INTRODUCTION
Endoscopic bariatric procedures are gaining traction as possible treatment modalities for obesity. A major reason for its intuitive appeal is the fact that endoluminal obesity therapy represents a much-needed minimally-invasive treatment option that is currently lacking in the management strategy of this worsening epidemic. For example, treatment of coronary artery disease spans a continuum, with cardiac stenting representing a point of intervention between medications/lifestyle changes and cardiac surgery. Similarly, for treatment of degenerative joint disease, arthroscopic surgery has an important role between medication/lifestyle changes and joint replacement surgery. By comparison, in the treatment of obesity, there is currently a glaring absence of minimally invasive options between medications / lifestyle changes, which have limited efficacy, and conventional bariatric surgery, which exhibits a not insignificant morbidity and mortality profile that causes many to avoid seeking treatment.
Endoscopic bariatric procedures can potentially satisfy the need for a minimally-invasive option in one or more different capacities. For example, they may be used as a (1) early-intervention to provide weight loss or weight stabilization in early-stage obese patients who do not yet qualify for traditional bariatric surgery. Alternatively, they could be used as a (2) bridge-to-surgery to reduce operative risk for various bariatric and non-bariatric surgeries. Endoscopic bariatric procedures could also be used as a (3) primary metabolic treatment to address comorbid illnesses such as diabetes. Furthermore, they could be used as a (4) primary bariatric treatment in the traditional surgical population, with the efficacy matching the specific device risk profile. Finally, they could be used as (5) revisional treatment for patients requiring repair following traditional bariatric surgery.i [TABLE 1]
TABLE 1.
Bariatric Endoscopy—Different Points of Intervention i
| PROCEDURE CATEGORY | PROCEDURE AIM |
|---|---|
| Early Intervention | Providing weight loss or stabilization in early-stage obese patients who do not yet qualify for traditional surgery |
| Bridge to Surgery | Reducing the obesity-related operative risk for various bariatric and non-bariatric surgeries |
| Metabolic | Primarily addressing co-morbid illness (e.g. diabetes) |
| Primary | Endoscopic option for the traditional surgical population, with outcomes and risk profiles similar to those of current surgeries |
| Revision | Repairing failed bariatric surgical procedures |
This chapter will focus its discussion on the various endoscopic devices and procedures that pertain to the last two points of intervention—primary and revisional treatments. However, it is important to keep in mind that many of these devices may also be applicable to one or more of the other three categories. This depends on various device-specific attributes; such as, safety, removability or reversibility, and effect on co-morbid illness. For example, intragastric balloons may be best applied as a bridge therapy because they require removal after 6 months, while endoscopic suturing may have more wide-ranging applications as a primary treatment, revisional treatment, early intervention, or bridge-to-surgery depending on how the suturing instrument is used. Another example may include endoscopic sleeves that could be applied as both primary metabolic therapy and bridge-to-surgery given the fact they currently require removal and achieve significant metabolic endpoints. This review will largely focus on the primary and revisional applications for specific devices, as the current literature is concentrated in these areas. Study results pertaining to applications in (1) early intervention, (2) bridge-to-surgery, and (3) primary metabolic categories will be highlighted where available. Devices that are currently in human use will be preferentially discussed, followed by references to devices that may see clinical use in the near future. [TABLE 2]
TABLE 2.
Procedures/Devices – Primary, Revisional, and Future Directions
| PROCEDURE CATEGORY | PROCEDURES / DEVICES |
|---|---|
| Primary |
|
| Revision |
|
| Future Directions |
|
PRIMARY TREATMENT
Intragastric Balloons
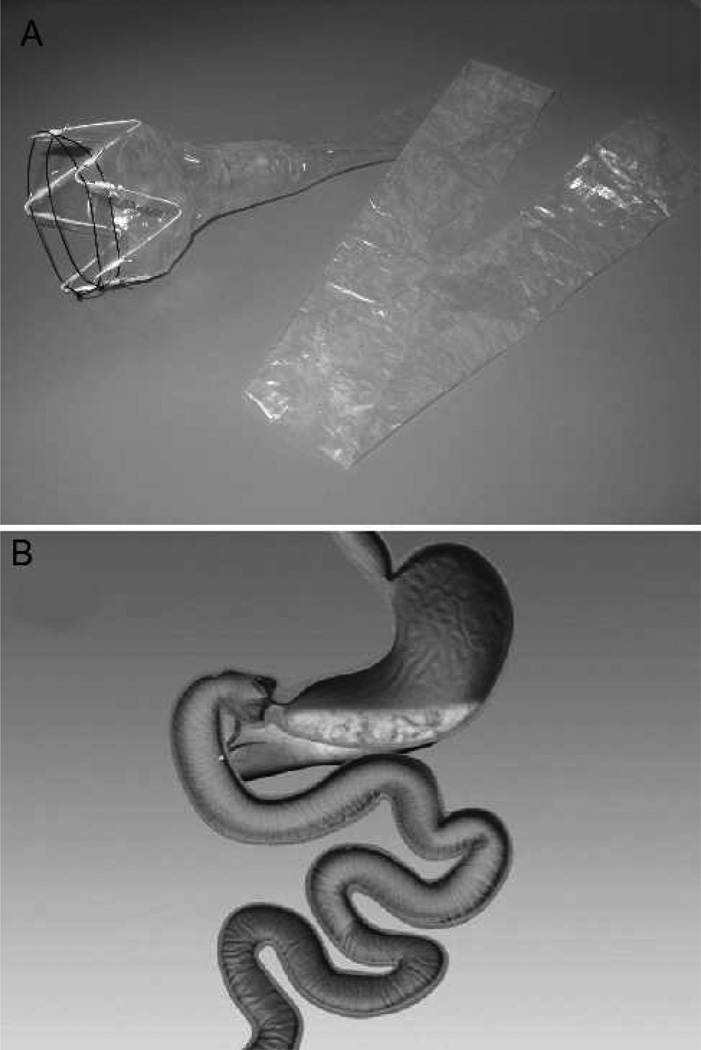
Intragastric balloons have been in use since 1982ii as a space-occupying device to induce a sense of satiety, and they represent the most frequently placed endoscopic device to date. The BioEnterics Intragastric Balloon (BIB, Allergan, Irvine, CA) has been the most extensively studied of the intragastric balloons. [FIGURE 1] The BIB is designed as a sphere to hold 400 to 800 mL of saline, and they require removal after 6 months. They are currently in use worldwide, although they are not yet available in the United States. This is in part due to a number of complications and premature balloon deflation associated mainly with the predecessor Garren-Edwards Gastric Bubble (GEGB).
FIGURE 1.
BioEnterics Intragastric Balloon (BIB, Allergan, Irvine, CA) (Permission to reuse image pending with Singapore Medical Journal)
Studies have demonstrated weight loss in the short-term and mid-term ranges, but long-term weight loss, especially following balloon removal, has been equivocal. Genco, et al retrospectively reviewed 2,515 Italian patients and showed a 6 month excess weight loss of 33.9% ± 18.7.iii However, in a randomized controlled trial comparing balloon treatment versus sham over a 3-month period in 43 patients (mean BMI of 43.3 kg/m2), Mathus-Vliegen et al found no statistical difference.iv Interestingly, after excluding 8 patients who either had not met the 3-month weight loss goal (n=5) or did not tolerate the balloon (n=3), the remaining balloon patients (n=12) exhibited a mean weight loss of 21.3 kg (%EWL of 17.1%) after 12 months. Following balloon removal at 12 months, these patients had still maintained weight loss of 12.6 kg (9.9%) at the end of the second, balloon-free year (with nutrition and physician counseling). In sum, these data suggested that in patients who tolerate therapy, balloon treatment could result in substantial 1-year weight loss, and the greater part of that weight loss could be maintained during a second, balloon-free year.
In another prospective study examining long-term outcomes after BIB placement, 100 consecutive morbidly-obese patients were enrolled to 6 months of BIB therapy with no structured weight maintenance program following balloon removal.v 97% of patients completed a mean final follow-up of 4.8 years. After 6 months, 63% of patients had ≥10% baseline weight loss. At final follow-up, only 28% were able to maintain this. At that time, 35 patients had undergone bariatric surgery and 34 patients had no significant weight change from baseline. These findings suggested that balloon implantation may be helpful in a minority of patients for long-term weight loss without a structured weight maintenance program.
Intragastric balloons have also been used in the super morbidly obese population as a bridge to conventional bariatric surgery. In one study, a BIB was placed in 26 high-risk super-obese patients with a mean BMI of 65.3 kg/m2 (+/− 9.8) and mean 4.33 +/− 1.12 severe comorbidities.vi Endoscopic placement was uneventful in all patients. However, one patient died from cardiac arrest following aspiration on the first post-insertion day. The mean weight loss was 28.5 +/− 19.6kg after the balloon was removed at 6 months. The mean reduction in comorbidity status was 4.33 to 2.23. Twenty patients underwent primary bariatric surgery the day after BIB removal, and two patients were rejected because of inadequate weight loss.
Intragastric balloons are designed for 6-month deployment, but recent studies suggest that repeated treatment may sustain weight loss at least to 1 year. In a prospective, nonrandomized multicenter study, patients with repeat treatment had greater weight loss than single-treatment patients at 1 year (12.0 kg versus 6.0 kg; 40.9% versus 20.8% EWL; p=0.008) but this difference became dampened by the 3 year mark (less than 2 kg difference).vii
In summary, intragastric balloons may represent a potential option for patients unwilling to undergo bariatric surgery or as a potential bridge to bariatric surgery with an eye towards reducing perioperative risk.
Transoral Gastric Volume Reduction (TRIM procedure) using the Bard Suturing System
The EndoCinch device (C.R. Bard, Inc., Murray Hill, N.J) was the first endoscopic suturing platform used for the treatment of obesity—originally designed for the treatment of GERD and then revisions of failed gastric bypass (discussed in the section REVISIONAL TREATMENT) and more recently for the primary treatment of obesity. The device features a hollow capsule that fits onto the endoscope tip and utilizes suction for tissue acquisition. A hollow needle is delivered through the acquired tissue to pass suture material back-and-forth. The most recent iteration of this device (the Restore Suturing System) allows for the creation of deeper, full-thickness plications and eliminates the need for device withdrawal for suture reloading as was required by its predecessor. [FIGURE 2]
FIGURE 2.
Restore Suturing System (C.R. Bard, Inc., Murray Hill, N.J) (Permission to reuse image obtained from Elsevier)
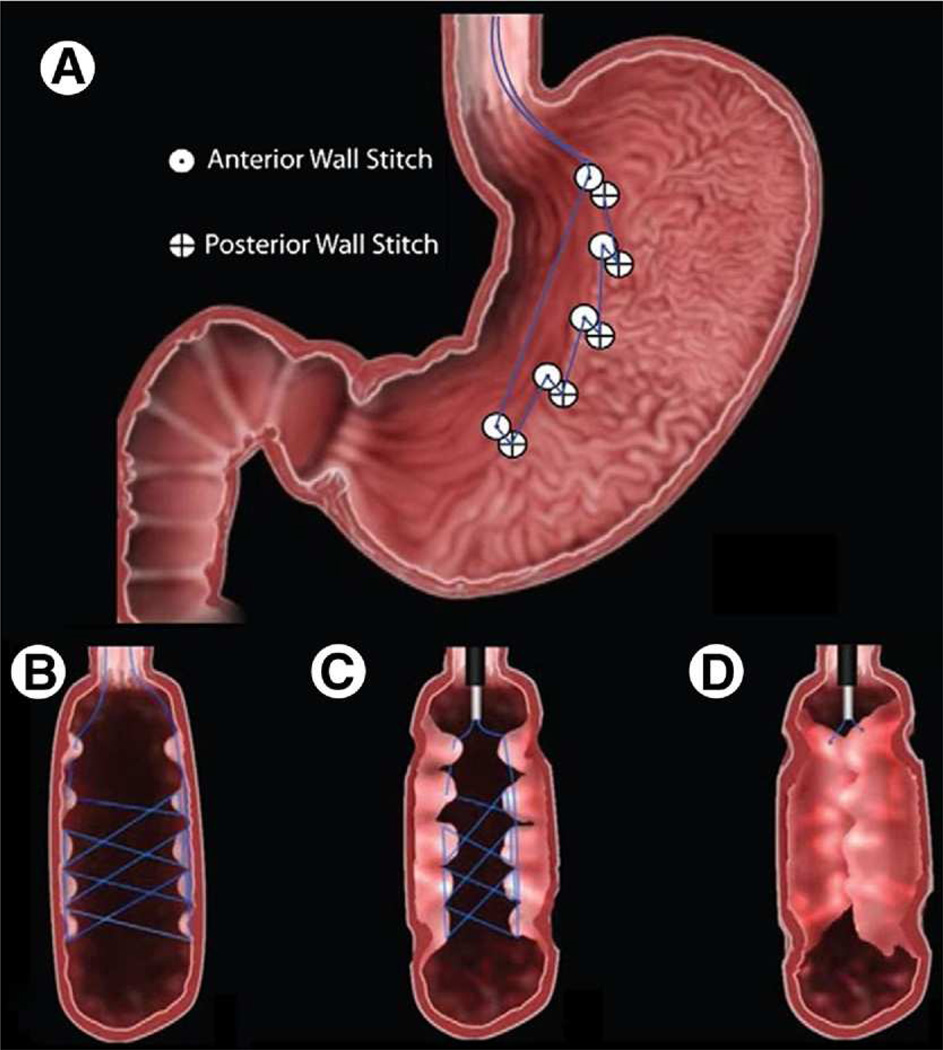
In 2008 Fogel, et al published a single-center, non-U.S. study using the EndoCinch system to perform transoral gastric volume reduction in 64 patients with a mean BMI of 39.9 (+/− 5.1) kg/m2.viii A running suture pattern was used to approximate a vertical gastroplasty. [FIGURE 3] The mean procedure time was 45 minutes, and no adverse events were reported. The mean %EWL at 12 months was 58.1 +/− 19.9. The mean BMI at 12 months was 30.6 +/− 4.7 kg/m2. When stratifying by original BMI groups, the patients with lowest BMI appeared to lose the most weight: BMI ≥40 kg/m2 had a %EWL at 12 months of 48.9 +/− 10.7. For BMI 35–40 kg/m2, the %EWL at 12 months was 56.5 +/− 13.9. For BMI <35 kg/m2, the %EWL at 12 months was 85.1 +/−24.0. These results raised the possibility of a role for transoral gastric volume reduction in early intervention of obesity.
FIGURE 3.
A running suture pattern was used to approximate a vertical gastroplasty. (A) A frontal cross-section view of the stomach after all stitches were placed. (B–D) Cross-sections of the stomach as the suture is pulled tight and secured to complete the procedure. (Permission to reuse image obtained from Elsevier)
A recent U.S. pilot study in 18 patients with a BMI of 30–56 kg/m2 was published by Brethauer et al.ix Gastric plications were created to approximate the anterior and posterior gastric walls to achieve functional volume reduction in the gastric body and fundus. [FIGURE 4] The procedure was successfully performed in all patients. The average number of plications was 6 [4–8]. The average procedure time was 125 +/− 23 minutes. There were no serious or significant procedure-related complications. The first ten patients were kept overnight per study protocol, and the remaining 8 patients were discharged on the day of the procedure. Data regarding weight loss, co-morbidity improvement, and durability are currently under assessment.
FIGURE 4.
Gastric plications were created to approximate the anterior and posterior gastric walls to achieve restriction of the upper stomach. (Permission to reuse image obtained from Elsevier)
In summary, the Restore Suturing Device places full-thickness gastric plications for gastric volume reduction. Early favorable results have been demonstrated in a wide range of BMI groups. Additional studies regarding durability of weight loss are forthcoming.
Transoral Gastroplasty using the TOGa Stapling Device
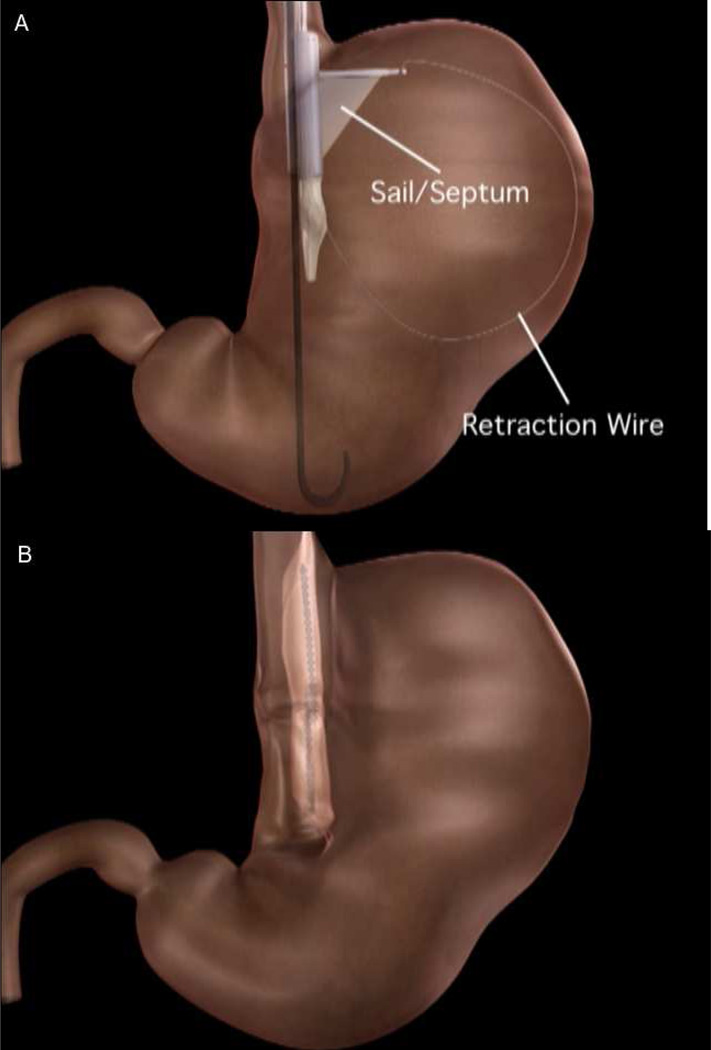
The TOGa system (Satiety Inc, Palo Alto, CA) uses a set of transoral endoscopically-guided staplers to create a restrictive sleeve by deploying a vertical staple line along the lesser curvature. [FIGURE 5] The TOGa stapler has a flexible 18-mm diameter shaft and is introduced over a guidewire. The device accommodates a standard endoscope to provide retroflexed visualization of the procedure. A septum from the device spreads and positions the anterior and posterior gastric walls, which are then apposed using vacuum. Two successive vertical staple lines are deployed to create a partial sleeve approximately 8–9 cm in length. A “restrictor” stapler is then used to staple pleats of tissue at the inferior end of the sleeve to create a restrictive “pouch.”
FIGURE 5.
(A) A depiction of the stapler in the stomach. The pediatric endoscope is positioned in the distal body and retroflexed to visualize the procedure. The stapler is advanced into the stomach and the retraction wire and sail properly align the device to optimize opposition of the anterior and posterior gastric wall when the stapler is fired. (B) A second staple line is created, resulting in a 7- to 8-cm sleeve along the lesser curvature. (Permission to reuse image obtained from Elsevier)
In a recent multicenter study enrolling 21 patients with mean BMI of 43.3 kg/m2 [35–53], the procedure was completed safely in all patients.x There were no serious adverse events. At 6 month endoscopy, all patients had full or partial stapled sleeves. However, staple line gaps were evident in 13 of the 21 patients. The average weight loss was 17.6 pounds (16.2% EWL) at 1 month, 24.5 pounds (22.6% EWL) at 3 months, and 26.5 pounds (24.4% EWL) at 6 months.
Following technical improvement, particularly in staple line deployment, the results of a second human trial were published.xi In 11 patients, the mean % EWL was 19% (1 month), 34% (3 months), and 46% (6 months). Average BMI decreased from 41.6 pre-procedure to 33.1 at 6 months. Results from a multicenter, randomized, sham-controlled study are anticipated in the near future.
Transoral Endoscopic Restrictive Implant System (TERIS)
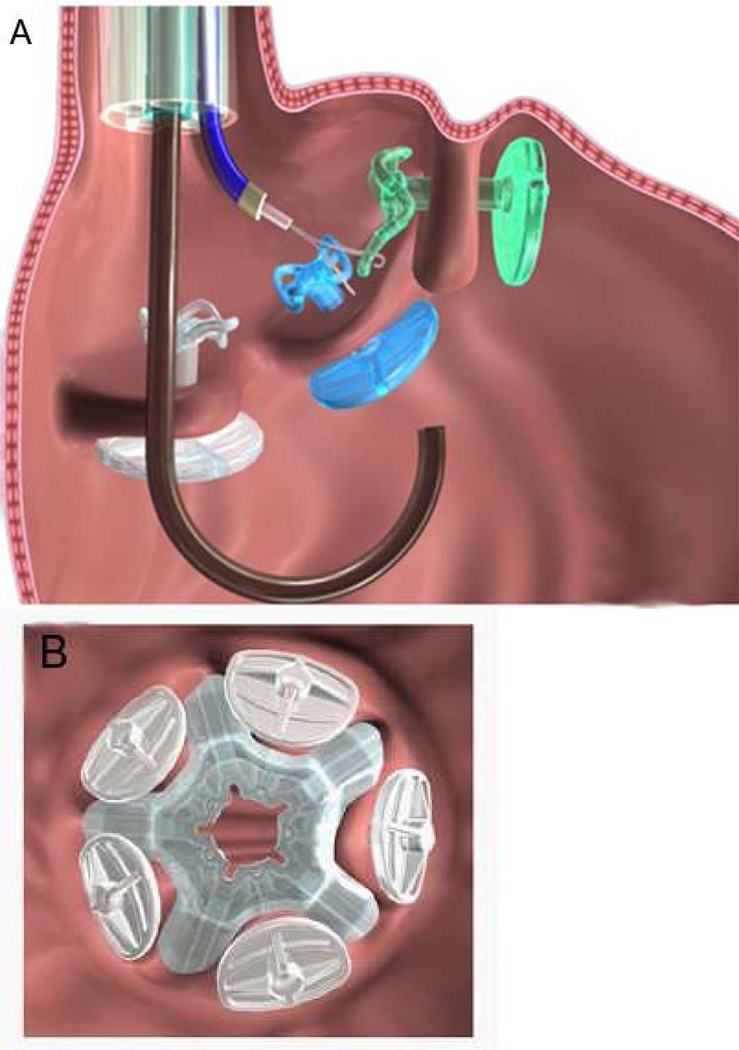
The endoscopic corollary of the gastric band, the TERIS system (Barosense, Inc, Redwood City, CA) is an endoscopic device designed to implant a prosthetic diaphragm in the gastric cardia via anchors to stapled plications. Although the device shares a similar location with the gastric band, the mechanism of action may in fact be rather different. The procedure involves the introduction of a stapler through an overtube to create full-thickness transmural plications in the cardia region. An anchor is then placed through a hole in the plication. This process is repeated until 5 plications with anchors are formed. The implant is then “parachuted” into place and locked to the anchors thereby creating a gastric pouch. [FIGURE 6]
FIGURE 6.
(A) Placement of locking graspers on anchors. (B) Gastric restrictor viewed from below. (Permission to reuse image obtained from Elsevier)
In a recent Phase I pilot study involving 13 patients with median BMI of 42.1 kg/m2, the TERIS implant was successfully deployed in 12 of the 13 patients.xii Procedural complications occurred in 3 patients, including gastric perforation related to stapler malfunctioning in 1 patient (procedure abandoned), and pneumoperitoneum in 2 patients treated with percutaneous needle decompression in one patient and conservative management in the other. At 3 months post-procedure, the median %EWL was 28%. The median BMI had decreased from 42.1 to 37.9 kg/m2. Further studies are underway.
Bypass Liners: the EndoBarrier and ValenTx Sleeves
Endoscopically-placed bypass sleeves are generating considerable interest as a type of metabolic surgery given the clinical observation that bypassing the proximal small bowel, as in procedures such as the Roux-en-Y gastric bypass, not only induces weight loss but also significantly improves Type 2 diabetes.xiii Mounting evidence indicates that proximal intestinal diversions improve glucose homeostasis by modulation of gut hormones independent of reduced food intake and body weight.xiv
There are currently two sleeve devices in human studies. The first, the EndoBarrier gastrointestinal liner, is a bypass sleeve that is seated in the duodenum and extends into the proximal jejunum. The second, the ValenTx sleeve, implants at the gastroesophageal junction and extends into the mid jejunum, thereby bypassing the stomach as well.
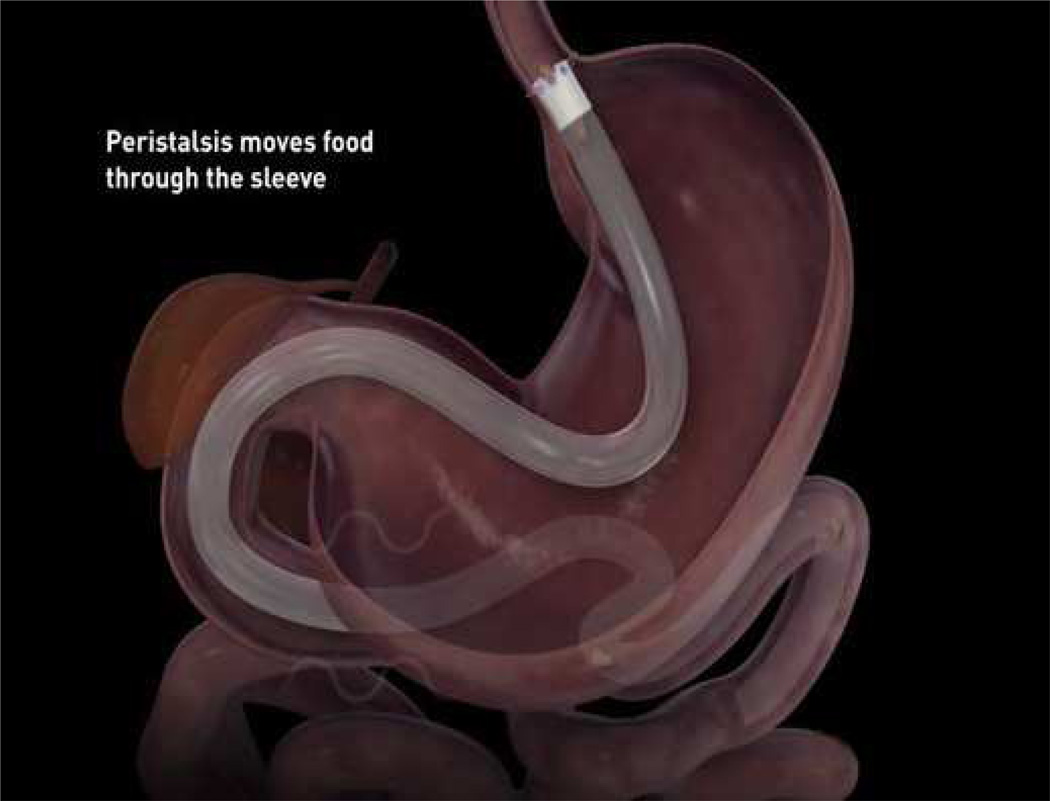
EndoBarrier
The EndoBarrier gastrointestinal liner (GI Dynamics, Lexington, MA) is endoscopically placed as a removable malabsorptive barrier that blocks both nutrient absorption and prevents mixing of food with biliopancreatic secretions in the duodenum. [FIGURE 7] The plastic liner is 60 cm long and extends into the proximal jejunum. It is attached to a self-expanding implant that seats in the duodenum. Recent published studies of the EndoBarrier have focused on its potential as both a stand-alone primary therapy for obesity as well as a bridge to bariatric surgery.
FIGURE 7.
(A) The EndoBarrier DJBS is comprised of an impermeable fluoropolymer sleeve of 60 cm and a nitinol anchor with barbs. The polypropylene drawstring is necessary for removal of the device. (B) Illustration of the EndoBarrier Gastrointestinal Liner. The device is endoscopically placed in the duodenum to form a barrier between chyme and the intestinal wall, creating a duodenal-jejunal bypass effect. (Permission to reuse images obtained from Elsevier)
Several trials have demonstrated the potential benefit of the EndoBarrier system. A pilot study of 12 patients demonstrated successful deployment in less than 30 minutes and successful removal in 40 minutes.xv Mean %EWL was 23.6%. Two patients required device explantation due to abdominal pain. All four diabetic patients who participated did not require their diabetes medications during the study duration, and all registered significant decreases in their hemoglobin A1C compared to the control group. A second, multicenter study from Chile showed significant 12 week weight loss in 24 patients treated with the EndoBarrier system compared to diet control group (22% versus 5% EWL, respectively).xvi Notably, in this study, 5 patients (20%) underwent early sleeve explantation due to bleeding (n=3), migration (n=1), and obstruction (n=1). In a recent study, 10 patients underwent EndoBarrier sleeve therapy combined with a restrictor orifice (flow restrictor) for 3 months, and the mean %EWL at the conclusion of the study was 40% +/−3%.xvii The mean total weight loss was 16.7 +/− 1.4 kg.
In a recent multicenter randomized clinical trial examining EndoBarrier therapy prior to conventional bariatric surgery, 41 patients were enrolled with 30 patients undergoing sleeve implantation and 11 patients serving as a diet control group.xviii Of the 30 patients randomized to sleeve therapy, 26 devices were successfully implanted and maintained for 3 months. Again, the 4 implantation failures were due to dislocation of the anchor (n=1), sleeve obstruction (n=1), sleeve migration (n=1), and continuous epigastric pain (n=1). Mean procedure time was 35 minutes. There were no procedure-related adverse events. Mean initial BMT was 48.9 kg/m2 for the device group and 47.4 kg/m2 for the control group. Mean %EWL at 3 months was 19.0% for device patients versus 6.9% for control (P<0.002). Absolute change in BMI at 3 months was 5.5 and 1.9 kg/m2, respectively. Type 2 diabetes mellitus was present at baseline in 8 patients of the device group and improved in 7 patients during the study period as evidenced by lower glucose levels, hemoglobin A1C and medication requirements.
In summary, the EndoBarrier has demonstrated significant weight loss at 12 weeks in a series of studies. It has also demonstrated considerable glycemic improvements in diabetic patients as well as potential utility as a bridge to bariatric surgery. Design modifications have been made to improve problems with bleeding, sleeve migration, and sleeve obstruction, and longer term trials are needed to answer questions about durability of response.
ValenTx Sleeve
ValenTx is a device that consists of a 120 cm sleeve secured at the gastric cardia.xix [FIGURE 8] The device ultimately extends into the mid-jejunum. The sleeve is impermeable and does not allow for proximal small bowel nutrient absorption. Metabolic effects occur when undigested material is presented to the mid small bowel. The device is currently implanted as a hybrid procedure, with endoscopic suturing under laparoscopic visualization. A critical part of this procedure is careful site selection and positioning of the internal cuff.
FIGURE 8.
ValenTx Sleeve (Permission to use image from ValenTx)
Early results from a pilot study were presented in abstract form in 2010. The study enrolled 22 patients with 17 patients able to maintain the device for the full 12 weeks. The primary reason for early explantation was dysphagia. Mean EWL at 8 weeks was 40.5%, and 39.9% at 12 weeks. Seven patients with preoperative diabetes mellitus all had normal blood glucose levels throughout the trial and none required anti-hyperglycemia medications. Hemoglobin A1c levels improved for all enrolled diabetic patients.
Incisionless Operating Platform (IOP) / POSE Procedure
The IOP (USGI Medical, San Clemente, CA) is a multifunctional endoscopic platform that has been applied to primary obesity therapy in a procedure called Primary Obesity Surgery, Endoluminal or POSE. [FIGURE 9] The IOP uses a specialized overtube with four channels—one for the endoscope itself and three for endoscopic instruments. One endoscopic instrument is a combined grasper with a curved hollow needle for tissue anchor deployment. The grasper has large jaws (2.5 cm in length) allowing for robust tissue acquisition and creation of full-thickness tissue plications. Plications are placed within the gastric body and fundus to reduce gastric volume.
FIGURE 9.
Incisionless Operating Platform (IOP) (USGI Medical, San Clemente, CA) (Permission from USGI Medical to use this image)
A U.S. registry of POSE patients is underway and results have not yet been reported. Presentation of early clinical results is anticipated in 2011.
REVISIONAL TREATMENT
While Roux-en-Y gastric bypass is considered one of the most effective weight loss procedures, up to 20% of bypass patients will fail to meet success criteria (defined as >50% EWL within 1 year of surgery)xx and up to 20% of bypass patients will experience significant weight regain (>15% from nadir).xxi The causes for failed Roux-en-Y gastric bypass are likely multifactorial, but mechanical etiologies such as dilation of the gastrojejunal anastomosis and/or gastric pouch are thought to contribute through loss of restriction. Recent studies have demonstrated that larger size of the gastric pouchxxii and larger diameter of the gastrojejunal stoma xxiii,xxiv correlate with increased weight gain. Surgical revision for weight regain following gastric bypass has a reported complication rate of 15% – 50% using standard approaches.xxv, xxvi Endoluminal revision of dilated gastrojejunal stomas and dilated gastric pouches theoretically has a lower risk profile and may provide a solution to some patients struggling with this problem.
Sclerotherapy
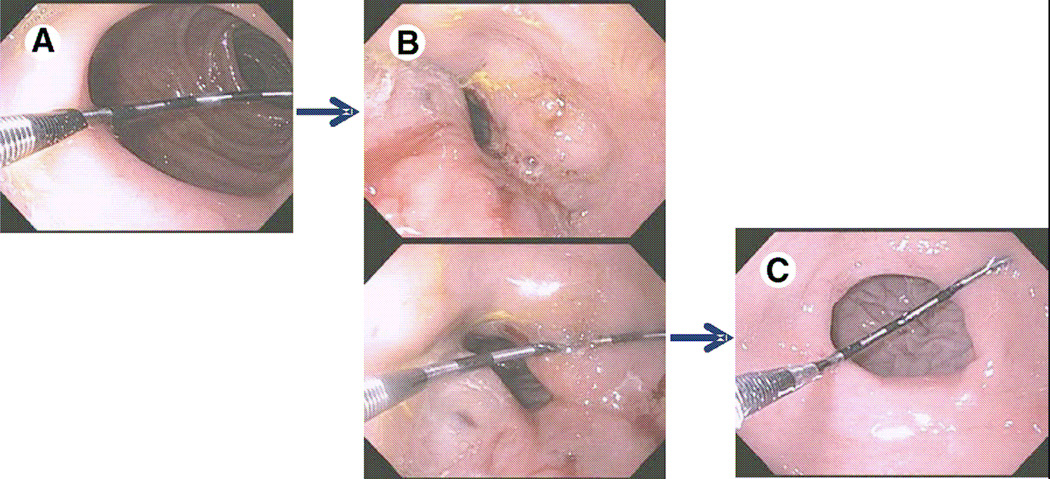
Sclerotherapy is a revisional therapy based on the injection of sclerosant in peri-stomal tissue. [FIGURE 10] Using an endoscopic injection needle, sodium morrhuate is injected around the gastrojejunal anastomosis. This is repeated at 8–12 week intervals and often requires a total of 2–3 sessions to achieve the desired outlet size of less than 12 mm. Approximately 2 cc per injection for a total of up to 20 cc per session is best. The proper technique is to create a submucosal bleb in the area and to avoid over-injection as this may lead to bleeding. On repeat procedures blebs may be difficult to create due to tissue sclerosis and care must be taken. Of note, if dark maroon or black discoloration is noted while creating a bleb, injection should be halted immediately as this may be a harbinger of bleeding.
FIGURE 10.
Gastrojejunostomy anastomosis (A) before, (B) immediately after sclerotherapy injections, and (C) 3 moths postprocedure. (Permission to reuse image obtained from Elsevier)
Published studies of sclerotherapy date to 2003.xxvii A 2007 GIE study involving 28 patient with dilated gastrojejunal stomas demonstrated that 64% of patients lost >75% of their weight regain following nadir. This weight loss was achieved after a mean of 2.3 sessions.xxviii A 2007 study retrospectively examined a cohort of 32 patients with 1 year follow-up and found that 91.6% of patients demonstrated weight loss and/or weight stabilization following sclerotherapy.xxix A subsequent 2008 study involving 71 patients showed that 72% of patients maintained or lost weight at 12 month follow-up.xxx
Sclerotherapy is procedurally straightforward. Since it uses standard endoscopic accessories, it could theoretically be performed in most endoscopy centers. Its exact mechanism of action is unknown and may involve more than restoration of a restrictive mechanism.
Bard EndoCinch Suturing System
In addition to primary obesity therapy, the Bard EndoCinch Suturing System (C.R. Bard, Inc., Murray Hill, NJ) can be used for revision of dilated gastrojejunal anastomoses. Again, the device features a hollow capsule that fits onto the endoscope tip and utilizes suction for tissue acquisition. A hollow needle is delivered through the acquired tissue to pass suture material back and forth. For revision of a dilated gastrojejunostomy, the device is used to place several interrupted stitches around a gastrojejunal outlet, after the stomal rim has undergone mucosal ablation with argon plasma coagulation. [FIGURE 11]
FIGURE 11.
(A) Mucosal ablation with APC. (B) Placement of sutures. (Permission to reuse image obtained from Springer)
Use of the Bard EndoCinch Suturing System for revision of dilated gastrojejunal anastomosis was initially reported in 2006.xxxi In this pilot study, 8 patients with a mean BMI of 40.5 kg/m2, an average stoma diameter of 25mm, and significant post-bypass weight regain (average 24kg from nadir) were included. The average post-reduction anastomosis diameter was 10mm. Six patients experienced a mean weight loss of 10kg at 4 months. Repeat procedures were performed on 3 subjects. Of those repeat reduction patients, 2 subjects showed a total weight loss of 19kg and 20kg respectively at 5 months. Out of the 11 total reduction procedures, no significant complications occurred. Average post-reduction BMI was 37.7, and percent excess weight loss was 23.4%.
In a randomized, double-blinded, sham-controlled U.S. multicenter trial (the RESTORe trial), 77 patients with mean BMI of 47.6 kg/m2 were randomized to either transoral sutured revision of a dilated gastrojejunal stoma (> 20 mm) or sham procedure.xxxii Technical success was achieved in 89% of cases (reduction of stoma to < 10 mm). There were no deaths or perforations. Patients were followed for 6 months. Two patients randomized to the suturing group could not undergo the procedure, however were kept blinded. Within this as-treated analysis, mean weight loss for the sutured group was 4.7 +/− 5.7 % versus 1.9 +/−5.2 % (p = 0.041). Mean weight loss using a last observation carried forward intent-to treat analysis was 4.2±5.4% and 1.9±5.2% (p=0.066). Weight loss or weight stabilization was achieved in 96% of the intervention group. The intervention group also demonstrated a reduction both systolic and diastolic blood pressure as well as a trend toward improvement in metabolic indices.
Notably, the EndoCinch device, the prior iteration to the Bard Suturing Device, has also been used for treatment of gastro-gastric fistulae, another cause of weight regain following Roux-en-Y gastric bypass. In a study of 95 patients who underwent gastrogastric fistulae closure either with endoscopic suturing (n=71; 75%) or endoscopic clipping (n=24; 25%), complete initial fistula closure was achieved in 90 patients (95%) with re-opening in 59 (65%) an average of 177 +/− 202 days. Patients with fistulae less than 10 mm in diameter had the best long-term results. xxxiii
Incisionless Operating Platform (IOP) / ROSE Procedure
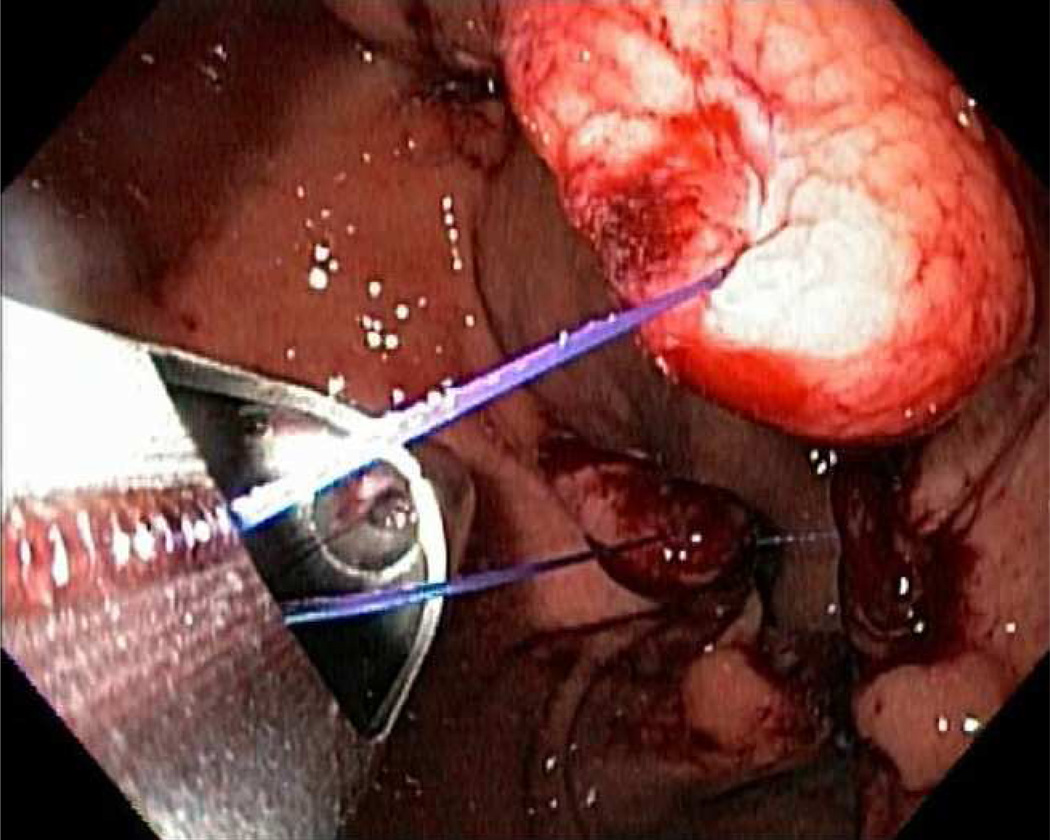
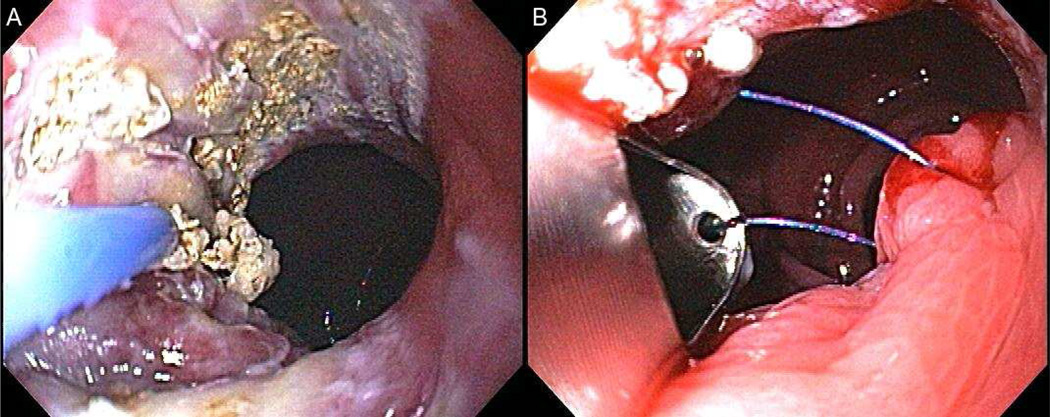
The IOP (USGI Medical, San Clemente, CA) can be used for revision of dilated gastric pouches and dilated gastrojejunal stomas. The system is the same that described above, however, the procedure is different from a technical standpoint. Plications are placed around a dilated stoma or within a dilated gastric pouch, instead of the gastric body and fundus. [FIGURE 12]
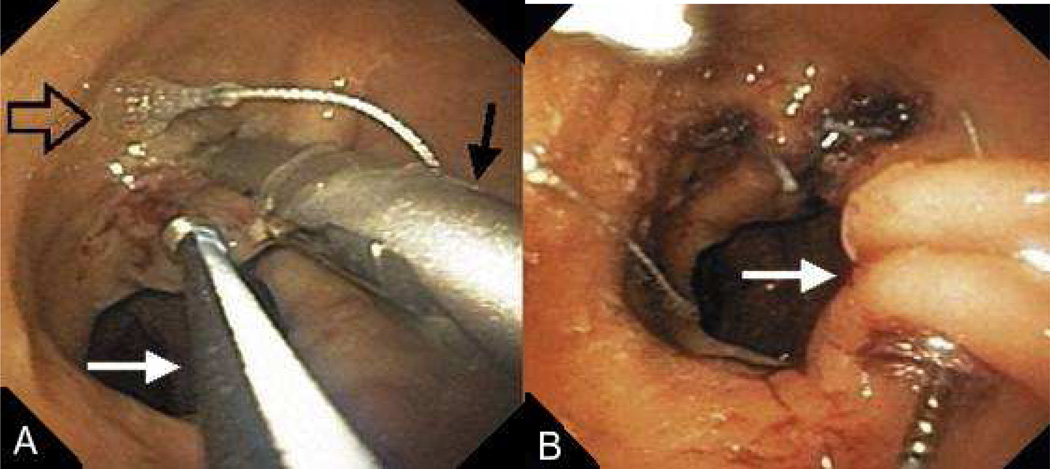
FIGURE 12.
(A) After tissue is grasped at rim of the GJA with a corkscrew-like tissue grasper (white arrow), the g-Prox is closed on tented tissue (black arrow) and the first anchor is deployed (open arrow). (B) after the g-Prox is released from the tissue, the second anchor is deployed, creating a tissue plication (arrow). (Permission to reuse image obtained from Elsevier)
Early prospective U.S. studies publishing the use of the IOP for post-bypass pouch and stoma reduction were first mentioned in 2009.xxxiv,xxxv Both studies demonstrated technical feasibility and minimal procedural complications. Mullady et al reported technical success in 85% of the cases (17/20), with a mean weight loss in successful cases of 8.8 kg at 3 months while Ryou et al reported technical success in 100% of patients (5/5) using a second gerneration device, with a mean weight loss in successful cases of 7.8 kg at 3 months.
More recently, in a prospective U.S. multicenter registry study, 116 patients were followed over 1 year following endoluminal revision using the IOP.xxxvi These patients had regained significant weight more than 2 years following RYGB after losing at least 50% of the excess body weight following RYGB. This patient cohort had also been screened for stomal and/or pouch dilation. Anchors were successfully placed in 112 (97%) of patients with an intraoperative reduction of stomal diameter and pouch length of 50% and 44%, respectively. The average procedure time was 87 minutes. No significant complications occurred. At 6 months post-procedure, an average of 32% of weight regain that had occurred after RYGB had been lost. The average %EWL was 18%. 12-month endoscopies confirmed the retention of anchors and durable plications.
StomaphyX
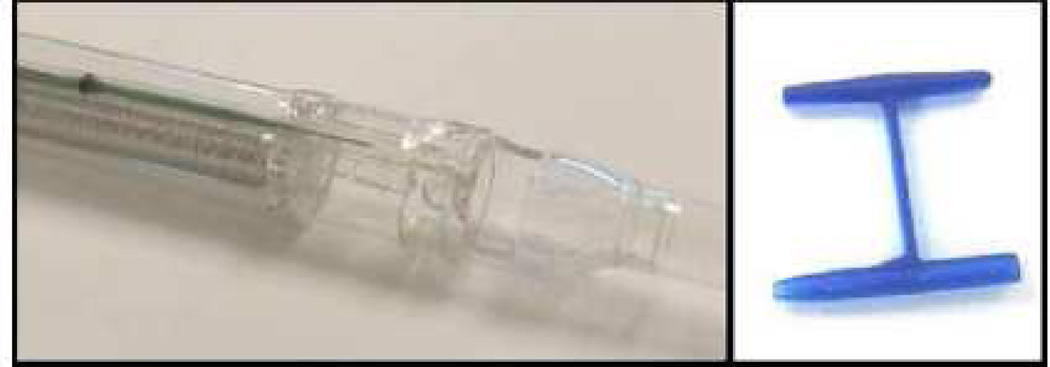
The StomaphyX device (EndoGastric Solutions, Inc., Redmond, WA) is a single-use endoluminal device for ligasure-based creation of plications in the gastric pouch. [FIGURE 13] The device uses suction for tissue acquisition and delivers polypropylene H-fasteners to secure full thickness plications. These plications are created in a circumferential manner in the gastric pouch, thereby reducing pouch volume
FIGURE 13.
StomaphyX delivery system (EndoGastric Solutions, Inc, Redmond, WA, USA). (Permission to reuse image obtained from Elsevier)
In a recent study of 39 patients with average BMI of 39.8 kg/m2, average % EWL at 1 month was 10.6% (n=34); 13.1% at 3 months (n=15); 17.0% at 6 months (n=14); 19.5% at 12 months (n=6).xxxvii Minor complications included transient sore throat in 87% of patients and transient epigastric pain in 76.9% of patients. In another study with 64 patients, the mean weight loss was 7.6 kg at a mean follow-up of 5.8 months.xxxviii
OverStitch Endoscopic Suturing System
The OverStitch (Apollo Endosurgery, Austin, TX) is in part an evolution of the original Eagle Claw suturing device. [FIGURE 14] The OverStitch mounts onto the tip of a double channel therapeutic gastroscope and uses a curved needle to deploy full-thickness sutures under direct visualization. Single-handed operation of suture deployment allows for the endoscopist to control the depth of suture placement while also maintaining visibility of the operative site. The device can deploy a running stitch as well as interrupted sutures and can also be re-loaded without scope removal.
FIGURE 14.
OverStitch (Apollo Endosurgery, Austin, TX) (Permission to use images from Apollo Endosurgery)
The OverStitch has demonstrated clinical versatility in its early human feasibility studies. It has been used in oversewing of chronic marginal ulcers and closure of various fistulae. Additionally, the OverStitch has been used for revision of dilated gastrojejunostomy in 9 patients, which was recently submitted in abstract form. Preliminary results are promising and longer term studies are underway.
OTSC(R)-Clip
An endoscopic over-the-scope clip (OTSC(R); Ovesco AG; Tubingen, Germany) has also been used to reduce the size of the gastrojejunal anastomosis. [FIGURE 15] In a recent study of 94 patients following gastric bypass with a starting mean BMI of 32.8 kg/m2 (+/− 1.9), the mean 3 month BMI was 29.7 kg/m2 (+/− 1.8), and the mean 1 year BMI was 27.4 7 kg/m2 (+/− 3.8).xxxix Best clinical results were obtained by narrowing the gastrojejunostomy by placing two clips at opposite sites, thereby reducing the outlet by more than 80%.
FIGURE 15.
Endoscopic over-the-scope clip (OTSC(R); Ovesco AG; Tubingen, Germany) (Permission to reuse image obtained from Springer)
FUTURE DIRECTIONS
As bariatric procedures have complex mechanisms of action, there is no shortage of endoscopic strategies in development. Three general developmental strategies in various stages of evaluation are discussed here.
One strategy is to induce a delayed gastric emptying state using gastric electrical stimulation xl,xli Experimental work in obese subjects of gastric electrical stimulation at a tachygastrial frequency has shown enhanced postprandial satiety and delayed gastric emptying. Further studies are required to quantify the type of weight loss that could be effected using this type of “neuromodulation.” Pyloric suturing has also been used in canine models to induce a delayed gastric emptying state.xlii While weight loss was shown in dogs that underwent pyloric suturing, long-term feasibility of this strategy remains to be determined.
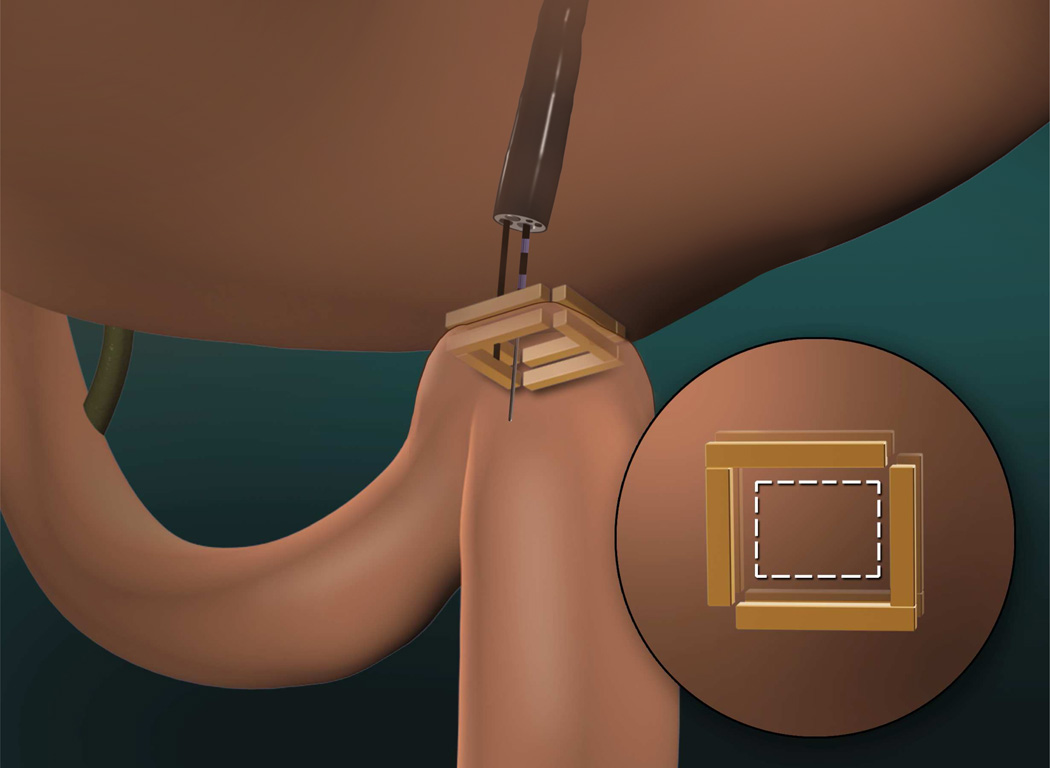
A second developmental strategy is to endoscopically re-create the most effective bariatric post-surgical anatomies, such as Roux-en-Y gastric bypass anatomy. An example is smart self-assembling magnets for endoscopy (SAMSEN) used for transoral creation of gastrojejunal anastomoses.xliii [FIGURE 16] Following endoscopic delivery, these magnets self-assemble into pre-determined configurations (e.g. square or hexagon). When reciprocal macromagnets occupy two different hollow organs (e.g. stomach and jejunum), they align and mate in order to create an anchoring window that can be cut through for an instant anastomosis. Compression anastomosis allows for the creation of a robust, large caliber fistula after several days and the magnets are then sloughed off. The implications for weight loss and for metabolic endpoints are with such approaches are obvious.
FIGURE 16.
Smart self-assembling magnets for endoscopy (SAMSEN) used for transoral creation of gastrojejunal anastomoses
Finally, endoscopic strategies specifically targeting the neuro-hormonal pathways of obesity may represent the most effective treatment options of the future—but will certainly require the most development. Potential targets include Ghrelin response to fasting or postprandial Ghrelin inhibition and/or stimulation of appetite frenators, such as peptide YY or glucagon-like peptide 1 (GLP-1). Enhanced understanding of physiologic effects of key component(s) of various bariatric surgeries and newer endoluminal therapies will undoubtedly allow more refined design of treatment options in the future.xliv
CONCLUSION
Endoluminal bariatric procedures meet a glaring need for a minimally-invasive option in the treatment of obesity. Given the increasing scope of endoluminal bariatrics, the gastroenterologist stands to play an important role in the treatment of obesity. Numerous devices and technologies are currently being developed, modified, and evaluated in early human studies. These procedures can potentially be offered as early interventions in premorbidly obese patients; as bridges to surgery in order to reduce operative risk; as primary metabolic therapies; as primary weight-loss procedures; or as revisional treatments of failed bariatric surgery. It is important for gastroenterologists to become more involved with the care of bariatric patients now, so that they better understand the disease and are fully equipped to assume more responsibility in the management of this condition moving ahead.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Ryou;s disclosures include: Beacon Endoscopy (consultant; royalties); Covidien (consultant)
Dr. Thompson’s disclosures include: Bard, Bariatric Advisory Board member/research support; USGI Medical, advisory board member; Valentx, consultant; Covidien, Endoluminal Advisory Board/educational continuing medical education activity grant; Boston Scientific, consultant and medical education activity grant in the area of endoluminal operating platforms; Beacon Endoscopy (consultant, royalties)
REFERENCES
- i.Thompson CC. Endoscopic therapy of obesity: a new paradigm in bariatric care. Gastrointest Endosc. 2010;72:505–507. doi: 10.1016/j.gie.2010.06.010. [DOI] [PubMed] [Google Scholar]
- ii.Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet. 1982;1:198–199. doi: 10.1016/s0140-6736(82)90762-0. [DOI] [PubMed] [Google Scholar]
- iii.Genco A, Bruni T, Doldi SB, et al. Bioenterics Intragastric Balloon: the Italian experiencewith 2515 patients. Obes Surg. 2005;15:1161–1164. doi: 10.1381/0960892055002202. [DOI] [PubMed] [Google Scholar]
- iv.Mathus-Vliegen EM, Tytgat GN. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc. 2005;61:19–27. doi: 10.1016/s0016-5107(04)02406-x. [DOI] [PubMed] [Google Scholar]
- v.Dastis NS, Francois E, Deviere J, et al. Intragastric balloon for weight loss: results in 100 individuals followed for at least 2.5 years. Endoscopy. 2009;41:575–580. doi: 10.1055/s-0029-1214826. [DOI] [PubMed] [Google Scholar]
- vi.Spyropoulos C, Katsakoulis E, Mead N, Vegenas K, Kalfarentzos F. Intragastric balloon for high-risk super-obese patients: a prospective analysis of efficacy. Surg Obes Relat Dis. 2007;3:78–83. doi: 10.1016/j.soard.2006.11.001. [DOI] [PubMed] [Google Scholar]
- vii.Dumonceau JM, Francois E, Hittelet A, Mehdi AI, Barea M, Deviere J. Single vs repeated treatment with the intragastric balloon: a 5-year weight loss study. Obes Surg. 2010;20:692–697. doi: 10.1007/s11695-010-0127-x. [DOI] [PubMed] [Google Scholar]
- viii.Fogel R, de Fogel J, Bonilla Y, de la Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51–58. doi: 10.1016/j.gie.2007.10.061. [DOI] [PubMed] [Google Scholar]
- ix.Bretahuer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction for weight management: technique and feasibility in 18 patients. Surg Obes Relat Dis. 2010;6:689–694. doi: 10.1016/j.soard.2010.07.012. [DOI] [PubMed] [Google Scholar]
- x.Deviere J, Ojeda Valdes G, Cuevas Herrera L, Closset J, Le Moine O, Eisendrath P, Moreno C, Dugardeyn S, Barea M, de la Torre R, Edmundowicz S, Scott S. Safety, feasibility and weight loss after transoral gastroplasty: First human multicenter study. Surg Endosc. 2008;22:589–598. doi: 10.1007/s00464-007-9662-5. [DOI] [PubMed] [Google Scholar]
- xi.Moreno C, Closset J, Dugardeyn S, Barea M, Mehdi A, Collignon L, Zalcman M, Baurain M, Le Moine O, Deviere J. Transoral gastroplasty is safe, feasible, and induces significant weight loss in morbidly obese patients: results of the second human pilot study. Endoscopy. 2008;40:406–413. doi: 10.1055/s-2007-995748. [DOI] [PubMed] [Google Scholar]
- xii.de Jong K, Mathus-Vliegen EM, Veldhuyzen EA, Eshuis JH, Fockens P. Short-term safety and efficacy of the Transoral Endoscopic Restrictive Implant System for the treatment of obesity. Gastrointest Endosc. 2010;72:497–504. doi: 10.1016/j.gie.2010.02.053. [DOI] [PubMed] [Google Scholar]
- xiii.Rubino F, Forigone A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xiv.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- xv.Rodriguez-Grunert L, Galvao Neto MP, Alamo M, et al. First human experience with endoscopically delivered and retrieved duodenal-jejunal bypass sleeve. Surg Obes Relat Dis. 2008;4:55–59. doi: 10.1016/j.soard.2007.07.012. [DOI] [PubMed] [Google Scholar]
- xvi.Tarnoff M, Rodriguez L, Escalona A, et al. Open label, prospective, randomized controlled trial of an endoscopic duodenal-jejunal bypass sleeve versus low calorie diet for pre-operative weight loss in bariatric surgery. Surg Endosc. 2009;23:650–656. doi: 10.1007/s00464-008-0125-4. [DOI] [PubMed] [Google Scholar]
- xvii.Escalona A, Yanez R, Pimentel F, Galvao M, Ramos AC, Turiel D, Boza C, Awruch D, Gersin K, Ibanez L. Initial human experience with restrictive duodenal-jejunal bypass liner for treatment of morbid obesity. Surg Obes Relat Dis. 2010;6:126–131. doi: 10.1016/j.soard.2009.12.009. [DOI] [PubMed] [Google Scholar]
- xviii.Schouten R, Rijs CS, Bouvy ND, Hameeteman W, Koek GH, Janssen IM, Greve JW. A multicenter, randomized efficacy study of the EndoBarrier Gastrointestinal Liner for presurgical weight loss prior to bariatric surgery. Ann Surg. 2010;251:236–243. doi: 10.1097/SLA.0b013e3181bdfbff. [DOI] [PubMed] [Google Scholar]
- xix.Sandler BJ, Swain CP, Rumbaut R, Torres G, Morales L, Gonzales L, Horgan S. First human experience with endoluminal, endoscopic gastric bypass [Abstract] Surg Endosc. 2010;24:S226–S227. [Google Scholar]
- xx.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–2796. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- xxi.McCormick JT, Papsavas PK, Caushaj PF, Gagne DJ. Laparoscopic revision of failed open bariatric procedures. Surg Endosc. 2003;17:413–415. doi: 10.1007/s00464-002-8533-3. [DOI] [PubMed] [Google Scholar]
- xxii.Roberts K, Duffy A, Kaufman J, Burrell M, Dziura J, Bell R. Size matters: gastric pouch size correlates with weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Endosc. 2007;21:1397–1402. doi: 10.1007/s00464-007-9232-x. [DOI] [PubMed] [Google Scholar]
- xxiii.Mali J, Jr, Fernandes FA, Valezi AC, Matsuo T, Menezes Mde A. Influence of the actual diameter of the gastric pouch outlet in weight loss after silicon ring Roux-en-Y gastric bypass: an endoscopic study. Obes Surg. 2010;20:1231–1235. doi: 10.1007/s11695-010-0189-9. [DOI] [PubMed] [Google Scholar]
- xxiv.Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2010 Nov 17; doi: 10.1016/j.cgh.2010.11.004. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxv.Coakley BA, Deveney CW, Spight DH, Thompso SK, Le D, Jobe BA, Wolfe BM, McConnell DB, O’Rourke RW. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008;4:581–586. doi: 10.1016/j.soard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- xxvi.Gagner M, Gentileschi P, de Csepel J, Kini S, Patterson E, Inabnet WB, Herron D, Pomp A. Laparoscopic reoperative bariatric surgery: experience from 27 consecutive patients. Obes Surg. 2002;12:254–260. doi: 10.1381/096089202762552737. [DOI] [PubMed] [Google Scholar]
- xxvii.Spaulding L. Treatment of dilated gastrojejunostomy with sclerotherapy. Obes Surg. 2003;13:254–257. doi: 10.1381/096089203764467162. [DOI] [PubMed] [Google Scholar]
- xxviii.Catalano MF, Rudic G, Anderson AJ, Chua TY. Weight gain after bariatric surgery as a result of a large gastric stoma: endotherapy with sodium morrhuate may prevent the need for surgical revision. Gastrointest Endosc. 2007;66:240–245. doi: 10.1016/j.gie.2006.06.061. [DOI] [PubMed] [Google Scholar]
- xxix.Spaulding L, Osler T, Patlak J. Long-term results of sclerotherapy for dilated gastrojejunostomy after gastric bypass. Surg Obes Relat Dis. 2007;3:623–626. doi: 10.1016/j.soard.2007.07.009. [DOI] [PubMed] [Google Scholar]
- xxx.Loewen M, Barba C. Endoscopic sclerotherapy for dilated gastrojejunostomy of failed gastric bypass. Surg Obes Relat Dis. 2008;4:539–542. doi: 10.1016/j.soard.2007.09.014. [DOI] [PubMed] [Google Scholar]
- xxxi.Thompson CC, Slattery J, Bundga ME, Lautz DB. Peroral endoscopic reduction of dilated gastrojejunal anastomosis after Roux-en-Y gastric bypass: a possible new option for patients with weight regain. Surg Endosc. 2006 Nov;20(11):1744–1748. doi: 10.1007/s00464-006-0045-0. Epub 2006 Oct 5. PubMed PMID: 17024527. [DOI] [PubMed] [Google Scholar]
- xxxii.Thompson CC, Roslin MS, Bipan C, Chen YK, DeMarco DC, Miller LS, Schweitzer M, Rothstein RI, Lautz DB, Ryan MB, Brethauer SA, Schauer PR, Mitchell MC, Starpoli AA, Haber GB, Catalano MF, Edmundowicz SA, Fagnant A, Kaplan LM. RESTORe: Randomized Evaluation of Endoscopic Suturing Transorally For Anastomotic Outlet Reduction: A Double-Blind, Sham-Controlled Multicenter Study for Treatment of Inadequate Weight Loss or Weight Regain Following Roux-en-Y Gastric Bypass. Gastroenterology. 2010;138(5, Supplement 1):S-388. [Google Scholar]
- xxxiii.Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis. 2010;6:282–288. doi: 10.1016/j.soard.2010.02.036. [DOI] [PubMed] [Google Scholar]
- xxxiv.Ryou MK, Mullady DK, Lautz DB, Thompson CC. Pilot study evaluating technical feasibility and early outcomes of second-generation endosurgical platform for treatment of weight regain after gastric bypass surgery. Surg Obes Relat Dis. 2009 Jul-Aug;5(4):450–454. doi: 10.1016/j.soard.2009.03.217. Epub 2009 Apr 8. PubMed PMID: 19632645. [DOI] [PubMed] [Google Scholar]
- xxxv.Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes (with video) Gastrointest Endosc. 2009 Sep;70(3):440–444. doi: 10.1016/j.gie.2009.01.042. Epub 2009 Jun 24. PubMed PMID: 19555944. [DOI] [PubMed] [Google Scholar]
- xxxvi.Horgan S, Jacobsen G, Weiss GD, Oldham JS, Jr, Denk PM, Borao F, Gorcey S, Watkins B, Mobley J, Thompson K, Spivack A, Voellinger D, Thompson C, Swanstrom L, Shah P, Haber G, Brengman M, Schroder G. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. 2010;6:290–295. doi: 10.1016/j.soard.2009.12.011. [DOI] [PubMed] [Google Scholar]
- xxxvii.Mikami D, Needleman B, Narula V, Durant J, Melvin WS. Natural orifice surgery: initial US experience utilizing the StomaphyX device to reduce gastric pouches after Roux-en-Y gastric bypass. Surg Endosc. 2010;24:223–228. doi: 10.1007/s00464-009-0640-y. [DOI] [PubMed] [Google Scholar]
- xxxviii.Letiman IM, Virk CS, Avgerinos DV, Patel R, Lavarias V, Surick B, Holup JL, Goodman ER, Karpeh MS., Jr Early results of trans-oral endoscopic placation and revision of the gastric pouch and stoma following Roux-en-Y gastric bypass surgery. JSLS. 2010;14:217–220. doi: 10.4293/108680810X12785289144197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- xxxix.Heylen AM, Jacobs A, Lybeer M, Prosst RL. The OTSC(R)-Clip in revisional endoscopy against weight regain after bariatric gastric bypass surgery. Obes Surg. 2010 Sep 3; doi: 10.1007/s11695-010-0253-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- xl.Liu J, Hou X, Song G, et al. Gastric electrical stimulation using endoscopically placed mucosal electrodes reduces food intake in humans. Am J Gastroenterol. 2006;101:798–803. doi: 10.1111/j.1572-0241.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- xli.Wang J, Song J, Hou X, Liu J, Chen JD. Effects of cutaneous gastric electrical stimulation on gastric emptying and postprandial satiety and fullness in lean and obese subjects. J Clin Gastroenterol. 2010;44:335–339. doi: 10.1097/MCG.0b013e3181d34572. [DOI] [PubMed] [Google Scholar]
- xlii.Vegesna A, Korimilli A, Besetty R, Bright L, Milton A, Agelan A, McIntyre K, Malik A, Miller L. Endosocpic pyloric suturing to facilitate weight loss: a canine model. Gastrointest Endosc. 2010;72:427–431. doi: 10.1016/j.gie.2010.03.1071. [DOI] [PubMed] [Google Scholar]
- xliii.Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling Magnets for Endoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video) Gastrointest Endosc. 2010 Dec 21; doi: 10.1016/j.gie.2010.10.024. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- xliv.Stylopoulos N, Aguirre V. Mechanisms of bariatric surgery and implications for the development of endoluminal therapies for obesity. Gastrointest Endosc. 2009;70:1167–1175. doi: 10.1016/j.gie.2009.01.022. [DOI] [PubMed] [Google Scholar]