Abstract
Nuclear transcription factor Stat3 is important for proper regulation of hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) proliferation, survival, and cytokine signaling responses. A new, noncanonical role for Stat3 in mitochondrial function has been discovered recently. However, there is little information on the role(s) of mitochondrial Stat3 in HSC/HPC function, especially potential effects of Stat3/mitochondrial dysregulation in human diseases. We investigated hematopoietic cell–targeted deletion of the STAT3 gene in HSCs/HPCs with a focus on mitochondrial function. We found that STAT3−/− mice, which have a very shortened lifespan, dysfunctional/dysregulated mitochondrial function and excessive reactive oxygen species production in HSCs/HPCs that coincides with pronounced defects in function. These animals have a blood phenotype with similarities to premature aging and to human diseases of myelodysplastic syndrome and myeloproliferative neoplasms such as erythroid dysplasia, anemia, excessive myeloproliferation, and lymphomyeloid ratio shifts. We show herein that the lifespan of STAT3−/− animals is lengthened by treatment with a reactive oxygen species scavenger, which lessened the severity of the blood phenotype. These data suggest a need for more detailed studies of role(s) of Stat3 in HSC/HPC mitochondrial function in human diseases and raise the idea that mitochondrial Stat3 could be used as a potential therapeutic target.
Introduction
Stat3 has been implicated in normal cell function and in human cancers and proliferative disorders.1 It is required for self-renewal in mouse embryonic stem cells and induced-pluripotent stem cell lines.2 We were interested in the role of Stat3 in hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) functions. Some information on Stat3 and HSCs exists,3 but mechanistic studies of Stat3 in human cells and mouse models, which were usually not in hematopoietic cells, have investigated activation of Stat3 almost solely in terms of tyrosine phosphorylation, dimerization, nuclear translocation, and transcription factor activity on specific gene targets.1 Recently, a new noncanonical role for Stat3 has been discovered in mitochondria.1 Stat3 augments mitochondrial oxidative phosphorylation4 and supports transformation by oncogenic Ras.5 Stat3 binds to and influences enzymatic activities of several subunit complexes of the mitochondrial electron-transport chain (ETC).6 The exact role of Stat3 in mitochondrial function and regulation is unknown, but new information indicates that there is a low Stat3-to-ETC complex ratio, suggesting that direct binding of Stat3 to mitochondrial complexes may only have a minimal effect on the overall function of Stat3 in mitochondria.7 Therefore, Stat3 may have a more global effect on mitochondrial metabolism in HSCs and HPCs other than direct ETC complex regulation.
Reactive oxygen species (ROS) can activate the JAK/STAT pathway.8 JAKs are nonreceptor tyrosine kinases that mediate signal transduction pathways regulating cell growth and survival.9 Recent studies have demonstrated dysregulated JAK/STAT signaling pathways to be a common link between at least 3 kinds of human Philadelphia chromosome–negative myeloproliferative neoplasms (MPNs),10 and to be implicated in chronic lymphocytic leukemia (CLL).11 Hematopoietic growth-factor signaling employs ROS as an important component,12 and mitochondrial metabolism and ROS generation regulates the lifespan of HSCs through a p38MAPK pathway,13 the same pathway that phosphorylates Stat3 on serine and that is essential for Ras-mediated tumorigenicity.5 We reported previously that SCF induces serine phosphorylation of Stat3 in hematopoietic cells.14 The BCR/ABL tyrosine kinase also induces ROS in hematopoietic cells during their oncogenic transformation.15
To understand potential Stat3/mitochondria/ROS and HSC/HPC interplay in hematopoiesis, in the present study, we investigated HSC and HPC function and mitochondrial activities in a mouse model of hematopoietic tissue–targeted deletion of STAT3 (STAT3−/−).16 We found that Stat3 deletion in mouse hematopoietic cells results in a significant reduction in phenotypically defined long-term marrow repopulating HSCs (LT-HSCs) and of serially repopulating functional HSCs. Conversely, more differentiated, phenotypically defined HPCs are increased in BM, but these HPCs are decreased in functional proliferative response and manifest loss of multicytokine synergistically stimulated proliferation in vitro. ROS levels were elevated in phenotyped HSCs and HPCs, potentially as a result of the loss of Stat3's role in mitochondrial function in these cells, which causes an apparent differentiation-stimulating influence on HSCs/HPCs (which ROS are known to do),15 and this results in pronounced myeloproliferative dysfunction and erythroid dysplasia in these animals. These manifested effects show some similarities to human MPNs. Treatment of these STAT3−/− animals with n-acetyl-cysteine (NAC), a ROS scavenger, extends their lifespan and lessened their MPN-like symptoms.
Methods
Animals
Stat3−/− and wild-type (WT) littermate control mice on a C57Bl/6 strain background were as described previously.16 Mouse BM cells were obtained and HSC and HPC function assessed as described previously.1718 The Indiana University Committee on Use and Care of Animals approved the mouse studies.
HPC assay
Mouse BM cells were usually plated at 5 × 104 cells/mL in 1% methylcellulose culture medium in the presence of 0.1mM hemin, 30% FBS (Hyclone), and the following growth factors unless otherwise noted: 1U/mL of recombinant human erythropoietin (Amgen), 50 ng/mL recombinant murine SCF (R&D Systems) and 5% vol/vol pokeweed mitogen mouse spleen cell conditioned medium.17 The percentage of HPCs in the S-phase of the cell cycle was estimated with the high specific activity–tritiated thymidine kill technique. Colonies were scored after 7 days of incubation at 5% CO2 and lowered (5%) O2 in a humidified chamber, and granulocyte-macrophage CFU (CFU-GM), erythrocyte burst-forming unit (BFU-E), and granulocyte, erythrocyte, monocyte, megakaryocyte CFU (CFU-GEMM) progenitors were distinguished by colony assay.19 Recombinant murine GM-CSF, IL-3, SCF, and Flt-3 ligand (FL) were from R&D Systems. Replating of individual colonies from primary to secondary plates, a measure of the limited self-renewal capacity of HPCs, was performed as described previously.18,19 In vitro and in vivo differences were assessed with a 2-tailed Student t test. P < .05 was considered significant.
Reagents and instruments
All Abs were from BD Biosciences. MitoTracker Green FM, JC-1, and Mitotracker-CM-H2TM-Orange probes were from Molecular Probes/Life Technologies and were used according to the manufacturer's instructions. Wright-Giemsa and May-Grünwald stain was from Thermo Fisher Scientific and used as described previously.20 Splenic sections and cytospin preparation were also as described previously.16 Automated blood cell counting was done using a Hemavet Blood Analyzer (Drew Scientific) and used according to the manufacturer's instructions for counting in mouse blood. Flow cytometry was performed with an LSR II flow cytometer (BD Biosciences). Cell surface labeling and other staining was as described previously.21 Flow cytometric data were analyzed using the Cyflogic program (Perttu Terho and CyFlo). Data were plotted and analyzed statistically using SigmaPlot Version 11.0 software (Systat Software). Oligomycin-A and NAC were from Sigma-Aldrich.
Transplantation assays
Competitive HSC engraftment studies were performed at a 1:1 donor (C57Bl/6 CD45.2+) to recipient (B6.BoyJ, CD45.1+) ratio into primary lethally irradiated B6.BoyJ mice and secondary transplantations were performed in a noncompetitive setting in lethally irradiated B6.BoyJ mice as described previously.17,22 Nucleated donor BM cells (5 × 105) were used for all injections into recipient mice (ie, 2.5 × 105 donor cells plus 2.5 × 105 competitor cells)
Respirometry
The cellular oxygen consumption rate (OCR) data were obtained using an XF96 extracellular flux analyzer from Seahorse Bioscience.23 Measurement of basal OCR data and OCR data after treatment with oligomycin-A were performed according to the manufacturer's instructions and as described previously.24
Results
Functional abnormalities in HSCs and HPCs in Stat3−/− mice
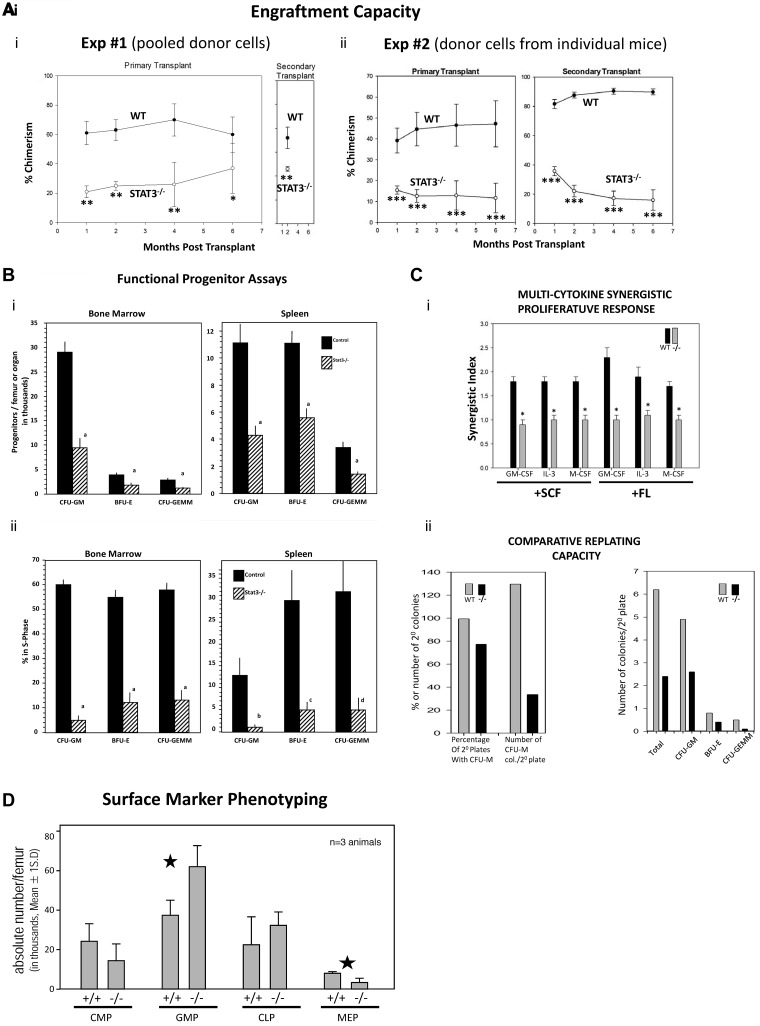
STAT3−/− mouse BM HSCs have decreased engrafting capability. We assessed functionally defined HSCs in Stat3−/− BM by performing competitive-repopulating primary transplantation and noncompetitive secondary transplantation assays in lethally irradiated female mouse recipients in 2 separate experiments, 1 with pooled male and female donor cells (Figure 1Ai), and 1 with female donor cells from individual mouse BM (Figure 1Aii). Recipients transplanted with STAT3−/− BM donor cells showed significantly reduced chimerism compared with WT donor BM-transplanted primary recipients at all posttransplantation time periods analyzed. Decreased STAT3−/− BM engraftment was also apparent in secondary mouse transplantations. Interestingly, when BM cells from individual female donors were transplanted into lethally irradiated female recipients (Figure 1Aii), the differences between STAT3−/− and WT donor cell engraftment was even more apparent than in pooled cells, and these differences were even further magnified in the secondary recipients. The increased percent change in chimerism from primary to secondary transplantations (Figure 1Aii) suggests that the self-renewal capacity of these cells may have been compromised. That there was little or no change in engraftment from primary to secondary mice in terms of chimerism for STAT3−/− cells when pooled donor cells were used (Figure 1Ai) argues against a homing defect in LT-HSCs. These results of decreased primary and secondary repopulation of STAT3−/− BM are consistent with a decrease in phenotypically defined LT-HSCs (see Figure 2B).
Figure 1.
Assessment of functional HPC repopulating ability and in vitro functional assessment of HPC numbers and cytokine-proliferative responses from WT and STAT3−/− mice. Results for primary mouse engraftment are shown as means ± SEM for 2 completely separate experiments. In experiment number 1, pooled male and female C57Bl/6 BM cells (CD45.2+) from 3 WT littermates or 3 Stat3−/− mice were injected at a 1:1 ratio with B6.BoyJ (CD45.1+) competitor cells each into 5 lethally irradiated B6.BoyJ recipient mice. (Ai) Only one time point is shown for the secondary transplantations because all lethally irradiated secondary transplantation recipients (WT and STAT3−/−) died before the fourth month after transplantation. (Aii) In experiment number 2, C57Bl/6 cells from 2 individual WT littermates or Stat3−/− female mice were each injected at a 1:1 ratio with B6.BoyJ competitor cells into 5 lethally irradiated B6.BoyJ recipient mice. Results for secondary engraftment are shown as means ± SEM. For the secondary transplantations, 106 cells from each primary group were injected into 5 lethally irradiated secondary female recipients. *P < .05; **P < .01; ***P < .001. (Bi) Influence of STAT3−/− on absolute numbers of HPCs. Results shown are the average ± SEM for 11 WT and 10 STAT3−/− mice individually assessed from a total of 3 different experiments. Cells were stimulated in vitro with erythropoietin, pokeweed mitogen mouse spleen cell conditioned medium, SCF, and hemin to detect the more immature subsets of progenitors. a indicates P < .001 compared with control. (Bii) Influence of STAT3−/− on cycling status of HPC for the same cells as in panel Bi. The percentage of HPCs in the S-phase was determined with the high specific activity tritiated thymidine kill technique. (Ci) Response in vitro of BM CFU-GM from WT and STAT3−/− mice to a colony stimulating factor (CSF) and a costimulating cytokine (SCF or FL). Results are presented as means ± SEM ratio shown for 7 WT and 6 STAT3−/− mice from a total of 2 separate experiments. *P < .001 for numbers of colonies stimulated by only a CSF plus either SCF or FL compared with the additive number of colonies stimulated by a CSF plus that only stimulated by SCF or FL. (Cii) Comparative replating capacity in vitro of HPC-derived colonies from WT and STAT3−/− BM cells. Results are based on 107 replated WT primary and 114 STAT3−/− primary colonies from a total of 3 different experiments. For CFU-M, primary and secondary colonies were grown in methylcellulose culture with M-CSF and SCF. For CFU-GM, BFU-E, and CFM-GEMM, primary and secondary colonies were grown with erythropoietin, SCF, pokeweed mitogen mouse spleen cell conditioned medium, and hemin. For CFU-M, colonies were scored after 7 days incubation. (D) Phenotype analysis of hematopoietic progenitors from WT and STAT3−/− mouse BM. Flow cytometric surface marker analysis was done as previously described.27,28
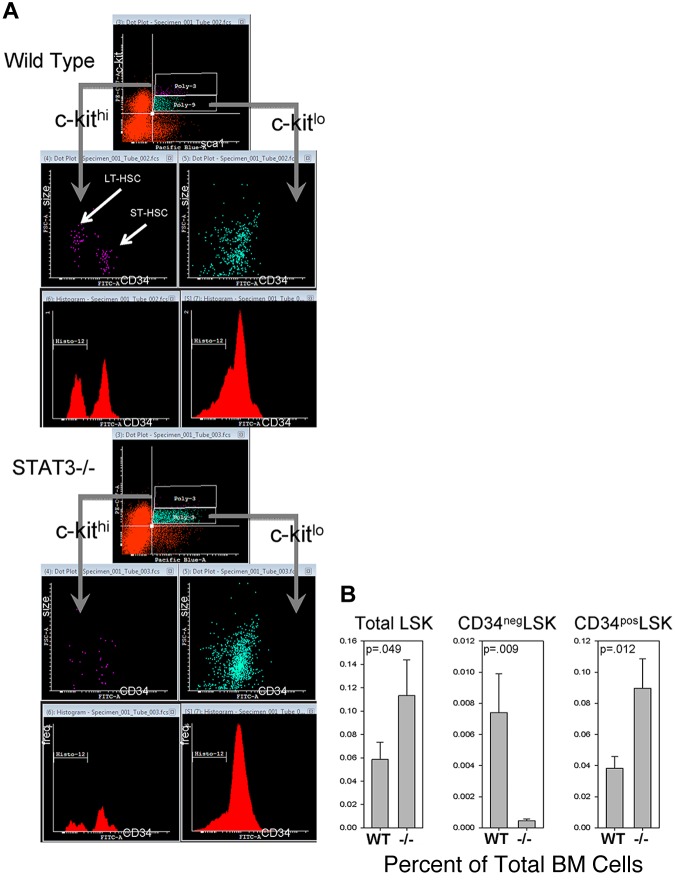
Figure 2.
Phenotypically defined HSCs and progenitors. (A) Representative flow cytometric dot-plots of c-kit, sca1, and CD34 surface marker expression, along with cell size, in the Lin− BM compartment of STAT3−/− and WT littermate mice at 5-6 weeks of age. See Figure 3 for details of the defined subpopulations. Isotype control Abs were used to established positive/negative quadrants and gates for lineage markers, lin cocktail, c-kit, sca1, and CD34 expression, as described previously.21 Histo-12 gate (in red histograms) represents cells with negative CD34 expression. (B) Comparative analysis of subpopulations of cells in the Lin− BM compartment from 4 WT/STAT3−/− mouse pairs. Data in panel B are expressed as means ± 1 SD.
STAT3−/− mouse BM has fewer functional myeloid progenitor cells/femur, is in a slower cell-cycle state, and has defective cytokine synergy response.
The numbers and cell-cycling status of functionally defined HPCs in STAT3−/− and WT BM and spleen were assessed using colony assays. Figure 1B shows the effects of STAT3 gene deletion on absolute numbers of BM and spleen progenitor cells (CFU-GM, BFU-E, and CFU-GEMM) numbers (Figure 1Bi) and their cell-cycle status (Figure 1Bii), all of which were reduced in the absence of Stat3. Proliferative synergy induced by certain combinations of cytokines in vitro is considered to play an important role in hematopoiesis. Stat3 signaling pathways in response to cytokine receptor activation are vital for proper regulation of hematopoiesis in vivo and in vitro.25 Synergistic proliferation responses to GM-CSF, IL-3, or M-CSF in combination with either SCF or FL was completely abrogated in STAT3−/− BM (Figure 1Ci, shown as a synergistic index: CSF + FL or SCF/CSF + either SCF or FL). Therefore, Stat3 is required for proliferative synergy in mouse BM progenitors. Replating capacity of HPCs provides an estimate of the limited self-renewal capacity of HPCs.26 Replating capacity of STAT3−/− macrophage CFU and CFU-GM, BFU-E, and CFU-GEMM was also significantly reduced, as assessed by numbers of secondary plates with colonies, and numbers of colonies per secondary plate (Figure 1Cii). These data show that there are highly significant deficiencies in functionally assessed HPC numbers and their activities. Interestingly, when we did quantitative phenotypic analysis of BM progenitor subcompartments as indicated by surface marker expression27,28 (Figure 1D), we observed a significant decrease in the megakaryocyte/erythroid progenitor population and a significant increase in the granulocyte/macrophage progenitor population, with no significant changes in the common myeloid progenitor or common lymphoid progenitor populations in Stat3−/− compared with WT mice. Therefore, for cells of the common myeloid progenitor and granulocyte/macrophage progenitor compartments, believed to be, respectively, similar to CFU-GEMM and CFU-GM when functional CFU-GEMM and CFU-GM were decreased in number, cycling, and sensitivity to synergistic stimulation, the phenotype analysis did not reflect functional defects and likely suggests that whereas the numbers of granulocyte/macrophage progenitors are increased, some or all of these progenitors have proliferative defects in response to cytokines.
Stat3−/− BM shows a shift to more phenotypically differentiated precursors
To obtain a more complete picture of the effects of STAT3 deletion, we performed a flow cytometric analysis of phenotypically defined BM HSCs/HPCs from these mice to compare with WT controls 5 weeks after birth. An analysis of proportions of phenotypically defined LT-HSCs and short-term marrow repopulating HSCs (ST-HSCs) based on CD34 expression and size of Lin−Sca1+c-Kit+ (LSK) cells is shown in Figure 2. LT-HSCs are considered to be CD34− LSK cells and ST-HSC are considered to be CD34+ LSK cells.29 In mouse BM, we routinely found 2 populations of LSK cells, one of which was c-kithigh and composed of larger and smaller CD34− cells in similar proportions. We also noticed this size-difference trend in a previous study using a different strain of mouse.21 This is also similar to our previous study describing true pluripotent human and mouse embryonic stem cells as reproducibly larger (as measured by laser light scatter) than early differentiated, low pluripotency, smaller embryonic stem cells in colonies in vitro.20 The second LSK population was c-kitlow, predominately CD34+, and contained predominately smaller cells. Figure 2A is a representative flow cytometric dot-plot analysis of CD34 expression in LSK cells from WT or Stat3−/− BM showing differences in size between CD34− and CD34+ LSK cells and differences in c-kit expression levels and numbers of LSK cells from BM of WT and STAT3−/− mice. Figure 2B is a statistical comparison of these LSK populations from 4 WT/STAT3−/− littermate pairs. These data demonstrate that, although total LSK cells were increased significantly in Stat3−/− BM (which has been observed to occur during normal mouse aging),30 the majority of STAT3−/− LSK cells were CD34+ c-kitlow in contrast to that found in WT BM. Therefore, the LSK compartment of Stat3−/− BM is composed predominately of phenotypically defined ST-HSCs with much lower numbers of LT-HSCs compared with WT BM.
Phenotyped Stat3−/− ST-HSCs and HPCs display mitochondrial dysfunction and increased ROS
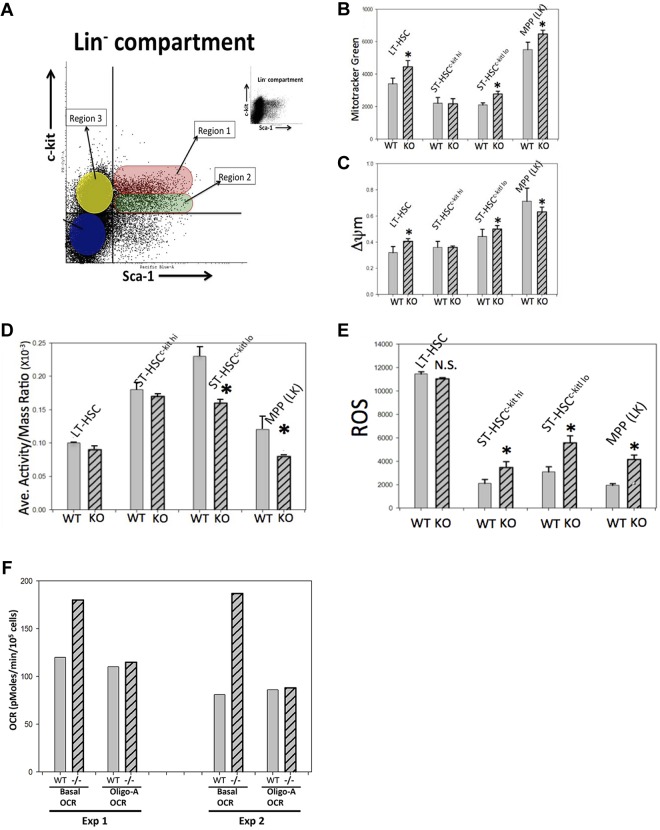
Stat3 has been shown recently to play a role in mitochondrial respiration,6,24,31–34 but the mechanisms remain unknown. Very little is known about mitochondrial metabolism in HSCs, so in the present study, we investigated mitochondrial mass and function (mitochondrial membrane potential, ΔΨm) in phenotypically defined Stat3−/− LT-HSCs, ST-HSCs, and LSK cells compared with WT littermate cells. In our previous studies,21 we identified several discrete subpopulations within the Lin− compartment of normal mouse BM based on mitochondrial mass, ΔΨm, CD34 expression, and size. Figure 3 summarizes the mitochondrial properties of the 3 discrete subpopulations that we defined in Stat3−/− and WT BM. Three regions that we investigated in Lin− BM cells are illustrated in Figure 3A. Data shown in Figure 2A had established that larger region 1 cells were CD34− LSKs. This population is highly enriched in LT-HSCs,29 and we refer to them as LT-HSCkit hi. Smaller region 1 (R1) cells are CD34+ and enriched in ST-HSCs; we refer to these as ST-HSCkit hi. The R2 subpopulation contains nearly all CD34+ cells and is composed of both smaller and larger cells, which we refer to as ST-HSCkit low. R3 cells are sca1− and are not in the LSK compartment (Lin−c-kit+sca1− [LK] cells). Figure 3B shows staining of these subpopulations with MitoTracker Green, which is taken up into cellular mitochondria in a ΔΨm-independent manner and is an indicator of cellular mitochondrial mass.21,35 Figure 3B compares average mitochondrial mass per cell in phenotypically distinct Lin− regions. Also shown is the influence of STAT3 deletion on cellular mitochondrial mass compared with that in WT cells, which was performed in a manner similar to that used in our previous study.21 Mitochondrial mass was lower in WT ST-HSCs compared with WT LT-HSCs, which may be because of the noted size difference, and this is consistent with our previous study.21 STAT3 deletion resulted in a significant increase in mitochondrial mass in LT-HSCs and ST-HSCkit lo cells but not in ST-HSCkit hi cells. The reasons for this lack of effect in ST-HSCkit hi cells, a functionally uncharacterized ST-HSC population, is unknown, but it is important to note that these cells are smaller than other cells in this LSK region. WT LK (R3; enriched for a phenotyped multipotential progenitor) cells contained greater mitochondrial mass than either WT LT-HSCs or WT ST-HSCs, and STAT3 deletion resulted in a small, but significantly increased mitochondrial mass in these populations. The percentage of total Lin− cells that were LK cells was 3.6 ± 0.5 for WT and 16.0 ± 2.3 for Stat3−/− (P = .0005). This demonstrates that the proportion of phenotyped LK cells was greatly increased in BM with STAT3 deleted. We also investigated mitochondrial activity using the probe JC-1, a rosamine derivative that is actively transported into mitochondria in a ΔΨm-dependent manner.36 Monomeric JC-1 has an emission spectrum similar to FITC (green) fluorescence, but its aggregated form has an emission spectrum similar to PE (red) fluorescence. As JC-1 accumulates in the mitochondria, it aggregates and shifts fluorescence color. Therefore, relative ΔΨm can be estimated on a per-cell basis by comparing the ratios of red/green fluorescence intensity. Supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) is an example of a flow cytometric dot-plot of gated LSK cells relating mitochondrial mass to size (supplemental Figure 1A) and the ratio of JC-1 monomers to aggregates (supplemental Figure 1B). The average ΔΨm/cell of R1-R3 cells was determined from the red/green fluorescence ratios (FITC/PE cytometer channels). This dot-plot also shows that LSK cells can generally be divided into 2 categories, high ΔΨm, in which the polymeric/monomeric JC-1 ratio is higher, and low ΔΨm, in which the ratio is lower. Gates such as polygram-9 and polygram-8 can be used to enumerate the percentages of each category in different populations, and the average fluorescence intensity ratio of these 2 categories provides a measure of mitochondrial activity in the population. A summary of average ΔΨm/cell measurements in LT-HSCs, ST-HSCs, and LK cells is shown in Figure 3C. Even though the mitochondrial activity of ST-HSCs is similar to mitochondrial activity in LT-HSCs, this average mitochondrial potential is generated by fewer mitochondria (ie, lower mitochondrial mass; Figure 3B). This suggests that, on a per-cell basis, the mitochondria in ST-HSCs are more active than those in LT-HSCs, which is consistent with our previous study.21 The WT LK compartment has an even higher average ΔΨm/cell. STAT3 deletion resulted in a modest but significant increase in ΔΨm/cell in LT-HSCs and R2 ST-HSCkit lo cells. STAT3 deletion also resulted in a small, but significantly decreased, average ΔΨm/cell in LK cells, which was the opposite of the effect that it had on other cells in the LSK compartment, but this is consistent with a positive influence of Stat3 on mitochondrial activity. Figure 3D shows a comparison of the mitochondrial activity/mass ratio, which is a useful monitor of “specific mitochondrial activity” (ie, mitochondrial activity/cell that is normalized for mitochondrial mass). The activity/mass ratio was significantly lower in ST-LSKc−kit lo cells and LK cells from STAT3−/− mice. This is consistent with the reported positive or stabilizing influence of Stat3 in mitochondria.32 The mitochondrial mass/activity data suggest that STAT3 deletion causes changes in mitochondria function and/or dysfunction/dysregulation in phenotypically defined HSCs and HPCs.
Figure 3.
Mitochondrial dysfunction and ROS overproduction in phenotypically defined STAT3−/− mouse HSCs and HPCs. (A) Graphic representation and dot-plot of mouse BM Lin− cells and c-kit and Sca1 expression performed as in Figure 2A. Shown are the 3 subpopulations that were gated and evaluated for mitochondrial properties and ROS levels. R1 and R2 are further subdivided into 2 discrete subpopulations based on size and CD34 surface expression (see Figure 2A). The small dot-plot in the top right part of panel A is the same data, but “de-cluttered” to more easily see the overall pattern. (B) Quantitative comparison of average MitoTracker Green staining per cell (a measure of mitochondrial mass per cell) on gated subpopulations described in panel A. Also shown is a statistical comparison of results from 3 WT and STAT3−/− pairs. (C) Staining of the indicated subpopulations with JC-1, a probe that measures mitochondrial membrane potential (δ ψ-m; ΔΨm), a measure of mitochondrial activity. Also shown is a quantitative and statistical analysis comparing ΔΨm in the 4 defined subpopulations (panel C), as well as a comparison of “activity/mass ratio” (panel D) for STAT3−/− and WT mouse cells from the same animals used for panel B. This ratio is a measure of mitochondrial activity per cell that is normalized for mitochondrial mass (derived from data in panels B and C) and reflects the average mitochondrial membrane potential per cell irrespective of mitochondrial mass (ie, a kind of “specific” mitochondrial activity measurement). (E) Analysis of average ROS levels per cell in the indicated subpopulations in a separate experiment using 3 different WT/STAT3−/− pairs. Error bars in panels B through E are SD of mean values and statistical significance. *P < .05 by 2-tailed Student t test. (F) OCR (obtained using a Seahorse XF96 extracellular flux analyzer/respirometer) from identical numbers of splenocytes/well from STAT3−/− or WT from 2 separate experiments each with 1 WT and 1 STAT3−/− mouse. Basal OCR and OCR after oligomycin-A treatment (an ATPase inhibitor) are shown. WT indicates littermate control cells; −/−, STAT3−/− cells. Data shown are the average OCR measured from 24 wells of each type of cell where the OCR (derived from slope of pM of O2 consumed per minute) was measured 16 times in each well simultaneously over a period of 2 hours until the OCRs were stabilized. The last stabilized average OCR measurement is presented.
Because mitochondria are one of the primary sources of ROS in cells,37,38 and because mitochondria are vital for balanced ROS regulation, we next assessed superoxide (the predominant ROS in cells) with another rosamine derivative, MitoTracker CM-H2TM-Orange.36,39 Unoxidized MitoTracker CM-H2TM-Orange is nonfluorescent but becomes highly red fluorescent after oxidation by superoxide, and this is used to quantitate average ROS levels/cell. Figure 3E shows the average results of 3 separate experiments, each with BM cells from 1 Stat3−/− and 1 WT littermate mouse. The data show that ROS levels were increased significantly in ST-HSCs and LK cells after STAT3 deletion. STAT3 deletion in LT-HSCs had no effect on ROS levels but, overall, LT-HSCs had higher ROS levels than ST-HSCs or LK cells.
Splenocytes from STAT3−/− mice have an increased mitochondrial respiration rate not associated with ATP production
Because STAT3 has been implicated in regulating mitochondrial respiration, we next assessed respiration rate as measured by OCR using a Seahorse extracellular flux analyzer.23 There are 2 principle sources of ROS in hematopoietic cells; the NADPH oxidase system and mitochondria. The inner mitochondrial membrane has a natural leak to protons and electrons can “leak” from the ETC complexes and convert molecular oxygen to superoxide. It is estimated that 1%-5% of oxygen consumed by mitochondria is converted to ROS38 (superoxide). We attempted to use sorted LSK populations for these experiments, but the numbers of these cells that could be obtained were too low to reliably measure OCR, so we used another abundant source of hematopoietic tissue, splenocytes, as a surrogate STAT3−/− cell source to evaluate the effects of STAT3 deletion on mitochondrial respiration. To determine of the source of overproduction of ROS in STAT3−/− cells, we performed respirometry experiments on splenocytes from 2 STAT3−/− mice and 2 WT mice. (Figure 3F). These experiments showed that basal OCR was increased in STAT3−/− splenocytes, which is consistent with increased mitochondrial respiration in these cells. ATP production-linked OCR, as measured by inhibition of ATPase (ETC complex V), with oligomycin-A showed that ATP-linked OCR was slightly lower in STAT3−/− cells compared with WT cells. However, this difference does not account for the total increased OCR in STAT3−/− cells compared with WT cells. This would be consistent with increased basal OCR in STAT3−/− cells being due, in part, to increased oxygen consumption by either increased NADPH oxidase activity or to increased proton leak and/or electron leak from the ETC. These data suggest that STAT3 deletion results in increased respiration/oxygen consumption in hematopoietic cells that is not related to ATP biosynthesis. This information, when coupled to potential mitochondria activity dysfunction/dysregulation in HSCs/HPCs (Figure 3B-E) in the absence of STAT3, supports the hypothesis that the excessive ROS generated in HSCs/HPCs in the absence of STAT3 could be because of dysregulation of a mitochondrial process not associated with the generation of ATP (ie, excessive proton/electron leak).
STAT3−/− mice display a “rapid-aging like” phenotype in their hematopoietic system
To relate the above functional information with the mice themselves, we looked at several hematopoietic parameters in STAT3−/− versus WT littermate control mice. Similar to humans, aged mice display shifts in ratios of lymphoid and myeloid blood cells, changes in erythroid cell morphology/function, and changes in HPCs.30,40
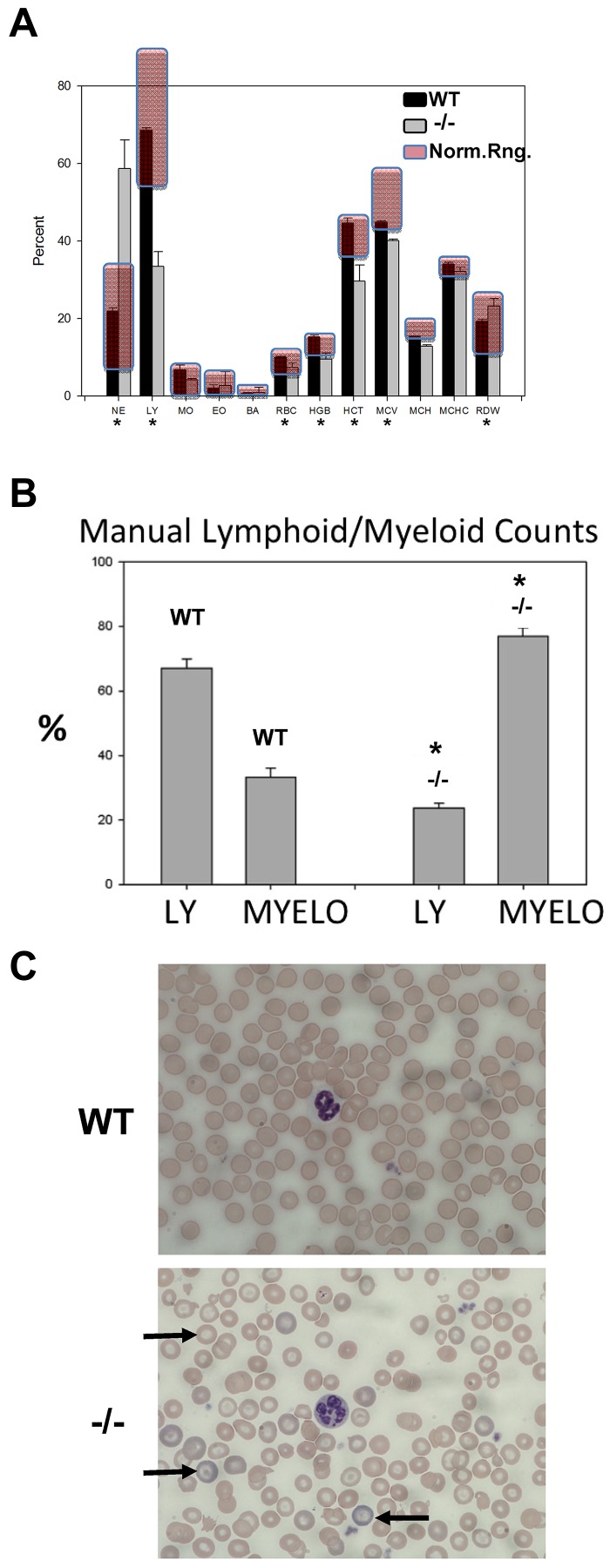
Stat3−/− mice are born normally in all observed respects, suggesting that Stat3 function during early development of the fetal hematopoietic system may be dispensable. However, by 2-3 weeks of age, a reduction in growth becomes apparent and at 4-6 weeks, these mice are approximately 20% smaller than WT littermates (as indicated by body weight) and they display a sedentary behavior, as described previously.16 Uniform death occurs at approximately 6 weeks of age in female mice, whereas male Stat3−/− mice survive approximately 1-2 weeks longer.16 Moreover, splenomegaly is apparent in 4- to 6-week-old animals. In our studies of 4- to 6-week-old mice, splenic architecture of STAT3−/− mice showed a profound reduction in white pulp structures with much larger areas of red pulp and myeloid cell infiltration (supplemental Figure 2A). There was a pronounced myeloid-lymphoid cell ratio shift, along with significant reduction of the total hemoglobin content and other erythrocyte indices in Stat3−/− blood compared with WT (Figure 4A automated peripheral blood counts). Erythrocyte counts were significantly lower in Stat3−/− mice, but were still within the normal range. Red cell distribution width (an indicator of anemic conditions) was also increased significantly in Stat3−/− compared with WT mice, but was still within the normal range. Manual lymphoid-myeloid counts were also performed in blood smears of 6 WT and 4 Stat3−/− animals from 3 separate litters at approximately 5 weeks of age (Figure 4B), and results were consistent with the automated counts. In addition, one blind study was performed in which the genotypes of the animals were not analyzed until the end of the experiment and the appearance of the animals was hidden from the “counters” and 1 litter of 4 animals was followed for 2-6 weeks (supplemental Figure 2B). The right-shifted lymphoid-myeloid ratio was apparent at 3 weeks and was very pronounced by 6 weeks of age in the 1 Stat3−/− mouse in this litter, which was later determined to be animal number 4. Femoral BM cytospin preparations stained with Wright-Giemsa (supplemental Figure 2C) showed severe erythroblast depletion in Stat3−/− BM compared with WT BM, along with a massive expansion of immature myeloid cells. Wright-stained blood smears revealed pronounced hypochromasia and many podocytes (target cells; Figure 4C). Hypersegmented neutrophils were also common in Stat3−/− blood compared with WT. These data indicate that Stat3−/− mice are born with apparently normal blood indices, but begin to become right shifted in lymphoid/myeloid ratio after 3 weeks of age, accompanied by pronounced erythroid dysplasia and anemia. Severe BM erythroblast depletion, along with splenomegaly and disrupted spleen morphology, suggest that the spleen may have become a site of extramedullary erythropoiesis. Alternatively, spleen hypertrophy could also be because of accumulation of defective erythrocytes in the spleen, where they are removed from the circulation. Abnormalities in peripheral blood neutrophil morphology and BM cell morphology/content and erythroid dysplasia are seen in human myelodysplastic syndrome (MDS) and MPNs, which are age-related diseases.41,42 Stat3−/− mice have diminished innate immunity.16 This, along with anemia and especially lymphoid-myeloid lineage shifts and increased LSK numbers, are all hallmarks of normal aging in mice and are typically seen in mice that are 1-2 years old.30,40 Therefore, STAT3−/− mice display morphologic abnormalities consistent with disease and/or aging processes.
Figure 4.
STAT3−/− mice have altered blood indices, spleen morphology, erythroid dysplasia, anemia, BM changes, and increased myeloproliferation. (A) Automated blood cell counts from 4 STAT3−/−/WT pairs at 5 weeks of age. Normal ranges for mice are shaded in red. NE indicates neutrophils; BA, basophils; HGB, hemoglobin; HCT, hematocrit; MCV, mean erythrocyte volume; MCH, mean erythrocyte hemoglobin; MCHC, mean erythrocyte hemoglobin content; and RDW, red cell distribution width (an indicator of anemia). (B) Manual counts of all lymphoid and myeloid blood cells from tail clipping blood smears stained with Wright-Giemsa and examined microscopically. At least 200 white cells per smear were counted and data are shown as means ± 1 SD from 6 STAT3−/−/WT mouse pairs. (C) Photomicrographs of blood smears stained with Wright-Giemsa representing data from 4 STAT3−/−/WT pairs. Arrows indicate podocytes (target cells). Also shown are hypochromatic erythrocytes, hyperchromatic reticulocytes, and an example of a normal and a hypersegmented neutrophil. *P < .05 by 2-tailed Student t test.
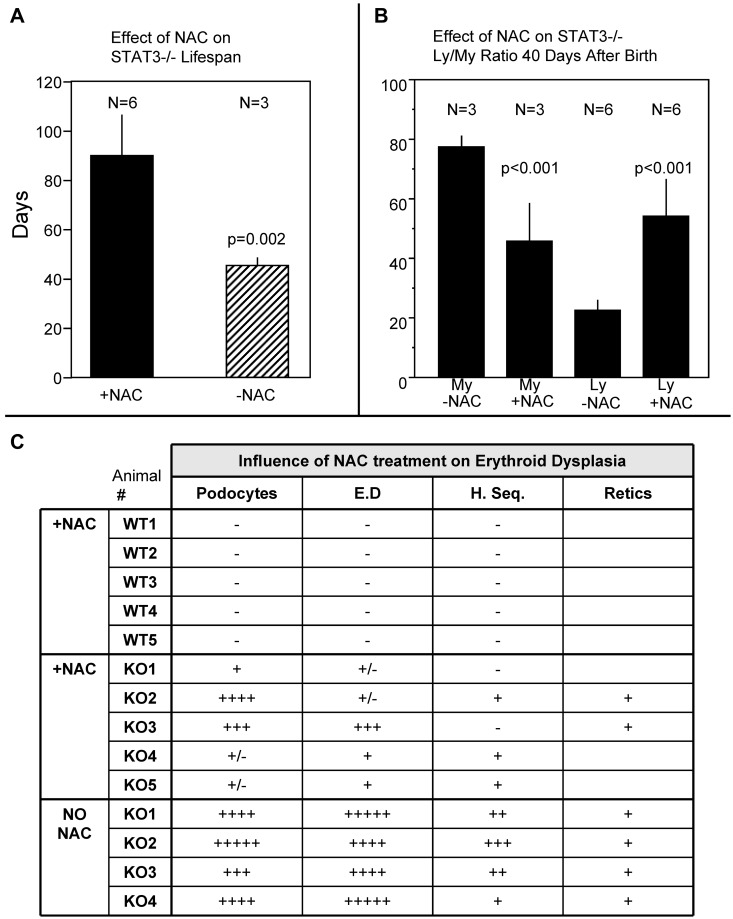
STAT3−/− mouse lifespan is increased by treatment with a ROS scavenger
Based on the data shown in Figure 4, we considered the possibility that excess ROS generation in HSC and/or HPC compartments may contribute, at least in part, to the blood phenotype and lifespan deficiency of STAT3−/− mice. We tested this by treating STAT3−/− and WT mice with periodic injections of the potent ROS scavenger NAC beginning 4 weeks after birth (Figure 5). The data show that NAC treatment nearly doubled the lifespan of the STAT3−/− mice (Figure 5A). During this experiment, blood smears from tail clippings were obtained at 40 days of age and manual lymphoid/myeloid cell counts were performed (Figure 5B). NAC treatment increased the lymphoid/myeloid ratio in STAT3−/− mice compared with that determined previously (ie, lymphoid cells were increased and myeloid cells were decreased at 40 days after birth in STAT3−/− mice treated with NAC compared with STAT3−/− mice without NAC treatment: a partial reversal of the STAT3−/− blood phenotype; Figure 4B-D). In addition, erythroid morphology and neutrophil nuclear segmentation were assessed (Figure 5C). Erythroid dysplasia, potocytosis, and neutrophil hypersegmentation were all decreased by NAC treatment of STAT3−/− mice. There was no effect of NAC treatment on these blood indices in WT mice. These data indicate that treatment with a ROS scavenger can lessen the severity of influence of STAT3 gene deletion on erythroid dysplasia and myeloproliferation (lymphoid/myeloid shift), which is associated with significant lifespan prolongation in these genetically defective mice.
Figure 5.
NAC injections extend the lifespan of STAT3−/− mice and lessen the severity of erythroid dysplasia phenotype. (A) Influence of NAC injections (1.5 mg per mouse 2 times/wk beginning 4 weeks after birth) on lifespan (in days) of STAT3−/− mice. (B) Results of manual lymphoid and myeloid blood cell counts on STAT3−/− blood smears taken 40 days after birth with and without NAC injections as in Figure 4C. My indicates myeloid cell counts (%); Ly, lymphoid cell counts (200 white cells were counted per smear stained with Wright-Giemsa). (C) Semiquantitative assessment (1+ to 5+) of podocytes (target cells), erythroid dysplasia (E.D; hypochromatic erythrocytes), hypersegmented neutrophils (H. Seg.), and reticulocytosis (Retics) on blood smears from WT and STAT3−/− animals 40 days after birth with or without NAC injections.
Discussion
In the present study, we have presented evidence that mice with a hematopoietic cell–targeted deletion of the nuclear transcription factor STAT3 have blood indices with similarities to those observed in some human MPNs beginning several weeks after birth. The stem cells from these mice are decreased and have a lessened ability to repopulate the BM of lethally irradiated recipient mice. In addition, STAT3−/− HPCs have a striking loss of normal synergistic cytokine proliferative responses in vitro. Stat3 activation is part of cytokine receptor signaling that is common in numerous cytokine/receptor systems,25 so it is likely that defective proliferative responses to the cytokines investigated herein were due to defects in these signaling cascades. It was reported recently that LSK cells expressing low levels of c-kit receptor are in a more quiescent state than cells expressing low levels of c-kit receptor, which was more pronounced in older animals.43 This data are consistent with our findings that STAT3 deletion results in BM LSK cells that are almost all c-kit low, that BM progenitors are not in active cell cycle, and with the LSK surface phenotype similar to that found in older mice. However, the results of the present study also indicate that there is no apparent difference in the efficiency of repopulating ability between c-kit low and c-kit high LSK cells; therefore, the c-kit expression level and LSK cell-cycle status had no discernible effect on the ability to repopulate primary and secondary transplantation recipients. This supports the notion that STAT3 deletion induced cell-cycle effects alone cannot account for the defective repopulation seen in STAT3−/− mouse HSCs, and that the loss of STAT3 has a more complex role in HSC repopulating ability than just effects on cytokine signaling cascades and cell-cycle status. However, we have also shown that HSCs and HPCs from STAT3−/− mice have dysfunctional mitochondrial activities/function associated with overproduction of ROS. ROS are well established as being integral for normal hematopoiesis and cytokine signaling,15 and their overproduction has been linked to various pathologic processes such as MDS, acute myeloid leukemia, and other neoplastic diseases.44 It appears unique that the deletion of an important signaling molecule that results in the loss of proliferative cytokine responses of myeloid HPCs in vitro also causes a pronounced myeloproliferative phenotype in vivo in whole animals. We found that this myeloproliferative phenotype was also associated with a defect in HSC maintenance or stem cell exhaustion, which is consistent with the loss of pluripotency maintenance for which Stat3 has been shown to be necessary.2 It is possible that the STAT3−/− mouse phenotype is linked, at least in part, to the overproduction of ROS that we observed in this model system. ROS are known to promote myeloid proliferation/differentiation,15 which is consistent with the observed STAT3−/− phenotype. We also have presented evidence herein that mitochondrial function/activity may be defective or dysregulated in HSCs and HPCs from STAT3−/− mice, which could also be linked to aberrant ROS production. Our respirometry experiments suggest that the source of overproduced ROS may be because of defects in mitochondrial ETC function not related to ATP production (ie, increased proton leak across the inner mitochondrial membrane or increased electron leak from the ETC). It is possible that increased electron leak from the mitochondrial ETC may be at the heart of the excessive mitochondrial ROS generation in STAT3−/− HSC/HPC cells. Stat3 has already been implicated in proper function of the mitochondrial permeability transition pore (mPTP),45,46 a little understood but important complex in mitochondria, and its omission from this mitochondrial complex could be the source of the proton leak and ROS generation. We recently reviewed the role of mPTP in the context of ROS production and its potential impact on stem cells.34 In that review, we highlighted the potential importance of a ROS-induced “feed-forward” overproduction of ROS in stem cells by a process known as ROS-induced ROS release45 by the mPTP and underscored the potential importance of Stat3 in regulating ROS production via this mitochondrial complex. Stem cells are, in general, thought to have fewer and/or less active mitochondria,21 and HSCs have been shown recently to be more dependent on glycolysis for their energy needs than other BM cells.47 The ability of cells to maintain a balanced ROS level depends on mitochondria and several antioxidant enzymes.21,34,48 Very little is known about this in HSCs47; nor is it known whether HSCs have robust adaptive responses to oxidatively damaging conditions. The importance of overproduced ROS in the pathophysiology of STAT3−/− mice seems clear from the fact that treatment of these mice with the ROS scavenger NAC has a pronounced prosurvival effect, which is commensurate with a reduction in the severity of the pathologic status in these animals.
Although mitochondrial DNA mutations have been implicated in MDS-like human diseases,49,50 to our knowledge, the role of Stat3 in mitochondrial ETC and in mPTP function has not been investigated in human diseases such as MDS, acute myeloid leukemia, CLL, or MPNs. However, Stat3 has been investigated previously in the context of constitutive activation by tyrosine phosphorylation and subsequent nuclear transcriptional activity. The results of the present study raise the possibility that lack of proper Stat3 activity in mitochondria could interfere with proper ROS regulation/production/balance and therefore contribute to the progression of diseases such as MDS, MPN, or perhaps acute or chronic leukemia. It is possible this could be due to a shift in Stat3 equilibrium between the nucleus and mitochondria, as we have already shown for the p53 protein.51 In this case, constitutive tyrosine phosphorylation, dimerization, and nuclear translocation could “over-sequester” Stat3 in the nucleus and lessen its stabilizing influence on mitochondria, resulting in an overproduction of ROS. Further studies to prove or disprove the existence of such an equilibrium and to determine whether this plays a role in various disease states involving dysregulated Stat3 function are needed. Our data presented herein suggest that the known link between constitutively activating mutations in the JAK/STAT pathways and human MPNs and CLL should be further studied in the context of the newfound roles of Stat3 in mitochondria.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (grants RO1 HL56416, RO1 HL67384, and POI DK90948 to H.E.B.). S.M.-G. was supported sequentially by National Institutes of Health training grants R25GM079657 and T32 DK07519 to H.E.B.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.M. contributed most of the concepts and performed most of the laboratory work; S.M.-G. contributed to the laboratory work; A.M. helped with the maintenance and genotyping of the STAT3−/− mice; S.C. and G.H. performed the colony and transplantation assays; X.-Y.F. contributed conceptually and provided the STAT3−/− mice; and H.E.B. was the principal investigator for the funding used, performed all colony counting, and assisted in editing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Charlie Mantel, Department of Microbiology and Immunology, Indiana University School of Medicine, Indianapolis, IN 46202-5181; e-mail: cmantel@iupui.edu.
References
- 1.Inghirami G, Chiarle R, Simmons WJ, et al. New and old functions of STAT3: a pivotal target for individualized treatment of cancer. Cell Cycle. 2005;4(9):1131–1133. doi: 10.4161/cc.4.9.1985. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright P. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132(5):885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 3.Oh I-H, Eaves CJ. Overexpression of a dominant negative form of STAT3 selectively impairs hematopoietic stem cell activity. Oncogene. 2002;21(31):4778–4787. doi: 10.1038/sj.onc.1205592. [DOI] [PubMed] [Google Scholar]
- 4.Reich NC. STAT3 revs up the powerhouse. Sci Signal. 2009;2(90):pe61. doi: 10.1126/scisignal.290pe61. [DOI] [PubMed] [Google Scholar]
- 5.Gough DJ, Corlett A, Schlessinger K, et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324(5935):1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wegrzyn J, Potla R, Chwae Y-J, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323(5915):793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips D, Reilley MJ, Aponte AM, et al. Stoichiometry of STAT3 and mitochondrial proteins: Implications for the regulation of oxidative phosphorylation by protein-protein interactions. J Biol Chem. 2010;285(31):23532–23536. doi: 10.1074/jbc.C110.152652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 9.Broxmeyer HE, Kohli L, Kim CH, et al. Stromal cell-derived factor-1/CXCL12 directly enhances survival/antiapoptosis of myeloid progenitor cells through CXCR4 and G(alpha)i proteins and enhances engraftment of competitive, repopulating stem cells. J Leukoc Biol. 2003;73(5):630–638. doi: 10.1189/jlb.1002495. [DOI] [PubMed] [Google Scholar]
- 10.Oh ST, Gotlib J. JAK2 V617F and beyond: role of genetics and aberrant signaling in the pathogenesis of myeloproliferative neoplasms. Expert Rev Hematol. 2010;3(3):323–337. doi: 10.1586/ehm.10.28. [DOI] [PubMed] [Google Scholar]
- 11.Hazan-Halevy I, Harris D, Liu Z, et al. STAT3 is constitutively phosphorylated on serine 727 residues, binds DNA, and activates transcription in CLL cells. Blood. 2010;115(14):2852–2863. doi: 10.1182/blood-2009-10-230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sattler M, Winkler T, Verma S, et al. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood. 1999;93(9):2928–2935. [PubMed] [Google Scholar]
- 13.Ito K, Hirao A, Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh A, Takahira H, Mantel C, et al. Steel factor induces serine phosphorylation of Stat3 in human growth factor-dependent myeloid cell lines. Blood. 1996;88(1):138–145. [PubMed] [Google Scholar]
- 15.Sattler M, Verma S, Shrikhande G, et al. The BCR/ABL tyrosine kinase induces production of reactive oxygen species in hematopoietic cells. J Biol Chem. 2000;275(32):24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 16.Welte T, Zhang SSM, Wang T, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci U S A. 2003;100(4):1879–1884. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broxmeyer HE, Lee MR, Hangoc G, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117(18):4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carow CE, Hangoc G, Broxmeyer HE. Human multipotential progenitor cells (CFU-GEMM) have extensive replating capacity for secondary CFU-GEMM: an effect enhanced by cord blood plasma. Blood. 1993;81(4):942–949. [PubMed] [Google Scholar]
- 20.Mantel C, Guo Y, Lee MR, et al. Checkpoint-apoptosis uncoupling in human and mouse embryonic stem cells: a source of karyotypic instability. Blood. 2007;109(10):4518–4527. doi: 10.1182/blood-2006-10-054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel C, Messina-Graham S, Broxmeyer HE. Upregulation of nascent mitochondrial biogenesis in mouse hematopoietic stem cells parallels upregulation of CD34 and loss of pluripotency: a potential strategy for reducing oxidative risk in stem cells. Cell Cycle. 2010;9(10):2008–2017. doi: 10.4161/cc.9.10.11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 23.Gerencser AA, Neilson A, Choi SW, et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Anal Chem. 2009;81(16):6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernier M, Paul RK, Martin-Montalvo A, et al. Negative regulation of STAT3 protein-mediated cellular respiration by SIRT1 protein. J Biol Chem. 2011;286(22):19270–19279. doi: 10.1074/jbc.M110.200311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Akira S. STAT family of transcription factors in cytokine-mediated biological responses. Cytokine Growth Factor Rev. 2000;11(3):199–207. doi: 10.1016/s1359-6101(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 26.Broxmeyer HE, Mejia JAH, Hangoc G, et al. SDF-1/CXCL12 enhances in vitro replating capacity of murine and human multipotential and macrophage progenitor cells. Stem Cells Dev. 2007;16(4):589–596. doi: 10.1089/scd.2007.0044. [DOI] [PubMed] [Google Scholar]
- 27.Ema H, Morita Y, Yamazaki S, et al. Adult mouse hematopoietic stem cells: purification and single-cell assays. Nat Protoc. 2006;1(6):2979–2987. doi: 10.1038/nprot.2006.447. [DOI] [PubMed] [Google Scholar]
- 28.Dykstra B, Kent D, Bowie M, et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1(2):218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly DS, Zelterman D, Sharkis S, Krause DS. Functional activity of murine CD34+ and CD34- hematopoietic stem cell populations. Exp Hematol. 1999;27(5):788–796. doi: 10.1016/s0301-472x(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 30.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192(9):1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw PE. Could STAT3 provide a link between respiration and cell cycle progression? Cell Cycle. 2010;9(21):4294–4296. doi: 10.4161/cc.9.21.13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers MG., Jr Cell biology: moonlighting in mitochondria. Science. 2009;323(5915):723–724. doi: 10.1126/science.1169660. [DOI] [PubMed] [Google Scholar]
- 33.Szczepanek K, Chen Q, Derecka M, et al. Mitochondrial-targeted Signal transducer and activator of transcription 3 (STAT3) protects against ischemia-induced changes in the electron transport chain and the generation of reactive oxygen species. J Biol Chem. 2011;286(34):29610–29620. doi: 10.1074/jbc.M111.226209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantel C, Messina-Graham SV, Broxmeyer HE. Superoxide flashes, reactive oxygen species, and the mitochondrial permeability transition pore: potential implications for hematopoietic stem cell function. Curr Opin Hematol. 2011;18(4):208–213. doi: 10.1097/MOH.0b013e3283475ffe. [DOI] [PubMed] [Google Scholar]
- 35.Oubrahim H, Stadtman ER, Chock PB. Mitochondria play no roles in Mn(II)-induced apoptosis in HeLa cells. Proc Natl Acad Sci U S A. 2001;98(17):9505–9510. doi: 10.1073/pnas.181319898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chazotte B. Labeling mitochondria with fluorescent dyes for imaging. Cold Spring Harb Protoc. 2009;2009(6) doi: 10.1101/pdb.prot4948. pdb.prot49. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge JM. Free radicals and antioxidant protection: mechanisms and significance in toxicology and disease. Hum Toxicol. 1988;7(1):7–13. doi: 10.1177/096032718800700102. [DOI] [PubMed] [Google Scholar]
- 38.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277(47):44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 39.Agnello M, Morici G, Rinaldi AM. A method for measuring mitochondrial mass and activity. Cytotechnology. 2008;56(3):145–149. doi: 10.1007/s10616-008-9143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers SM, Shaw CA, Gatza C, et al. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoekstra J, Bresser EL, Smalberg JH, et al. Long-term follow-up of patients with portal vein thrombosis and myeloproliferative neoplasms. J Thromb Haemost. 2011;9(11):2208–2214. doi: 10.1111/j.1538-7836.2011.04484.x. [DOI] [PubMed] [Google Scholar]
- 42.Polednak AP. US death rates from myeloproliferative neoplasms, and implications for cancer surveillance. J Registry Manag. 2011;38(2):87–92. [PubMed] [Google Scholar]
- 43.Matsuoka Y, Sasaki Y, Nakatsuka R, et al. Low level of c-Kit expression marks deeply quiescent murine hematopoietic stem cells. Stem Cells. 2011;29(11):1783–1791. doi: 10.1002/stem.721. [DOI] [PubMed] [Google Scholar]
- 44.Abdel-Wahab O, Levine RL. Metabolism and the leukemic stem cell. J Exp Med. 2010;207(4):677–680. doi: 10.1084/jem.20100523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192(7):1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105(6):771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 49.Farquhar MJ, Bowen DT. Oxidative stress and the myelodysplastic syndromes. Int J Hematol. 2003;77(4):342–350. doi: 10.1007/BF02982641. [DOI] [PubMed] [Google Scholar]
- 50.Gattermann N. From sideroblastic anemia to the role of mitochondrial DNA mutations in myelodysplastic syndromes. Leuk Res. 2000;24(2):141–151. doi: 10.1016/s0145-2126(99)00160-5. [DOI] [PubMed] [Google Scholar]
- 51.Han M-K, Song E-K, Guo Y, et al. SIRT1 regulates apoptosis and Nanog expression in mouse embryonic stem cells by controlling p53 subcellular localization. Cell Stem Cell. 2008;2(3):241–251. doi: 10.1016/j.stem.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.