhibition of HO or genetic ablation of astrocyte HO-2 eliminates glutamate-induced KCa channel activation in cerebral arteriolar smooth muscle cells in contact with these astrocytes; 4) Glutamate decreases brain slice arteriolar smooth muscle [Ca2+]i through a mechanism that is attenuated by pharmacological inhibition of HO, and 5) Glutamate-induced dilation of piglet neocortical brain slice arterioles is blocked by either HO inhibition, KCa channel inhibition, or astrocyte injury.
Between the present and a recent study9, we use 3 approaches, isolated cells, brain slices, and cranial windows in vivo. Each has different strengths and weaknesses and the combination is mutually complimentary. Brain slices have no descending pressure or blood flow. But slices allow arteriole wall [Ca2+]i measurements and remove both neuronal influences from beyond the study area and blood borne mediators. The results from slices mirror the in vivo experiments9 that show glutamate causes arteriolar dilation and brain CO production9 which are inhibited by astrocyte injury. In vivo, arterioles have normal pressure and flow and CO production can be measured, but specific cell types involved in communication or CO production, channels involved, and [Ca2+]i measurements are not possible. Isolated cells allow specific communication between myocytes and astrocytes to be investigated, inhibitor treatment of only astrocytes, using HO-2 knockout astrocytes with wild type arteriolar smooth muscle, and measurement of CO production specifically by astrocytes. Thus, the combined 3 approaches lead to a more complete picture of the functional control mechanisms.
CO is produced physiologically by catabolism of heme to CO, iron, and biliverdin36. HO catalyzes this reaction with oxidation of NADPH. HO is expressed as three known isoforms: HO-1, HO-2 and a third isoform (HO-3) with much lower heme degrading activity and expression37. The present study shows that glutamate increases CO production by HO-2+/+ mouse astrocytes but not HO-2−/− astrocytes that do not express HO-2. CO reduces vascular smooth muscle tone both in vitro38,39 and in vivo27, 40. Indications of the functional significance of CO are supported by alterations of responses to important physiological cerebrovascular regulatory stimuli upon HO inhibition. In the newborn pig in vivo, pial arteriolar dilations to glutamate, hypotension, and hypoxia are inhibited following topical treatment with CrMP that blocks CO production26, 41.
While CO has been demonstrated to increase transient KCa currents when applied directly to isolated vascular smooth muscle cells or arterioles, the present study is the first to show that a HO-2 product produced by another cell, an astrocyte, can activate transient KCa currents in an associated arteriolar smooth muscle cell. Because HO products other than CO do not activate KCa channels28, the HO-2 product activating the KCa channel must be CO. Of additional significance, the signal, glutamate, is a primary mediator of brain function that does not directly affect KCa currents in cerebrovascular myocytes (Fig 1). The signal from astrocytes is necessary to provide the functional hyperemic response.
Our studies using cranial windows in vivo, found that either astrocyte injury or inhibition of CO production blocks dilation of newborn pig pial arterioles to topical glutamate application9, 26. In the present study, we show that in the absence of astrocytes or with astrocytes pretreated with CrMP, glutamate does not increase either smooth muscle cell transient KCa currents or KCa channel activity. To confirm that HO-2 is involved in this signaling pathway, piglet smooth muscle cells were allowed to contact mouse HO-2+/+ and HO-2−/− astrocytes. Glutamate increased transient KCa currents as well as KCa channel activity of smooth muscle cells attached to HO-2+/+ astrocytes, but not those attached to HO-2−/− astrocytes. We show that glutamate-induced dilation of brain slice arterioles is abrogated by either blocking the enzyme that produces CO or by blocking the KCa channel that CO activates. Further, HO inhibition prevented glutamate-induced reduction of [Ca2+]i in brain slice arterioles. All these data are consistent with the hypothesis that glutamate stimulates astrocyte HO-2, producing the diffusible gasotransmitter, CO, which activates KCa channels in adjacent cerebral microvascular smooth muscle cells.
In contrast to rat hypothalamus42 and newborn pig cerebral cortex26, the sole reported influence of the HO/CO system on vascular tone in adult rat cerebral cortex has been constrictor43. Endogenous CO appears to have a tonic inhibitory effect on NO production in rat cerebrum because the HO inhibitor, zinc protoporphrin (ZnPP) dilated pial arterioles over a 1h period and enhanced cerebral NO production as estimated using diaminofluorescein-243. The dilation was blocked by L-NAME and by addition of CO to the superfusion solution. Conceivably, contributions of CO to cerebrovascular regulation could be biphasic with basal production providing tonic inhibition of NOS with resultant increased tone, while acute CO elevations in response to stimulation lead to dilation. Also, available data on responses of isolated adult cerebral arteries to CO suggest that species differences exist. Dilation to exogenous CO added to the bathing solution has not been detected in rabbit, rat, or mouse cerebral arteries in vitro44, 45. On the other hand, CO dilates dog basilar artery segments46. In addition, in pigs, responses of pial arterioles to CO are age-dependent with juvenile pigs, and adult rats, having reduced dilations compared to baby pigs47.
CO causes dilation by activating smooth muscle cell KCa channels26,29, 48. Absence of dilation of rat cerebral arteries and arterioles to CO in vitro (above) is surprising because CO dilates peripheral rat arterioles via KCa channel activation49,50. In vascular smooth muscle cells, several KCa channels are activated by spatially localized intracellular Ca2+ transients, termed Ca2+ sparks, that elevate the local [Ca2+] into the micromolar range51. Transient KCa currents induce membrane hyperpolarization that reduces voltage-dependent Ca2+ channel activity, and thus decreases global [Ca2+]i, producing dilation. CO increases transient KCa currents by enhancing the coupling of Ca2+ sparks to KCa channels28. The KCa channel α-subunit contains a heme-binding pocket and binding of heme inhibits KCa channel activity52. CO, by binding to channel-bound heme, changes the association of heme with the KCa channel and causes activation48. Therefore, the KCa channel is functionally a heme-protein, with heme acting as the binding site for CO48.
Species and/or age differences exist in Ca2+ spark-to-KCa channel coupling efficiency that could influence arteriole smooth muscle sensitivity to CO and possibly even the role of CO in regulation of cerebrovascular circulation. In adult rat cerebrovascular smooth muscle cells, virtually all Ca2+ sparks produce transient KCa currents53, 54. Conversely, not all Ca2+ sparks produce transient KCa currents in either newborn pig28, 55 or adult human56 cerebral arterioles. Therefore, the ability of CO to hyperpolarize and dilate rat cerebral arterioles may be less44 than in newborn pig cerebral arterioles because CO increases the coupling of Ca2+ sparks to KCa channels and rat Ca2+ spark-to-KCa channel coupling is essentially unitary at physiological membrane potentials.
In the present study, rather than report transient KCa current amplitude as the mean of all transient KCa currents, we divided transient KCa currents into smaller (7–20 pA), larger (21–40 pA), and largest (>40 pA) currents. As can be seen in Table 1, transient KCa current frequency in all size categories increased upon treatment with glutamate. In piglets, smaller amplitude Ca2+ sparks do not cause transient KCa currents28,34. CO increases the number of smaller amplitude transient KCa currents because smaller, uncoupled Ca2+ sparks become coupled to KCa channels28. The addition of these smaller currents depresses the overall mean, which explains the minimal change in mean transient KCa current amplitude, even though large amplitude transient KCa currents are markedly increased. An accurate depiction of the effect of glutamate on transient KCa current amplitude requires accounting for the increased number of smaller currents. Glutamate increases frequencies of transient KCa currents across the amplitude spectrum because glutamate uses CO to increase Ca2+ spark-to-KCa channel coupling.
The present study shows glutamate increases CO production by astrocytes, but coupling mechanisms are not known. Stimulation of mGluR and iGluR increase Ca2+ oscillations in astrocytes (20–24), electrical field stimulation increases brain slice astrocyte endfoot [Ca2+]i (14,22), and inhibition of calmodulin blocks glutamate stimulation of CO production in cerebral microvessels and cortical neurons (57,58). Therefore, it is reasonable to speculate that glutamate stimulates CO production in astrocytes via elevation of astrocytic [Ca2+]i. Increasing [Ca2+]i could increase HO-2 activity directly to elevate CO production or by a variety of indirect actions including delivery of electrons from NADPH via cytochrome P450 reductase, delivery of heme to HO-2, or activation of kinases that elevate HO-2 activity. Glutamate also could increase HO-2 activity via Ca2+ independent kinase pathways. In endothelium, vascular smooth muscle, and neurons HO-2 activity can be increased by phosphorylation (31, 58). mGluR elevates diacylglycerol that stimulates protein kinase C, which could phosphorylate and activate HO-2.
In summary, the present study indicates that the neurotransmitter glutamate activates transient KCa currents and KCa channel activity in newborn pig cerebral arteriole smooth muscle cells by increasing CO production by associated astrocytes. Either inhibition of CO production or treatment with astrocyte toxin prevents glutamate-induced arteriolar dilation in piglet brain slices. These data support the conclusion that astrocytes employ CO as a diffusible messenger to cause cerebral arteriole dilation and enhance local blood flow to match increased glutamatergic neuronal activity.
Acknowledgments
SOURCES OF FUNDING: Research supported by NHLBI/NIH and NEI/NIH.
Footnotes
DISCLOSURES: none
REFERENCES
- 1.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29:229–234. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 2.Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci. 2007;27:2468–2471. doi: 10.1523/JNEUROSCI.3204-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 4.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 5.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 6.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 8.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–317. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributiions of astrocyte CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–H2904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of the glia limitans on pial arteriolar relaxation in the rat. Am J Physiol Heart Circ Physiol. 2004;287:H331–H339. doi: 10.1152/ajpheart.00831.2003. [DOI] [PubMed] [Google Scholar]
- 11.Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol. 2005;288:H382–H388. doi: 10.1152/ajpheart.00727.2004. [DOI] [PubMed] [Google Scholar]
- 12.Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, Koehler RC. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am J Physiol Heart Circ Physiol. 2000;279:H1616–H1624. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- 13.Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med. 2001;11:38–42. doi: 10.1016/s1050-1738(01)00082-2. [DOI] [PubMed] [Google Scholar]
- 14.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 15.Paulson OB, Newman EA. Does the release of potassium from astrocyte endfeet regulate cerebral blood flow? Science. 1987;237:896–898. doi: 10.1126/science.3616619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia. 2006;54:700–715. doi: 10.1002/glia.20367. [DOI] [PubMed] [Google Scholar]
- 17.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 18.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- 21.Beck A, Nieden RZ, Schneider HP, Deitmer JW. Calcium release from intracellular stores in rodent astrocytes and neurons in situ. Cell Calcium. 2004;35:47–58. doi: 10.1016/s0143-4160(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 22.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- 23.Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Straub SV, Bonev AD, Wilkerson MK, Nelson MT. Dynamic inositol trisphosphate-mediated calcium signals within astrocytic endfeet underlie vasodilation of cerebral arterioles. J Gen Physiol. 2006;128:659–669. doi: 10.1085/jgp.200609650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 26.Leffler CW, Nasjletti A, Yu C, Johnson RA, Fedinec AL, Walker N. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 27.Robinson JS, Fedinec AL, Leffler CW. Role of carbon monoxide in glutamate receptor-induced dilation of newborn pig pial arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H2371–H2376. doi: 10.1152/ajpheart.00911.2001. [DOI] [PubMed] [Google Scholar]
- 28.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 29.Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of KCa channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaggar JH. Intravascular pressure regulates local and global Ca2+ signaling in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2001;281:C439–C448. doi: 10.1152/ajpcell.2001.281.2.C439. [DOI] [PubMed] [Google Scholar]
- 31.Leffler CW, Balabanova L, Fedinec AL, Waters CM, Parfenova H. Mechanism of glutamate stimulation of CO production in cerebral microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H74–H80. doi: 10.1152/ajpheart.01081.2002. [DOI] [PubMed] [Google Scholar]
- 32.Leffler CW, Balabanova L, Fedinec AL, Parfenova H. Nitric oxide increases carbon monoxide production by piglet cerebral microvessels. Am J Physiol Heart Circ Physiol. 2005;289:H1442–H1447. doi: 10.1152/ajpheart.00464.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed A, Waters CM, Leffler CW, Jaggar JH. Ionic mechanisms mediating the myogenic response in newborn porcine cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;287:H2061–H2069. doi: 10.1152/ajpheart.00660.2004. [DOI] [PubMed] [Google Scholar]
- 34.Li A, Adebiyi A, Leffler CW, Jaggar JH. KCa channel insensitivity to Ca2+ sparks underlies fractional uncoupling in newborn cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2006;291:H1118–H1125. doi: 10.1152/ajpheart.01308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 37.Scapagnini G, D'Agata V, Calabrese V, Pascale A, Colombrita C, Alkon D, Cavallaro S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002;954:51–59. doi: 10.1016/s0006-8993(02)03338-3. [DOI] [PubMed] [Google Scholar]
- 38.Barkoudah E, Jaggar JH, Leffler CW. The permissive role of endothelial NO in CO-induced cerebrovascular dilation. Am J Physiol Heart Circ Physiol. 2004;287:H1459–H1465. doi: 10.1152/ajpheart.00369.2004. [DOI] [PubMed] [Google Scholar]
- 39.Coceani F. Carbon monoxide and dilation of blood vessels. Science. 1993;260:739. doi: 10.1126/science.8484109. [DOI] [PubMed] [Google Scholar]
- 40.Nishikawa Y, Stepp DW, Merkus D, Jones D, Chilian WM. In vivo role of heme oxygenase in ischemic coronary vasodilation. Am J Physiol Heart Circ Physiol. 2004;286:H2296–H2304. doi: 10.1152/ajpheart.00671.2003. [DOI] [PubMed] [Google Scholar]
- 41.Kanu A, Whitfield J, Leffler CW. Carbon monoxide contributes to hypotension-induced cerebrovascular vasodilation in piglets. Am J Physiol Heart Circ Physiol. 2006;291:H2409–H2414. doi: 10.1152/ajpheart.01368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horvath B, Hrabak A, Kaldi K, Sandor P, Benyo Z. Contribution of the heme oxygenase pathway to the maintenance of the hypothalamic blood flow during diminished nitric oxide synthesis. J Cereb Blood Flow Metab. 2003;23:653–657. doi: 10.1097/01.WCB.0000071890.63724.C9. [DOI] [PubMed] [Google Scholar]
- 43.Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–e114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- 44.Andresen JJ, Shafi NI, Durante W, Bryan RM., Jr Effects of carbon monoxide and heme oxygenase inhibitors in cerebral vessels of rats and mice. Am J Physiol Heart Circ Physiol. 2006;291:H223–H230. doi: 10.1152/ajpheart.00058.2006. [DOI] [PubMed] [Google Scholar]
- 45.Brian JE, Jr, Heistad DD, Faraci FM. Effect of carbon monoxide on rabbit cerebral arteries. Stroke. 1994;25:639–643. doi: 10.1161/01.str.25.3.639. [DOI] [PubMed] [Google Scholar]
- 46.Komuro T, Borsody MK, Ono S, Marton LS, Weir BK, Zhang ZD, Paik E, Macdonald RL. The vasorelaxation of cerebral arteries by carbon monoxide. Exp Biol Med. 2001;226:860–865. doi: 10.1177/153537020122600909. [DOI] [PubMed] [Google Scholar]
- 47.Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exptl. Biol. Med. doi: 10.3181/0705-BC-136. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Kaide J, Wei Y, Jiang H, Yu C, Balazy M, Abraham NG, Wang W, Nasjletti A. Carbon monoxide produced by isolated arterioles attenuates pressure-induced vasoconstriction. Am J Physiol Heart Circ Physiol. 2001;281:H350–H358. doi: 10.1152/ajpheart.2001.281.1.H350. [DOI] [PubMed] [Google Scholar]
- 50.Naik JS, Walker BR. Heme oxygenase-mediated vasodilation involves vascular smooth muscle cell hyperpolarization. Am J Physiol Heart Circ Physiol. 2003;285:H220–H228. doi: 10.1152/ajpheart.01131.2002. [DOI] [PubMed] [Google Scholar]
- 51.Perez GJ, Bonev AD, Nelson MT. Micromolar Ca2+ from sparks activates Ca2+-sensitive K+ channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281:C1769–C1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- 52.Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 53.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient KCa currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–H618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 56.Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 57.Boehning D, Sedaghat L, Sedlak TW, Snyder SS. Heme oxygenase-2 is activated by calcium-calmodulin. J Biol Chem. 2004;279:30927–30930. doi: 10.1074/jbc.C400222200. [DOI] [PubMed] [Google Scholar]
- 58.Leffler CW, Balabanova L, Sullivan CD, Wang X, Fedinec AL, Parfenova H. Regulation of CO production in cerebral microvessels of newborn pigs. Am J Physiol. 2003;285:H292–H297. doi: 10.1152/ajpheart.01059.2002. [DOI] [PubMed] [Google Scholar]