Abstract
In the past, manufacturers’ labeling of sunscreen varied greatly, confusing the consumers regarding efficacy and the appropriate photoprotection provided by their products. Therefore, in June 2011, the United States Food and Drug Administration issued new guidelines for sunscreen labeling. Sunscreen products are over-the-counter drugs; therefore, they are regulated by the United States Food and Drug Administration to determine safety, efficacy, and labeling. This article discusses ultraviolet radiation and the positive and negative effects of ultraviolet radiation, provides a review of sunscreens, and discusses the new United States Food and Drug Administration regulations for sunscreens.
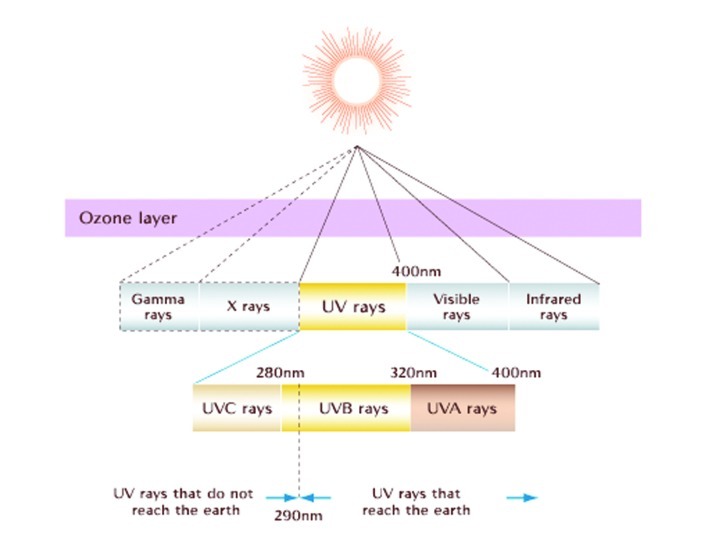
Since the skin is the largest organ of the human body, the importance of maintaining homeostasis and protecting the skin from ultraviolet radiation (UVR) is important. Imbalances can result in wrinkles, hair loss, blisters, rashes, life-threatening cancers, and disorders in immune regulation. There are three types of UV radiation: UVA, UVB, and UVC. UVC is not as much of a concern because its rays are blocked by the ozone layer and therefore do not reach the earth's surface.1 Photoprotection from both UVA and UVB radiation is more of a concern for patients.
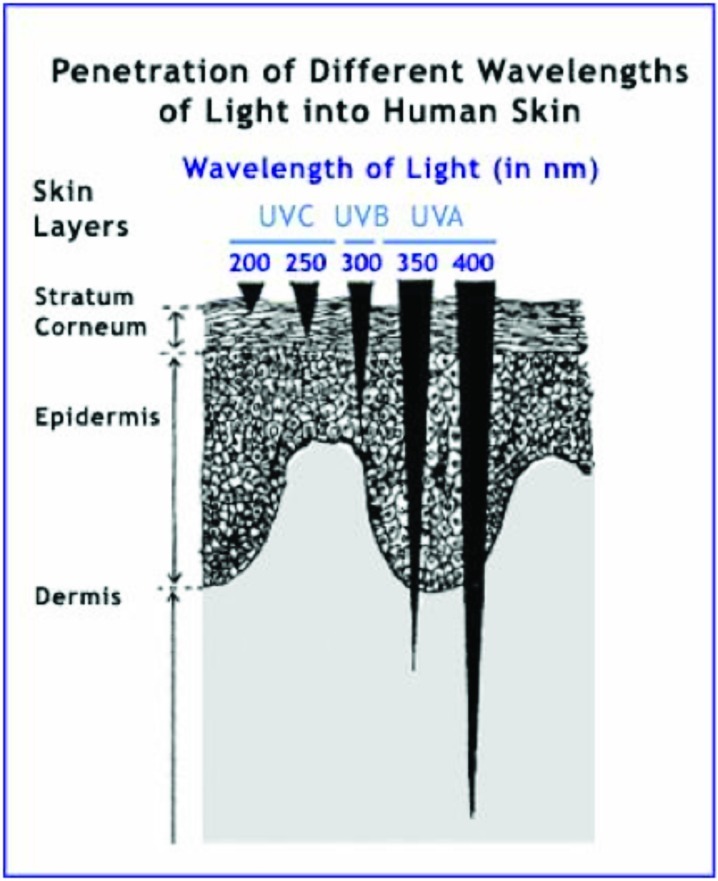
UVA (320–400nm) has a longer wavelength; therefore, its rays penetrate deeper into the skin through both the epidermis and dermis (Figure 1). UVA can be further subdivided into UVA I (320–400nm or “far UVA”) and UVA II (320–340nm or “near UVA”). UVA rays are present throughout the day, even in the morning and late afternoon. Because UVA can penetrate window glass, a very photosensitive patient may even have difficulty indoors. Studies document that multiple low-dose UVA exposures in humans are associated with significant dermal and epidermal histological changes.2,3 UVB (290–320 nm), also known as the “burn rays,” are typically what we think of with sunscreen coverage. Most automobile glass and windows block these rays (Figure 2).
Figure 1.
Penetration of different wavelengths of light into human skin
Sources: 1. Shaath NA. Sunscreens, development, evaluation, and regulatory aspects. New York, NY: Marcel Dekker, Inc; 1997:211‒233. 2. Moyal D, Fourtanier A. Photoaging. New York, NY: Marcel Dekker, Inc; 2004:15–32. 3. Kullavanijaya P, Lim HW. J Am Acad Dermatol. 2005;52:937–958.
Figure 2.
The ultraviolet component of the electromagnetic spectrum Source: http://www.bioscience.org/1997/v2/d/soehnge/3.htm
POSITIVE EFFECT OF UVR
Exposure to UVR is not always considered bad. In fact, UVR has been found to be particularly helpful in treating vitamin D deficiency, seasonal affective disorders, psoriasis, sarcoidosis, mycosis fungoides, and numerous other cutaneous conditions.
Vitamin D is important for calcium absorption from the intestinal tract to help maintain strong bones. Vitamin D has to go through a series of steps to become activated and useful within the body. Within the epidermis, 7-dehydrocholesterol is converted to vitamin D (cholecalciferol) by UVB light. Then through a series of steps in the liver and kidneys, vitamin D's activated component 1,25 dihydroxyvitamin D3 stimulates intestinal absorption of calcium. Short and limited solar exposure is usually sufficient to maintain adequate vitamin D levels. The elderly and young children are the ones who are particularly susceptible to vitamin D deficiency. Vitamin D deficiency can lead to rickets in children, osteomalacia in adults, osteopenia/osteoporosis, and factures in the elderly. The Institute of Medicine recommends the following vitamin D allowances: 400 IU for 0 to 12 months, 600 IU for 1 to 70 years, and 800 IU for greater than 70 years.4
Light therapy is an inexpensive treatment and can be beneficial in treating certain diseases. The use of UVR in the treatment of some patients with seasonal affective disorders has been successful. In fact, a meta-analysis of randomized trials found that bright light and dawn stimulation therapies reduce the severity of depression in patients with seasonal affective disorder.5 Additionaly, difficult-to-treat psoriasis patients sometimes find relief with UVR. It is thought that UVR has both antiproliferative and anti-inflammatory effects through downregulation of T-cell response to antigens.6 Studies have also shown improvement of the cutaneous effects of sarcoidosis with UVA-1 light and topical psoralen plus UVA (PUVA) therapy.7–10 PUVA and narrowband UVB has been shown to induce and maintain remissions of mycosis fungoides.11–16
NEGATIVE EFFECT OF UVR
The negative effects of the sun have been documented in the literature. Chronic sun exposure creates premature cutaneous aging, decreases immune response to environmental pathogens, and increases the risk for developing premalignant and malignant neoplasms. On the molecular level, exposure to UV radiation can result in a covalent joining of pyrimidine (usually thymine) dimers. If deoxyribonucleic acid (DNA) repair mechanisms, such as nucleotide excision repair, base excision repair, or mismatch repair genes, do not recognize dimers, the mutations go uncorrected to the cell cycle. When mutated genes reach the cell cycle, if not repaired by the induction of the p53 pathway, a series of changes can result in malignant transformation and immunosupression.17
UV-induced immunosuppression contributes to skin cancer due to damage to DNA and inhibition of protective mechanism within the skin. A common type of sun-related skin damage is actinic keratosis (AK). Age, Fitzpatrick skin type 1 or 2, and UV light are the major risk factors for developing AK.18,19 Most AKs do not progress into invasive squamous cell carcinoma (SCC), but the risk is still present. The risk of malignant transformation of an AK to SCC within one year is approximately 1 in 1,000.20 However, approximately 60 percent of invasive SCCs of the skin probably arise from AKs.21,22 If not treated or protected against additional sun damage, AKs may eventually progress to invasive SCC. Avoiding sun exposure and daily application of sunscreen statistically decreases the number of AKs.23
The diagnosis of SCC has increased over the past 20 years. Tanning beds, higher levels of sun exposure, the aging population, and improved skin cancer detection are likely to blame.24,25 The most important risk factor for SCC is cumulative sun damage and age. In a case-controlled study of 58 patients with cutaneous SCC, the risk was greatest in those with more than 30,000 hours of cumulative lifetime sun exposure.26 UVA, UVB, PUVA, and tanning beds have been shown to increase the incidence of cutaneous SCC. Prevention of SCC includes protection from the sun, including the use of protective clothing and application of sunscreen.
Basal cell carcinoma (BCC) is the most common skin cancer and occurs most frequently on the face and head. In Caucasians, the incidence of BCC has steadily increased and the lifetime risk of developing BCC is 30 percent.27 BCC arises from the basal layer of epidermis and its appendages. The most important risk factor is chronic UVR. Other known risk factors include fair skin, light eyes, red hair, chronic arsenic exposure, therapeutic radiation, immunosuppression, basal cell nevus syndrome, and various other genetic pre-dispositions. Primary prevention is protection from sun exposure beginning at an early age.
Melanoma is the most serious form of skin cancer. Despite early screening and detection programs, the overall mortality rate from melanoma has remained stable or continues to rise.28 The incidence of melanoma has more than tripled in the Caucasian population in the United States over the past 20 years. It is estimated that approximately 68,130 new cases of invasive melanoma have been diagnosed in 2010.29 Intense and intermittent sun exposure at a young age increases a patient's risk of melanoma. Individuals with five or more severe sunburns in childhood or adolescence have an estimated twofold greater risk of developing melanoma.30 Melanoma is commonly found on areas sporadically exposed to UVR, such as the back of the legs in women and the backs of men.30–32 UVB, UVA, and PUVA therapy all have been proven to increase the risk of melanoma. Tanning beds have been found to particularly increase a patient's risk for melanoma. A meta-analysis of 19 population based, case-controlled studies and one cohort study found a modest increase of risk for “ever” versus “never” exposed to tanning bed (summary RR1.15, 95% CI 1.00–1.31). There was a 75-percent increase in the risk of cutaneous melanoma found among individuals who utilized tanning beds before the age of 35 (RR 1.75, 95% CI 1.35–2.25).33 Appropriate UVR protection decreases the risk of developing a melanoma or having a secondary melanoma. Studies indicate that decreasing recreational sun exposure following the diagnosis of primary melanoma can significantly decrease the chance of developing a second melanoma.34,35
UVR IS INEVITABLE SO WHY ENCOURAGE PHOTOPROTECTION?
Sunscreens represent a practical approach to photoprotection for skin. The importance of beginning sun protection at a young age cannot be overstated. In humans, the regular use of sunscreens has been shown to reduce AKs,36,37 solar elastosis,37 UV-induced immunosupression,38 and photosensitivities. Sunscreens also prevent the formation of SCCs in animals.39 A thorough understanding of the mechanism of action of sunscreens, different sunscreen vehicle choices, and adverse effects can help educate patients on their choice of sunscreens. Sunscreens are classically divided into physical or chemical sunscreens.
Chemical sunscreens are organic and generally aromatic compounds conjugated with a carbonyl group.40 They are designed to absorb high-intensity UVR, produce excitation to a higher energy state, and, with the return to the ground state, result in conversion of the absorbed energy into a longer, lower energy wavelength.41 Chemical sunscreens can be classified based on their portion of UV coverage. Commonly known ingredients for UVB sunscreen protection are Padimate O, octinoxate, octisalate, octocrylene, and ensulizole. Commonly known UVA sunscreen ingredients are oxybenzone, meradimate, avobenzene, and tetraphthalydine dicamphor sulfonic acid. Broad spectrum is a term designed to mean protection from both UVA and UVB.41 Physical blockers are inorganic and reflect, scatter, and/or absorb UVR.42 Examples of physical blockers are titanium dioxide and zinc oxide.
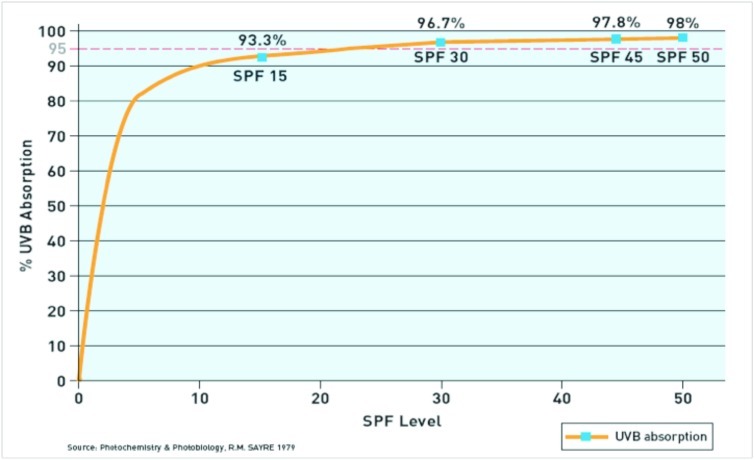
Sunscreens were designed to protect the skin from the sun. In June 2011, the United States Food and Drug Administration (FDA) released a sunscreen monograph providing qualitative definitions for labeling sun protection products. Sun protection factor (SPF) is primarily a measure of UVB absorption. SPF is defined as the dose of UVR required to produce one minimal erythema dose (MED) on protected skin after application of 2mg/cm2 of product divided by the UVR to produce one MED on unprotected skin.41 An SPF of 15 correlates with 93.3 percent of UVB absorption, whereas SPF 30 correlates with 96.7 percent, SPF 45 correlates with 97.8 percent, and SPF 50 correlates with 98 percent UVB absorption (Figure 3).43 The formula to calculate sunscreen percentage absorption based on SPF is: absorption = 100 – (100/SPF).
Figure 3.
Ultraviolet B protection level by sun protection factor (SPF)
Although a variety of methods have been proposed, there is currently no consensus as to the best method for measuring UVA protection.44
SUNSCREEN VEHICLES
The vehicle used for sunscreen protection is important to consider because ultimately it can affect the strength of UV absorbance and patient adherence.45
Oil-in-water and water-in-oil systems are the most commonly used sunscreens and for good reasons. They are easy to apply and the oil provides the UV absorption. The only real drawback is that the lotions may be thick or leave a greasy feel. Products marketed as “sports lotions” or “ultrasheer” are less oily.46
Gels are water based; therefore, they are a good option for people who have oily skin. Gel-based sunscreens are less greasy than oil based but they are more easily removed by perspiration or water. Gels also tend to cause facial and eye stinging.46
Sprays are convenient, but are often difficult to apply evenly and may leave a film. Sprays are good for applying sun protection to the scalp. Sticks are lipid-soluble formulas and are useful in protecting areas of the body, such as the lips, nose, or around the eyes.
Cosmetics, such as foundation makeup, help provide an everyday protection. The SPF in cosmetics ranges from 4 to 30, and by virtue of its opacity, foundation makeup also provides some UVA protection.46
ADVERSE EFFECTS OF SUNSCREEN
In some people, sunscreen protection can cause adverse reactions that cause them to choose not to protect their skin. In a longitudinal study of 603 subjects applying sunscreen daily, 19 percent developed adverse reactions and the majority of reactions were irritant in nature.47 The most common irritation complaint is stinging or burning of the eye area when applying the sunscreen.46 Contact dermatitis is a longer lasting irritation that can be classified as an irritant or allergic contact dermatitis. Para-aminobenzoic acid (PABA), PABA esters, benzophenones, fragrances, and preservatives account for most of the reactions.48,49 Acne is another common complaint caused predominantly by the vehicle rather than the ingredients within the sunscreen. Gels or sprays with less oil may reduce this adverse effect.46
CHANGES EXPECTED WITH NEW FDA REGULATIONS ON SUNSCREEN
On June 14, 2010, the FDA announced significant changes to sunscreen products. The final rule was effective as of June 18, 2012. The changes ensure that sunscreen products are appropriately labeled and tested and provide greater consumer protection from the skin damage caused by excessive sun exposure. The most important revisions are clarifying the meaning of broad spectrum, allowing consumers to understand the risks of using an SPF of less than 15, and defining more precisely how long the SPF can retain its protection.
SPF will be required to read as “SPF numerical number” or “broad spectrum numerical number” depending on whether the sunscreen product passes or fails the broad-spectrum test. In order to be labeled as broad spectrum, the sunscreen has to pass the critical wavelength equal to or greater than 370nm, and there must also be an increase in UVA protection as SPF protection increases.50 Any sunscreen product that fails the test or has an SPF value less than 15 will require a warning statement of the adverse effects of sun damage. The labeled warning must read, “Skin cancer/skin aging alert: spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early aging.”50
At this time, the proposed UVA star rating will not be required on the principal display panel (PDP) because the belief is that the presence of stars will lead to consumer confusion. A new direction statement must also be included in the labeling section informing consumers that exposure to the sun increases the risk of skin cancer and early skin aging. The label must also provide a list of specific sun protection measures that can decrease their risk.
Manufactures are now prohibited from making claims that are considered unproven or absolute, such as “waterproof,” and “all day protection,” or labeling products as “sunblocks.” PDP for water resistance must now specify how much time the sunscreen product was shown to retain the labeled SPF level of protection. The two times permitted in labeling are 40 minutes or 80 minutes.50
The FDA is currently proposing that the maximum sunscreen rating be SPF 50, unless there is adequate data showing added benefit to consumers for SPF values higher than 50. The FDA feels it is misleading to consumers because no added benefit above SPF 50 has been established.
CONCLUSION
Although there are some positive effects of UVR, the negative effects can potentially be life threatening. Encouraging photoprotection is currently the best preventative measure to maintain homeostasis within the skin. It is important to educate patients on how to pick an appropriate sunscreen because this will increase compliance. New FDA regulations recommend using broad-spectrum sunscreens with an SPF of at least 15, applying sunscreen 15 minutes before sun exposure, and reapplying no less than every two hours. Dermatologists should encourage their patients to limit time in the sun especially between 10am and 2pm. If a patient must be out in the sun, dermatologists should recommend wearing protective clothing, such as hats, long-sleeved shirts, pants, and eye protection.50
| FAST FACTS FOR PATIENTS |
|---|
|
Footnotes
DISCLOSURE:The authors report no relevant conflicts of interest.
REFERENCES
- 1.Harber LC, Bickers DR. Toronto: BC Decker: 1989. Photosensitivity Diseases: Principles of Diagnosis and Treatment; p. 18. [Google Scholar]
- 2.Lavker RM, Gerberick GF, Veres D, et al. Cumulative effects from repeated exposures to suberythemal dose of UVB and UVA in human skin. J Am Acad Dermatol. 1995;32:53–62. doi: 10.1016/0190-9622(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 3.Lowe NJ, Meyers DP, Wieder JM, et al. Low doses of repetitive ultraviolet A include morphologic changes in human skin. J Invest Dermatol. 1995;105:739–743. doi: 10.1111/1523-1747.ep12325517. [DOI] [PubMed] [Google Scholar]
- 4.IOM (Institute of Medicine) Dietary reference intakes for calcium and vitamin D. In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Washington, DC: The National Academies Press; 2011. p. 363. [PubMed] [Google Scholar]
- 5.Golden RN, Gaynes BN, Ekstrom RD, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psych. 2005;162(4):656. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- 6.Cooper KD, Oberhelman L, Hamilton TA. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci USA. 1992;89(18):8497. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahnke N, Medve-Koenigs K, Megahed M, Neumann NJ. Medium-dose UV-A1 phototherapy. Successful treatment of cutaneous sarcoidosis. Hautarzt. 2003;54(4):364. doi: 10.1007/s00105-003-0503-8. [DOI] [PubMed] [Google Scholar]
- 8.Graefe T, Konrad H, Barta U, Wollina U, Elsner P. Successful ultraviolet A1 treatment of cutaneous sarcoidosis. Br J Dermatol. 2001;145(2):354. doi: 10.1046/j.1365-2133.2001.04356.x. [DOI] [PubMed] [Google Scholar]
- 9.Patterson JW, Fitzwater JE. Treatment of hypopigmented sarcoidosis with 8-methoxypsoralen and long wave ultraviolet light. Int J Dermatol. 1982;21(8):476. doi: 10.1111/j.1365-4362.1982.tb03187.x. [DOI] [PubMed] [Google Scholar]
- 10.Gleeson CM, Morar N, Staveley I, Bunker CB. Treatment of cutaneous sarcoid with topical gel psoralen and ultraviolet A. Br J Dermatol. 2011;164(4):892. doi: 10.1111/j.1365-2133.2010.10175.x. [DOI] [PubMed] [Google Scholar]
- 11.Diederen PV, van Weelden H, Sanders CJ, Toonstra J, van Vloten WA. Narrowband UVB and psoralen-UVA in the treatment of early-stage mycosis fungoides: a retrospective study. J Am Acad Dermatol. 2003;48(2):215. doi: 10.1067/mjd.2003.80. [DOI] [PubMed] [Google Scholar]
- 12.Boztepe G, Sahin S, Ayhan M, Erkin G, Kilemen F. Narrowband ultraviolet B phototherapy to clear and maintain clearance in patients with mycosis fungoides. J Am Acad Dermatol. 2005;53(2):242. doi: 10.1016/j.jaad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay DL, Lish KM, Yalowitz CB, Soter NA. Ultraviolet-B phototherapy for early-stage cutaneous T-cell lymphoma. Arch Dermatol. 1992;128(7):931. [PubMed] [Google Scholar]
- 14.Resnik KS, Vonderheid EC. Home UV phototherapy of early mycosis fungoides: long-term follow-up observations in thirty-one patients. J Am Acad Dermatol. 1993;29(1):73. doi: 10.1016/0190-9622(93)70155-m. [DOI] [PubMed] [Google Scholar]
- 15.Gathers RC, Scherschun L, Malick F, Fivenson DP, Lim HW. Narrowband UVB phototherapy for early-stage mycosis fungoides. J Am Acad Dermatol. 2002;47(2):191. doi: 10.1067/mjd.2002.120911. [DOI] [PubMed] [Google Scholar]
- 16.Querfeld C, Rosen ST, Kuzel TM. Long-term follow-up of patients with early-stage cutaneous T-cell lymphoma who achieved complete remission with psoralen plus UV-A monotherapy. Arch Dermatol. 2005;141(3):305. doi: 10.1001/archderm.141.3.305. [DOI] [PubMed] [Google Scholar]
- 17.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Anwar J, Wrone DA, Kimyai-Asadi A, Alam M. The development of actinic keratosis into invasive squamous cell carcinoma: evidence and evolving classification schemes. Clin Dermatol. 2004;22(3):189. doi: 10.1016/j.clindermatol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Salasche SJ. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1 Pt 2):4. doi: 10.1067/mjd.2000.103342. [DOI] [PubMed] [Google Scholar]
- 20.Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet. 1988;1(8589):795. doi: 10.1016/s0140-6736(88)91658-3. [DOI] [PubMed] [Google Scholar]
- 21.Jeffes EW, 3rd Tang EH. Actinic keratosis. Current treatment options. Am J Clin Dermatol. 2000;1(3):167. doi: 10.2165/00128071-200001030-00004. [DOI] [PubMed] [Google Scholar]
- 22.Criscione VD, Weinstock MA, Naylor MF, Luque C, Eide MJ, Bingham SF. Department of Veteran Affairs Topical Tretinoin Chemoprevention Trial Group. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 23.Naylor MF, Boyd A, Smith DW. High sun protection factor sunscreens in the suppression of actinic neoplasia. Arch Dermatol. 1995;131(2):170. [PubMed] [Google Scholar]
- 24.Marks R. The epidemiology of nonmelanoma skin cancer: who, why and what can we do about it. J Dermatol. 1995;22(11):853. doi: 10.1111/j.1346-8138.1995.tb03935.x. [DOI] [PubMed] [Google Scholar]
- 25.Hacker SM, Flowers FP. Squamous cell carcinoma of the skin. Will heightened awareness of risk factors slow its increase? Postgrad Med. 1993;93(8):115. doi: 10.1080/00325481.1993.11701720. [DOI] [PubMed] [Google Scholar]
- 26.Vitaliano PP, Urbach F. The relative importance of risk factors in nonmelanoma carcinoma. Arch Dermatol. 1980;116(4):454. [PubMed] [Google Scholar]
- 27.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 28.Geller AC, Miller DR, Annas GD. Melanoma incidence and mortality among US whites, 1969-1999. JAMA. 2002;288(14):1719. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 29.Jemal A, Seigel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 30.Nelemans PJ, Groenendal H, Kiemeney LA. Effect of intermittent exposure to sunlight on melanoma risk among indoor workers and sun-sensitive individuals. Environ Health Perspect. 1993;101(3):252. doi: 10.1289/ehp.93101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elwood JM, Gallagher RP, Hill GB, Pearson JC. Cutaneous melanoma in relation to intermittent and constant sun exposure—the Western Canada Melanoma Study. Int J Cancer. 1985;35(4):427. doi: 10.1002/ijc.2910350403. [DOI] [PubMed] [Google Scholar]
- 32.Zanetti R, Rosso S, Martinez C, et al. Comparison of risk patterns in carcinoma and melanoma of the skin in men: a multi-centre case-case-control study. Br J Cancer. 2006;94(5):743. doi: 10.1038/sj.bjc.6602982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Agency for Research on Cancer Working Group on Artificial Ultraviolet (UV) Light and Skin Cancer. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: a systematic review. Int J Cancer. 2007;120(5):1116. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 34.Kricker A, Armstrong BK, Goumas C, et al. for the GEM Study Group Ambient UV personal sun exposure and risk of multiple primary melanomas. Cancer Causes Control. 2007;18(3):295. doi: 10.1007/s10552-006-0091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckel TB, Goldstein AM, Fraser MC, Rogers B, Tucker MA. Recent tanning bed use: a risk factor for melanoma. Arch Dermatol. 2006;142(4):485. doi: 10.1001/archderm.142.4.485. [DOI] [PubMed] [Google Scholar]
- 36.Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. N Engl J Med. 1993;329:1147–1151. doi: 10.1056/NEJM199310143291602. [DOI] [PubMed] [Google Scholar]
- 37.Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol. 2003;139:451–455. doi: 10.1001/archderm.139.4.451. [DOI] [PubMed] [Google Scholar]
- 38.Roberts LK, Beasley DG. Commercial sunscreen lotions prevent ultraviolet-radiation-induced immune suppression of contact hypersensitivity. J Invest Dermatol. 1995;33:941–946. doi: 10.1111/1523-1747.ep12320339. [DOI] [PubMed] [Google Scholar]
- 39.Gurish MF, Roberts LK, Krueger GG, et al. The effect of various sunscreen agents on skin damage and the induction of tumor susceptibility in mice subjected to ultraviolet irradiation. J Invest Dermatol. 1975;65:543–546. doi: 10.1111/1523-1747.ep12526084. [DOI] [PubMed] [Google Scholar]
- 40.Shaath NA. The chemistry of sunscreens. Cosmet Toilet. 1868;101:55–70. [Google Scholar]
- 41.Wolverton SE, Levy SB. Comprehensive Dermatologic Drug Therapy. 2nd ed. 2007;38:704–708. [Google Scholar]
- 42.Sayre RM, Killias N, Roberts RL, et al. Physical sunscreens. J Soc Cosmet Chem. 1990;41:103–109. [Google Scholar]
- 43.Levy SB. Sunscreen for photoprotection. Dermatol Ther. 1997;4:59–71. [Google Scholar]
- 44.Cole C. Sunscreen protection in the ultraviolet A region: how to measure the effectiveness. Photodermatol Photoimmunol Photomed. 2001;17:2–10. doi: 10.1034/j.1600-0781.2001.017001002.x. [DOI] [PubMed] [Google Scholar]
- 45.Aprapidis-Paloympis FE, Nash RA, Shaath NA. The effect of solvents on the ultraviolet absorbance of sunscreens. J Soc Cosmet Chem. 1987;38:209–221. [Google Scholar]
- 46.Wolverton SE, Levy SB. Comprehensive Dermatologic Drug Therapy. 2nd ed. 2007;38:711–712. [Google Scholar]
- 47.Foley P, Nixon R, Marks R, et al. The frequency of reactions to sunscreens: results of a longitudinal population-based study on the regular use of sunscreens in Australia. Br J Dermatol. 1993;128:512–518. doi: 10.1111/j.1365-2133.1993.tb00227.x. [DOI] [PubMed] [Google Scholar]
- 48.Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. Review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37(5):221–232. doi: 10.1111/j.1600-0536.1997.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 49.Murphy ME, Montemarano AD, Debboun M, et al. The effect of sunscreen on the efficacy of insect repellant: a clinical trial. J Am Acad Dermatol. 2000;43:219–222. doi: 10.1067/mjd.2000.107960. [DOI] [PubMed] [Google Scholar]
- 50.Food and Drug Administration. Labeling and Effectiveness Testing; Sunscreen Drug Products for Over-the-Counter Human Use. Federal Registar/Vol.76,No.117/Friday, June 17,2001/Rules and Regulations. [PubMed]