Abstract
Sequestration in the bone marrow niche may allow leukemic stem cells to evade exposure to drugs. Because the CXCR4/SDF-1 axis is an important mechanism of leukemic stem cell interaction with marrow stroma, we tested whether plerixafor, an antagonist of CXCR4, may dislodge chronic myeloid leukemia (CML) cells from the niche, sensitizing them to tyrosine kinase inhibitors. We initially treated mice with retrovirally induced CML-like disease with imatinib plus plerixafor. Plerixafor mobilized CXCR4+ cells, but no difference was observed in leukemia burden, possibly reflecting insufficient disease control by imatinib. In a second series of experiments, we tested the combination of plerixafor with dasatinib in the same as well as an attenuated CML model. Despite much improved leukemia control, plerixafor failed to reduce leukemia burden over dasatinib alone. In addition, mice receiving plerixafor had an increased incidence of neurologic symptoms in association with CNS infiltration by BCR-ABL–expressing cells. We conclude that plerixafor is ineffective in reducing leukemia burden in this model but promotes CNS infiltration. Beneficial effects of combining tyrosine kinase inhibitors with plerixafor may be observed in a situation of minimal residual disease, but caution is warranted when disease control is incomplete.
Introduction
Chronic myeloid leukemia (CML) is caused by BCR-ABL, a constitutively active tyrosine kinase that is the result of the t(9;22) reciprocal translocation, cytogenetically visible as the Philadelphia chromosome.1 More than 80% of newly diagnosed CML patients treated with the BCR-ABL tyrosine kinase inhibitor (TKI) imatinib attain durable complete cytogenetic responses.2 However, BCR-ABL transcripts often remain detectable by RT-PCR3 and disease recurrence usually occurs on discontinuation of drug, even in patients who are negative by PCR.4 This implies that fully leukemogenic stem cells survive, even in the best responders, a state termed disease persistence.
Various mechanisms have been proposed for the persistence of CML leukemic stem cells in the presence of TKIs, including quiescence of CML stem cells,5 mutations in the kinase domain of BCR-ABL that confer low level drug resistance,6 drug transport out of leukemia cells,7,8 and protection by factors from the bone marrow microenvironment.9,10 The latter mechanism is consistent with observations in multiple myeloma and acute myeloid leukemia (AML), where “niches” in the marrow protect malignant stem cells from the effects of chemotherapy, by direct contact and/or by soluble factors present at concentrations exceeding those in the plasma of patients.11 Similarly, it was shown that bone marrow stroma is highly protective toward CML progenitor cells treated with BCR-ABL kinase inhibitors.9 Therefore, the interactions between CML cells and their microenvironment probably mediate disease persistence in vivo, despite effective inhibition of BCR-ABL kinase activity.10
CML progenitor cells exhibit defective adhesion to bone marrow stroma, which is partially BCR-ABL–independent and restored by IFN-α.12,13 Two mechanisms have been implicated. First, β1 integrin function is abnormal in CML progenitor cells, impairing their ability to bind to fibronectin.14 Second, CXCR4, the receptor for stromal-derived factor-1 (SDF-1; also named CXCL12) is down-regulated in BCR-ABL expressing cells, resulting in impaired migration toward SDF-1 and impaired SDF-1–mediated adhesion.15,16 Conversely, imatinib induces up-regulation of CXCR4 in CML cells.17,18 The up-regulation of CXCR4 in CML cells in response to imatinib may allow the cells to reestablish homing to niches in the bone marrow, where they are subsequently protected from the effects of BCR-ABL inhibition.18 This is consistent with the observation that clearance of blasts from the blood of patients with advanced CML is much more easily achieved than clearance of blasts from the bone marrow.
Interactions between CXCR4 on hematopoietic progenitor cells and SDF-1 produced by the niche are crucial for retention of progenitor cells in the marrow.19,20 Pharmacologic disruption of this axis using the CXCR4 antagonist plerixafor is widely used clinically for mobilization of hematopoietic stem cells into the peripheral blood for stem cell harvest.21–23 Furthermore, it has been shown that plerixafor enhances sensitivity of multiple myeloma24 and AML25–27 cells to multiple therapeutic agents in in vivo murine models.
We hypothesized that the reestablishment of CXCR4/SDF-1 interactions on imatinib therapy may provide a survival signal to CML stem cells that contributes to clinical disease persistence. If so, interrupting this interaction with plerixafor should deprive CML progenitor and stem cells of microenvironmental protection and eliminate residual leukemia cells. We tested this hypothesis in a murine transduction/transplantation model of CML.
Methods
Bone marrow transplantation
BALB/C mice were purchased from The Jackson Laboratory and maintained under standard conditions at the Oregon Health & Science University (OHSU) animal care facility. An murine stem cell virus (MSCV)–based retroviral transduction/transplantation model of p210BCR-ABL was used to induce CML-like disease in mice as previously described.28 After transplantation, the mice were inspected twice daily. Mice were subjected to weekly blood counts using a Vet ABC machine (Heska) beginning at day 9 after transplantation along with flow cytometric analysis of GFP in peripheral blood to quantify disease burden using a FACSAriaII (BD Biosciences). Treatment was initiated after hematopoietic reconstitution occurred as defined by normal blood counts (normal ranges: white blood cells [WBCs]; 3.0-15.0 × 103/mm3, hemoglobin; 11.1-18.0 g/dL, platelets; 140-600 × 103/mm3).
Treatment with imatinib in combination with plerixafor
Treatment was initiated at day 9, when elevated WBC counts and GFP+ cells in the peripheral blood were confirmed. Treatment schedules were as follows: (1) imatinib 100 mg/kg, twice daily, orally (n = 10); (2) imatinib 100 mg/kg, twice daily, orally plus plerixafor 5 mg/kg daily subcutaneously (n = 10); (3) plerixafor 5 mg/kg daily subcutaneously (n = 10); and (4) mock treatment (n = 10). Blood was drawn 2 hours after plerixafor dosing.
Treatment with dasatinib in combination with plerixafor
In an independent experiment, treatment was initiated with dasatinib only (10 mg/kg, twice daily, orally) on hematopoietic reconstitution (day 12) in an attempt to establish a state of residual disease, whereas a control group (n = 9) was mock-treated to confirm subsequent development of leukemia. Once the WBC counts of treated mice remained consistently in the normal range and the number of GFP+ cells remained stable at 65%-70%, the cohort was divided into 2 treatment arms: (1) dasatinib only (n = 19) and (2) dasatinb 10 mg/kg plus plerixafor 5 mg/kg daily subcutaneously (n = 17). Because plerixafor causes mobilization of CXCR4+ cells into the peripheral blood between 1 and 3 hours (Figure 1) and dasatinib plasma levels peak at 3 hours,29 plerixafor was dosed 1.5 hours after dasatinib to ensure that peaks of both drugs occur approximately simultaneously. Blood draws were performed before drug administration.
Figure 1.
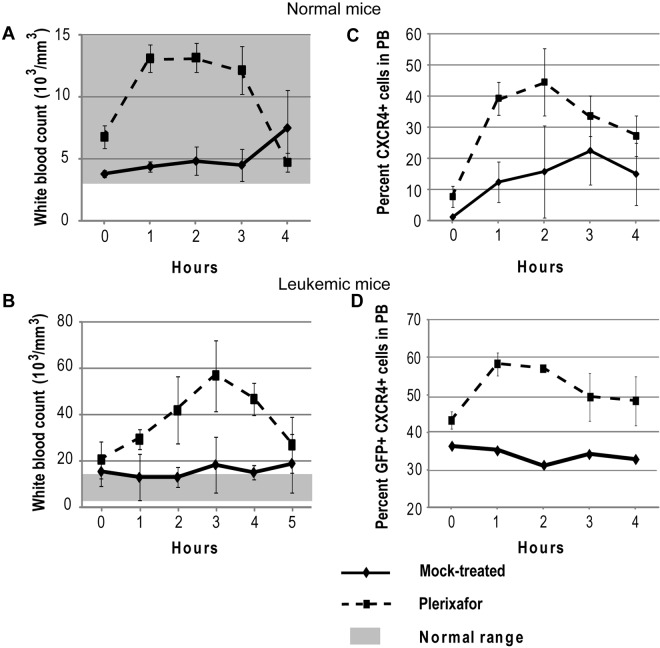
Plerixafor mobilizes CXCR4+ cells into the peripheral blood. Normal (A,C) and leukemic (B,D) mice were treated with a single dose of plerixafor, and WBC and CXCR4 expression in peripheral blood was measured over time. (A-B) Peripheral blood WBC count of normal and leukemic mice treated with plerixafor. (C-D) CXCR4 expression in peripheral blood of normal and leukemic mice treated with plerixafor.
Deceased and moribund mice were killed according to OHSU Institutional Animal Care and Use Committee guidelines, which mandated that animals are killed when they exhibit a hunched position, weight loss > 20%, excessive vomiting/diarrhea, skin necrosis, large tumors, or paralysis. A detailed autopsy was performed, including measurement of spleen and liver weight, histology of lung, spleen, liver, bone marrow, and brain (in the case of the dasatinib experiments). In addition FACS analysis was performed to determine the percentage of GFP+ cells in the spleen and bone marrow.
Flow cytometry
Peripheral blood was collected in tubes containing EDTA. Lysis of red blood cells was performed with ammonium chloride lysis buffer (0.8% NH4Cl with 0.1mM EDTA). Cells were resuspended in PBS containing 2% FBS and immediately subjected to analysis for GFP. To measure cell surface expression of CXCR4, a PE-conjugated anti–mouse CXCR4 antibody (clone 2B11; BD Biosciences) was used. All flow cytometry was performed on a FACSAriaII.
Immunoblot analysis
Whole cell lysates from spleen and bone marrow cells were prepared in NP-40 lysis buffer (1% NP40, 150mM NaCl, 20mM Tris, pH 8.0, 10% glycerol, 1mM EDTA, and 1% protease and phosphatase inhibitors; Sigma-Aldrich). A total of 50 μg of protein was resolved on SDS-PAGE, and immunoblotting was performed using pCRKL (Santa Cruz Biotechnology) and α-tubulin (Sigma-Aldrich) antibodies.
Immunohistochemistry
Brain tissue from autopsies was fixed in 10% buffered formalin for at least 7 days, and fixed 2-mm-thick coronal sections were processed in paraffin, cut as 7-μm sections, deparaffinized, subjected to standard antigen retrieval techniques (5 minutes treatment at room temperature with 95% formic acid, followed by incubation at 85°C-90°C in citrate buffer, pH 6.0, for 30 minutes), and stained with rabbit monoclonal antibody to GFP (clone D5.1 at 1:500 dilution, Cell Signaling Technologies). Sections were developed using the Vectastain ABC technique (Vector Laboratories).
Statistical analysis
Continuous variables were compared by pairwise Student t test for 2 independent samples using Excel software. Survival curve analysis was performed with GraphPad Prism software using the log-rank test with 2-tailed P value. P < .05 was considered statistically significant. Fisher exact test was performed to calculate the relative risk and odds ratio with CIs using GraphPad Prism software.
Results
Plerixafor mobilizes CXCR4+ cells into the peripheral blood
To confirm that plerixafor is able to mobilize WBCs into the peripheral blood, 5 normal mice were treated with a single dose of plerixafor. Hourly blood counts were taken to follow changes in WBC count, and CXCR4 expression was measured by flow cytometry. Treatment with plerixafor resulted in a 2-fold increase of the WBC count over baseline (Figure 1A). To determine whether plerixafor has similar effects in leukemic mice, 3 mice with a complete hematologic response to dasatinib and not previously treated with plerixafor were given 1 dose of plerixafor. The average WBC increased almost 3-fold over baseline, whereas the WBC counts of mock-treated mice remained constant (Figure 1B). These results are consistent with previous reports, which also showed 2- to 3-fold increases in leukocyte counts on plerixafor treatment.30–32 The percentage of CXCR4+ cells also increased in both plerixafor-treated groups compared with the mock-treated groups (Figure 1C-D). Overall, these data show that plerixafor is able to mobilize CXCR4-expressing WBCs into the peripheral blood both in normal and leukemic mice, consistent with disruption of the CXCR4/SDF-1 interaction.
Addition of plerixafor to TKIs does not improve survival or decrease disease burden
Imatinib combined with plerixafor.
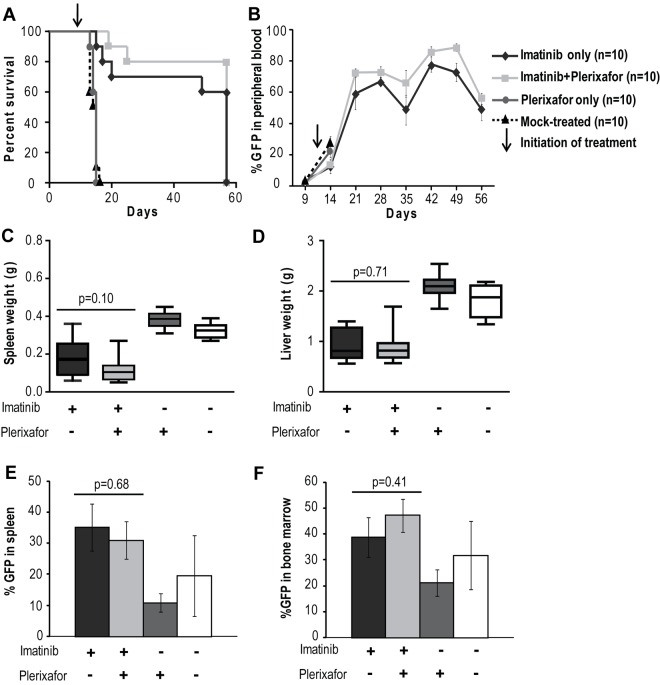
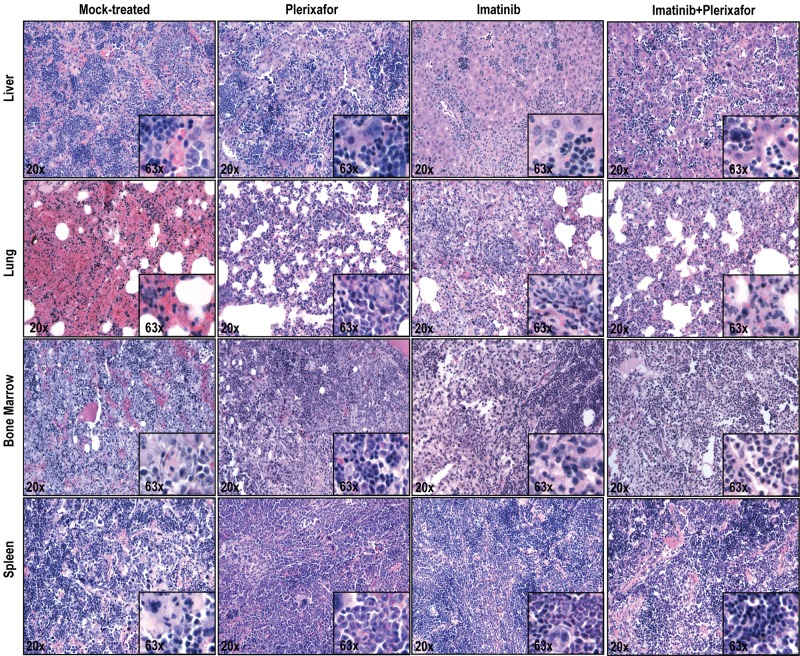
A previous study has shown that, in a retroviral murine model of CML, treatment with imatinib results in a marked reduction in peripheral WBC counts but fails to eradicate residual leukemia.33 We hypothesized that the bone marrow microenvironment provides a protective niche to leukemia stem cells, allowing them to evade the effects of BCR-ABL inhibition. In an initial experiment we tested whether disruption of the CXCR4/SDF-1 axis with plerixafor in addition to tyrosine kinase inhibition with imatinib would reduce disease burden compared with imatinib treatment alone. As expected, we saw no significant difference in disease manifestations or survival in the cohort treated with plerixafor alone compared with the mock-treated cohort; all animals developed overt leukemia and died or required death by day 16 (Figure 2A). Mice treated with the combination of imatinib + plerixafor (n = 10) as well as the group treated with imatinib alone (n = 10) were followed for 57 days after bone marrow transplantation. Both groups achieved a hematologic response with almost normal blood counts (supplemental Figure 1A-C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We observed a slight, but not statistically significant, increase in the WBC of mice treated with the combination of imatinib + plerixafor compared with the imatinib only treated mice, probably resulting from increased mobilization of WBCs into the peripheral blood by plerixafor. Despite a decrease in the absolute WBC count, there was an increase in the percentage of myeloid cells in the peripheral blood with a predominance of granulocytes in both treatment groups, suggesting persistence of leukemic cells in the peripheral blood (supplemental Figure 1D-F). Neither imatinib treatment alone nor the combination of imatinib + plerixafor led to a decrease in the proportion of leukemic cells (measured as GFP+ cells in the peripheral blood), with persistence of 60%-80% GFP+ cells in the blood of both cohorts (Figure 2B). There was no statistically significant difference in survival of mice treated with the combination of imatinib + plerixafor compared with imatinib only treated mice, and the experiment was terminated at day 57 because of the morbid condition of the mice (Figure 2A). Autopsy revealed that treatment with imatinib reduced spleen size compared with mock-treated or plerixafor-treated mice. We noticed a small reduction of GFP+ cells in the spleen and bone marrow of plerixafor-treated mice that did not receive imatinib, this is likely attributed to the need for sacrifice at an early time point following transplant (Figure 2E-F). However, none of these differences was statistically significant, and overall plerixafor had no significant effects on spleen or liver size, or the proportion of GFP+ cells in spleen, bone marrow, and peripheral blood, irrespective of whether the mice received imatinib or not (Figure 2C-F). In all groups, there was almost complete effacement of splenic architecture with extensive infiltration by hematopoietic cells, predominantly granulocytes. The bone marrow was hypercellular with granulocyte predominance. The lungs showed gross hemorrhaging with extensive infiltration of alveolar spaces, although there was a slight decrease in the extent of infiltration in the lungs of plerixafor-treated mice groups (Figure 3). Overall, these data show that, in this murine CML model, imatinib induces a hematologic response but does not significantly reduce the proportion of leukemic cells in the blood. Plerixafor leads to a mild, but statistically insignificant (P > .05), increase in leukemic (GFP+) cells in the peripheral blood, probably reflecting mobilization. However, the addition of plerixafor to imatinib had no appreciable effect on overall disease burden. We concluded that more profound TKI-induced suppression of the leukemia may be required for plerixafor to exhibit a positive effect. Therefore, to obtain a better model of residual disease, we repeated this experiment using dasatinib as a more potent TKI and added plerixafor after normalization of blood counts rather than simultaneously with the TKI.
Figure 2.
Addition of plerixafor to imatinib does not improve survival or decrease disease burden of leukemic mice. (A) Kaplan-Meier survival analysis of mock-treated, plerixafor only, imatinib only, or imatinib + plerixafor treatment groups (P = .3228, log-rank test). The experiment was terminated at day 57. (B) FACS analysis for GFP to assess the proportion of leukemia cells in the peripheral blood. (C-D) Spleen and liver weights measured at necropsy. (E-F) Percentage GFP+ cells in spleen and bone marrow measured by FACS.
Figure 3.
Histopathologic examination of imatinib and imatinib + plerixafor treated mice shows no significant differences. Spleen, liver, lung, and bone marrow were stained with hematoxylin and eosin.
Dasatinib combined with plerixafor.
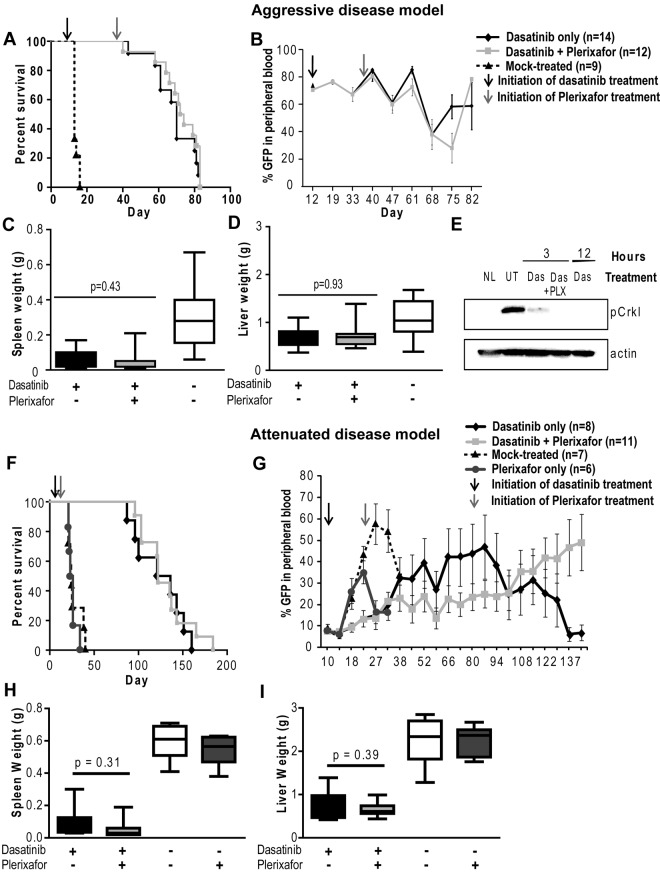
Mice (N = 36) were started on dasatinib (10 mg/kg twice daily) at day 12 after transplantation, when an elevation of the WBC count and GFP+ cells in the peripheral blood indicated hematopoietic reconstitution and establishment of leukemia. A control group (N = 9) was mock-treated. All these mice died of a CML-like myeloproliferative neoplasm by day 14 (Figure 4A). In contrast, blood counts normalized by day 19 in the mice treated with dasatinib and remained in the normal range thereafter in the majority of mice (supplemental Figure 2A-C). At day 36, when the WBC counts were in the normal range and GFP positivity was stable at 65%-70%, plerixafor was started in 1 group of mice (N = 17), whereas the other mice (N = 19) continued on dasatinib only. Over the following weeks, no significant difference was observed in the peripheral blood counts between the dasatinib and dasatinib + plerixafor groups. Both groups demonstrated a similar increase in the relative percent of granulocytes and a decrease in both monocytes and lymphocytes (supplemental Figure 2D-F), and the percentage of GFP+ cells remained similarly elevated (60%-80%) in both groups throughout the study (Figure 4B). Further, at autopsy, there were no significant differences in spleen or liver weight (Figure 4C-D) or in the proportion of GFP+ cells in bone marrow and spleen (supplemental Figure 6A-B).
Figure 4.
Addition of plerixafor to dasatinib does not improve survival or decrease disease burden of leukemic mice. Experiment was performed with both aggressive (A-E) and attenuated disease models (F-I). (A,F) Kaplan-Meier survival analysis of mice treated with dasatinib only or in combination with plerixafor (P = .2934, log-rank test). (B,G) FACS analysis for GFP+ cells to assess the proportion of leukemia cells in the peripheral blood. (C-D,H-I) Spleen and liver weight measured at necropsy of leukemic mice. (E) pCrkL levels measured by Western blot analysis in bone marrow cell lysates of dasatinib (Das) and dasatinib + plerixafor (Das + PLX) treated mice after indicated time of treatment compared with normal mice (NL) and untreated leukemic mice (UT).
To determine whether the combination of dasatinib + plerixafor prolongs survival after TKI discontinuation, 5 mice from each group were randomly selected and taken off all treatment at day 68. No significant difference was observed in survival (supplemental Figure 3), blood counts, and spleen or liver weights (data not shown) between these 2 subgroups.
Treatment was continued in the remaining mice, but no significant difference in survival was observed (median, 73 days; range, 40-83 days for dasatinib only; and median, 70 days; range, 43-83 days for dasatinib + plerixafor; Figure 4A). Importantly, several of the animals required death because of neurologic symptoms. These findings are described in more detail under “Combined treatment of plerixafar and dasatinib increases the incidence of CNS involvement.”
To exclude the possibility that dasatinib was unable to adequately reduce leukemia burden because of ineffective inhibition of BCR-ABL kinase activity in vivo, we performed immunoblot analysis using pCrkL as a surrogate marker for BCR-ABL kinase activity. In previous studies, CrkL has been demonstrated to be a major substrate of BCR-ABL in vivo and shown to be aberrantly phosphorylated in BCR-ABL transgenic mice.34 Bone marrow was harvested at 3 and 12 hours after dasatinib dosing (10 mg/kg). We found that pCrkL levels were reduced in bone marrow cells from mice treated with dasatinib only or with dasatinib and plerixafor, suggesting that the failure to eliminate leukemia cells is not the result of a lack of target inhibition (Figure 4E).
Dasatinib combined with plerixafor in an attenuated disease model.
Given the lack of discernible effects of plerixafor on leukemia burden, we reasoned that demonstrating such effects may require a less aggressive disease model more akin to persistent disease in humans. To address this question, we developed an attenuated disease model by reducing the number of BCR-ABL transduced bone marrow cells injected. Mice received 5 × 104 lineage-negative cells with 2.5% BCR-ABL-expressing (GFP+) cells, for a total of 1250 BCR-ABL+ cells/mouse. A total of 105 noninfected lineage-positive cells were also injected to provide support until engraftment had occurred. This represents a 74-fold smaller inoculum of GFP+ cells compared with the previous experiment where 5 × 105 bone marrow donor cells per mouse were injected (18.5% GFP+ cells). Nineteen mice were started on dasatinib (10 mg/kg, twice daily) at day 10 after transplantation, when the WBC count (3-10 × 103/mm3) and GFP+ cells (5%-10%) in the peripheral blood indicated hematopoietic reconstitution and establishment of leukemia. Seven mice were mock-treated and 6 mice were treated with plerixafor (5 mg/kg daily) only; all these mice died of a CML-like myeloproliferative neoplasm by day 40 with no significant difference in survival (Figure 4F). At day 22, plerixafor was added to the regimen in 11 of the 19 dasatinib-treated mice, whereas the remaining 8 mice continued on dasatinib only. Serial GFP measurements showed that plerixafor retained the ability to mobilize leukemic cells, even after prolonged treatment (supplemental Figure 4). Over the following weeks, no significant difference was observed in peripheral blood counts between the dasatinib and dasatinib + plerixafor groups (supplemental Figure 5). Between day 38 and 94, the median percentage of GFP+ cells was slightly lower in the combination treatment group (6%-18%) compared with the dasatinib only treated group (11%-35%; Figure 4G). However, this trend was not maintained at later time points, when several mice showed progression (evident by an increase in GFP+ cells in the blood) and required death. Similar results were observed by Chien et al in an AML xenograft mouse model, which showed an initial reduction in leukemia burden in mice treated with plerixafor in combination with chemotherapy, with progression at later time points.35 Survival was not different between the 2 groups (dasatinib only: median, 128.5 days; range, 87-160 days; dasatinib + plerixafor: median, 122 days; range, 96-165 days; Figure 4F). No significant differences were observed in spleen or liver weight (Figure 4H-I). The proportions of total GFP+ cells and GFP+Lin−Kit+ progenitor/stem cells in bone marrow and spleen were not significantly different, although we noted a trend toward increased GFP+ cells in the spleens of combination-treated mice (supplemental Figure 6D,F).
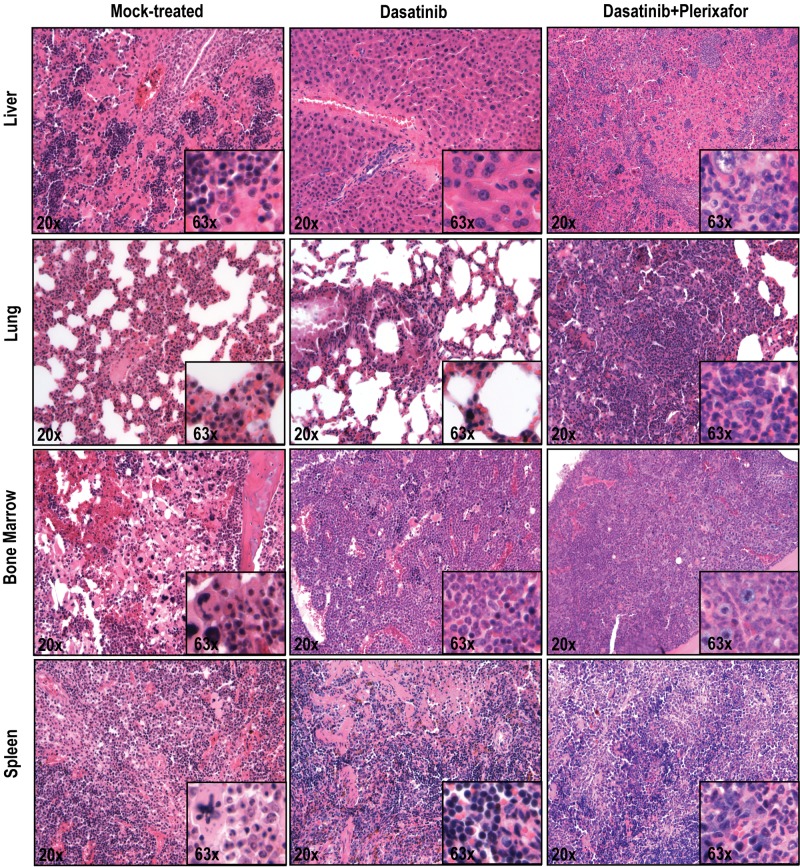
To ascertain an objective assessment, all histopathologic assessments were performed by 2 independent observers blinded to the treatment group (dasatinib only vs dasatinib + plerixafor). In the aggressive disease model, there was increased infiltration by hematopoietic cells in the liver, lung, and spleen in the dasatinib + plerixafor combination group compared with the dasatinib only group (Figure 5). In the attenuated disease model, these differences were less consistent (supplemental Figure 7). No significant differences were noted in the bone marrow, which demonstrated 100% cellularity with granulocytic dominance in both groups. All in all, despite the decrease in leukemic burden, as evidenced by decreased spleen size and extended survival, the results with the attenuated disease model with respect to the effect of addition of plerixafor were similar to those with the aggressive model.
Figure 5.
Histopathologic analysis of mice treated with dasatinib + plerixafor shows slightly increased infiltration of leukemic cells in all organs compared with dasatinib only treatment. Spleen, liver, lung, and bone marrow sections were stained with hematoxylin and eosin.
Combined treatment of plerixafor and dastinib increases the incidence of CNS involvement
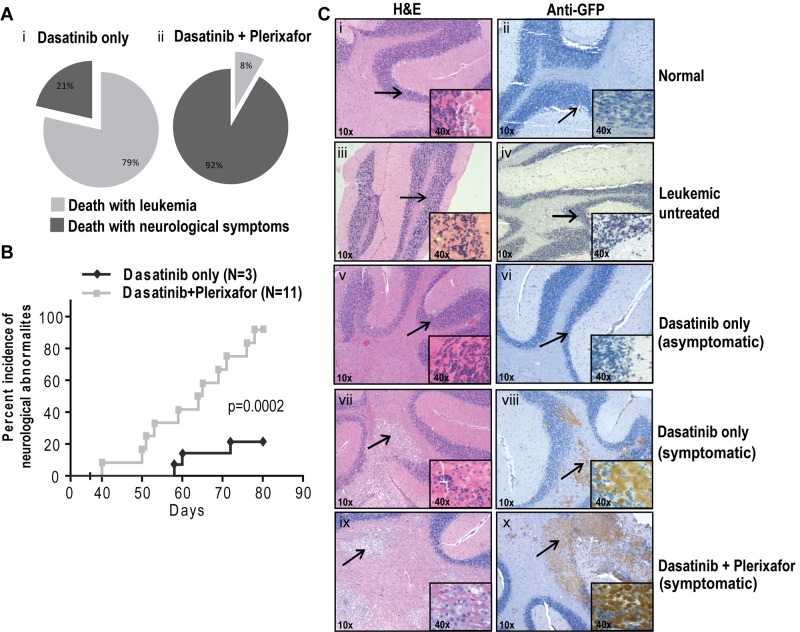
Unexpectedly, many experimental mice developed focal and diffuse neurologic signs indicative of CNS involvement. The milder cases exhibited rear limb paralysis and ataxia, whereas severely affected animals showed complete loss of motor control. Rear limb paralysis progressed from a reduction in movement of hind limbs to complete loss of function. Loss of motor control typically started with compulsive bending of the head to 1 side, which was followed by halting or jerking movements. The terminal state was characterized by uncontrolled random movements of the head, limbs, and torso. Mice with progressive neurologic symptoms were euthanized. In the first experiment that tested the dasatinib/plerixafor combination in the aggressive CML model, of 14 mice in the dasatinib only group, 3 (21%) developed neurologic symptoms, compared with 11 of 12 (92%) in the dasatinib + plerixafor group (P < .001, Fisher exact test). The remaining mice died or were sacrificed because of overt leukemia, without neurologic symptoms (Figure 6Ai-ii). The odds of developing neurologic symptoms while on dasatinib + plerixafor treatment are estimated to be 40.33 times greater compared with dasatinib only treatment (95% CI, 3.613-450.216). When we limited our analysis to central neurologic symptoms (pronounced leaning, shaking, and twisting), excluding hind limb paralysis, we found that 9 of 12 (75%) dasatinib + plerixafor combination mice showed central neurologic abnormalities, compared with only 1 of 14 (7%) dasatinib only treated mice (P = .01, Fisher exact test; estimated odds ratio 39, 95% CI, 3.477-437.490). Further, the onset of neurologic symptoms was earlier in the dasatinib + plerixafor group (first neurologic symptom observed at day 40) compared with the dasatinib only group (first neurologic symptom observed at day 58; P = .0002, log-rank test; Figure 6B). Similarly, in the attenuated disease model, the incidence of neurologic impairment was also higher in the group receiving dasatinib + plerixafor. By day 95, 1 of 8 (12.5%) mice in the dasatinib only group had developed neurologic symptoms compared with 5 of 11 (45.5%) in the dasatinib + plerixafor combination group. By day 170, the incidence of neurologic impairment was 50% in the dasatinib and 100% in the combination group with a median time to occurrence of 153 versus 108 days, respectively (P = .018, log-rank test; supplemental Figure 8A-C).
Figure 6.
Plerixafor combination with dasatinib increases neurologic symptoms of leukemic mice. (A) Percentage incidence of neurologic symptoms in dasatinib only and combination group (i-ii) after excluding 5 mice from each group, which were taken off treatment at day 68. (B) Incidence of neurologic symptoms, from first occurrence of symptoms to death in symptomatic mice. Significant difference was observed between dasatinib only and dasatinib + plerixafor groups (P = .0002, log-rank test) in the occurrence of neurologic symptoms. (C) Hematoxylin and eosin (left panel) and anti-GFP immunohistochemistry (right panel) on brain sections. Arrow indicates the area of the brain, which is shown in higher magnification normal brain structure (i-vi) and leukemic infiltrates (vii-x).
To exclude the possibility that the neurologic findings were a direct neurotoxic effect of dasatinib or plerixafor in mice that had undergone bone marrow transplant, mice (n = 3 in each group) were injected with normal bone marrow after lethal irradiation. Treatment with dasatinib, plerixafor, or dasatinib + plerixafor was initiated on hematologic reconstitution and continued for 4 months. None of these animals developed neurologic symptoms.
To determine the cause of the neurologic symptoms, histopathologic analysis was performed on brains of mice in both groups as well as normal controls, using hematoxylin and eosin staining (Figure 6C; supplemental Figure 8D left panel). Brains of mice with neurologic symptoms demonstrated infiltration of cells morphologically appearing as macrophages similar to previous reports of CNS involvement (Figure 6Cvii-x).36 These cells are absent in normal or leukemic mice without neurologic symptoms (Figure 6Ci-vi). To confirm that the abnormal infiltrates in the brain sections represent leukemia, we performed immunohistochemistry on corresponding brain sections using a GFP antibody. We observed that all nodular infiltrates observed in symptomatic mice are GFP+ mononuclear cells corresponding to the infiltrates in hematoxylin and eosin sections (Figure 6Ciii-iv; Table 1). All mice with larger (> 1 mm in greatest dimension) nodular infiltrates in the brainstem and pons were symptomatic. Other brain regions, including the striatum, thalamus, and cerebellum, also contained nodular infiltrates that were less tightly correlated with symptoms (Table 1). In addition, leptomeningeal and scant perivascular GFP+ cells were present in multiple areas of the brain regardless of the presence of symptoms. Brains from normal BALB/c mice (Figure 6Ci-ii; Table 1), untreated leukemic mice (Figure 6Ciii-iv; Table 1), and radiation only treated mice (Table 1) were devoid of any infiltrates. Thus, although symptoms of CNS involvement were observed in both treatment groups, their incidence was significantly higher in the dasatinib + plerixafor combination group.
Table 1.
Summary of histopathologic analysis of leukemic infiltration in brain
| Treatment | CNS symptoms | Death | Striatum | Thalamus | Brainstem/pons | Cerebellum |
|---|---|---|---|---|---|---|
| None* | No | Killed | 0 | 0 | 0 | 0 |
| None* | No | Radiation | 0 | 0 | 0 | 0 |
| None | No | Leukemia | 0 | 0 | 0 | 0 |
| Dasatinib | No | Leukemia | 1 | 1 | 1 | 2 |
| Dasatinib | No | Leukemia | 1 | 1 | 1 | 1 |
| Dasatinib | Yes | Leukemia | 3 | 2 | 4 | 2 |
| Dasatinib + plerixafor | Yes | Killed because of neurosymptoms | 2 | 2 | 2 | 4 |
| Dasatinib + plerixafor | Yes | Killed because of neurosymptoms | 1 | 3 | 4 | 1 |
| Dasatinib + plerixafor | Yes | Killed because of neurosymptoms | 3 | 3 | 4 | 4 |
The presence of GFP-positive infiltrates in various brain structures, as established by anti-GFP immunohistochemistry with a semiquantitative scheme of assessment: 1 indicates perivascular leukemic infiltrates; 2, nodular infiltrates < 0.5 mm; 3, nodular infiltrates 0.5-1 mm; and 4, nodular infiltrates > 1 mm. The presence of CNS symptoms was most closely associated with involvement of the brainstem and pons by nodular leukemic infiltrates, although the number of animals undergoing necropsy examination was too small to establish a statistically significant association between treatment and specific pathologic findings.
Nonleukemic mice that were killed to use as controls.
Discussion
The CXCR4/SDF-1 axis has attracted considerable attention as a therapeutic target in hematologic malignancies.17–19,24–27,37 Several studies have shown that addition of plerixafor, a small-molecule antagonist of CXCR4, to chemotherapy can reduce disease burden in murine models of AML,25,26 multiple myeloma,24 and BCR-ABL+ acute lymphoblastic leukemia.38 Moreover, a recent study found that combining plerixafor with nilotinib in mice injected with 32D cells expressing p210BCR-ABL cells reduced leukemia burden and prolonged survival over mice treated with nilotinib only.32 A large body of data support the notion that BCR-ABL disrupts the physiologic CXCR4/SDF-1 interaction in CML by reducing its expression or interfering with its function.17,18,37 It is thought that this may contribute to the mobilization of leukemic cells into the circulation, a hallmark of CML. Interestingly, it has been demonstrated that inhibition of BCR-ABL with TKIs, including imatinib and dasatinib, restored CXCR4 function, thereby promoting the redistribution of leukemia cells into the bone marrow, where they may be protected from TKI effects by the microenvironment.18,39 Based on this, we hypothesized that addition of plerixafor to BCR-ABL TKIs should reduce leukemia burden by dislodging CML cells from their protective marrow environment, and tested this hypothesis in the standard retroviral murine model. In contrast to our expectations, the addition of plerixafor to imatinib or dasatinib in this CML model did not result in a reduction of disease burden (measured by WBC counts and percentage of GFP-expressing cells), and did not increase survival or delay the return of overt leukemia after the discontinuation of TKI therapy (Figures 2 and 4), although plerixafor was able to mobilize leukemia cells (Figure 1). It is conceivable that plerixafor's failure to reduce disease burden is the result of the relative inefficacy of TKIs, particularly imatinib, in this disease model. Although both reduced (imatinib) or normalized (dasatinib) WBC counts, GFP+ cells persisted in the blood, equivalent to a hematologic but not a cytogenetic response in patients (Figures 2B and 4B). Immunoblotting for pCrkL showed that this was not the result of a lack of BCR-ABL inhibition (Figure 4E), suggesting that alternative survival signals compensate for BCR-ABL, which in turn may explain the lack of a beneficial plerixafor effect. Our results were largely consistent both in the standard retroviral CML model and an attenuated model produced by reduction of the initial inoculums of GFP+ cells, although we observed a slight, not statistically significant and transient reduction of GFP+ cells in the attenuated model (Figure 4G).
Mice treated with the dasatinib + plerixafor combination were more likely to develop severe neurologic deficits that correlated with infiltration of the brain by hematopoietic cells (Figure 6; supplemental Figure 8). A previous study using the same CML model has reported CNS involvement in imatinib-treated mice,36,40 whereas CNS involvement is not usually seen in untreated mice. This is thought to reflect the fact that imatinib prolongs survival by efficiently controlling leukemia, thereby providing a window for CNS involvement to develop. The symptoms and pathologic disease features in these mice were very similar to our observations. It is possible that we missed neurologic involvement in our initial experiment combining imatinib with plerixafor because of the rapid leukemia progression and the fact that we did not specifically monitor for neurosymptoms. In the dasatinib experiments, where disease control was much improved and survival prolonged, neurosymptoms were much more common in the mice receiving the dasatinib/plerixafor combination compared with mice receiving dasatinib alone. Although the degree of infiltration in symptomatic mice from both groups was similar (Table 1; Figure 6C; supplemental Figure 8), these data indicate that plerixafor promotes CNS involvement by BCR-ABL+ disease in this model, raising concerns about the safety of plerixafor in patients with active CML that is insufficiently controlled with TKIs. Importantly, no neurosymptoms were observed in lethally irradiated mice that were treated with dasatinib and plerixafor after transplantation of normal bone marrow.
Our results are at odds with a recent study that tested the combination of plerixafor with nilotinib in mice transplanted with 32D cells engineered to express p210BCR-ABL.32 The authors reported a significant reduction of leukemia burden with the plerixafor/nilotinib combination compared with nilotinib alone. Although they found meningeal involvement, this did not differ between mice treated with plerixafor and controls. The discrepancies between the 2 studies probably reflect the differences in the disease models. The 32Dp210BCR-ABL cells are derived from IL-3–dependent parental cells, which were transformed to growth factor independence by BCR-ABL expression. On TKI exposure, they revert to growth factor dependence, which explains their improved survival in cytokine-rich environments, such as the marrow.41 In contrast, the retroviral model, although imperfect, is a more faithful reflection of the clinical disease. It remains possible that the total body irradiation used in the retroviral model may promote CNS involvement in the first place by disrupting the blood-brain barrier, which may enhance infiltration by bone marrow-derived progenitor cells.42
Several additional factors deserve consideration. Continuous plerixafor dosing, as used in our study, was shown to promote extramedullary hematopoiesis in rats43 and could have contributed to CNS involvement in our study. Of note, no neurotoxicity was observed with the use of plerixafor in addition to cytotoxic chemotherapy in phase 1 or 2 studies of AML, where the drug was administered only during the 5 days of chemotherapy.44 Moreover, TKI-induced minimal residual disease in human CML is clearly different from the TKI-induced hematologic control in our murine model.
In conclusion, our results suggest that plerixafor in combination with TKIs may have limited efficacy in patients with poorly controlled CML, and such persons should be monitored closely for neurologic symptoms. This notwithstanding, it is entirely conceivable that plerixafor will be effective in targeting minimal residual leukemia in patients with well-controlled CML.
Acknowledgments
The authors thank Sarah Bowden and Suzanne Wickens for administrative support.
This work was supported by the National Institutes of Health (grants HL082978-01 and CA04963920A2, M.W.D.), the Leukemia & Lymphoma Society (grant 7036-01, M.W.D.), Genzyme (M.W.D.), Howard Hughes Medical Institute (B.J.D.), and Leukemia & Lymphoma Society (grant SCOR 7393-06, B.J.D.). A.A. is a recipient of Lady Tata Memorial Trust and OHSU Knight Cancer Institute's Cancer Research Development Award. A.G.F. is a recipient of an Amgen Oncology Fellowship. M.W.D. is a Scholar in Clinical Research of the Leukemia & Lymphoma Society.
Footnotes
Presented in abstract form at the 52nd Annual Meeting of the American Society of Hematology, Orlando, FL, December 6, 2010.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.A., A.G.F., C.L.P., R.M., and S.L. performed the experiments and analyzed results; A.A., A.G.F., and M.W.D. designed the research and wrote the manuscript; B.J.D. reviewed the manuscript; and M.L. and R.L.W. performed the histopathologic analysis.
Conflict-of-interest disclosure: M.W.D. serves as a consultant for Novartis and Bristol-Myers Squibb (consulting income < $10 000). M.W.D. receives research support from Bristol-Myers Squibb (no salary from these contracts). B.J.D. is currently principal investigator or coinvestigator on Novartis and Bristol-Myers Squibb clinical trials. His institution has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead. He does not derive salary, nor does his laboratory receive funds from these contracts. OHSU and B.J.D. have a financial interest in MolecularMD. OHSU has licensed technology used in some of these clinical trials to MolecularMD. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interests.
Correspondence: Michael W. Deininger, Huntsman Cancer Institute, 2000 Circle of Hope, Room 4261, Salt Lake City, UT 84112-5550; e-mail: michael.deininger@hci.utah.edu.
References
- 1.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–3356. [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 3.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349(15):1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 4.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109(1):58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 5.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 6.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105(5):2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]
- 7.White DL, Saunders VA, Dang P, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110(12):4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 8.Mahon FX, Belloc F, Lagarde V, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101(6):2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- 9.Damiano JS, Hazlehurst LA, Dalton WS. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia. 2001;15(8):1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 10.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114(6):1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93(5):1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 12.Dowding C, Guo AP, Osterholz J, Siczkowski M, Goldman J, Gordon M. Interferon-alpha overrides the deficient adhesion of chronic myeloid leukemia primitive progenitor cells to bone marrow stromal cells. Blood. 1991;78(2):499–505. [PubMed] [Google Scholar]
- 13.Wertheim JA, Forsythe K, Druker BJ, Hammer D, Boettiger D, Pear WS. BCR-ABL-induced adhesion defects are tyrosine kinase-independent. Blood. 2002;99(11):4122–4130. doi: 10.1182/blood.v99.11.4122. [DOI] [PubMed] [Google Scholar]
- 14.Sattler M, Salgia R, Shrikhande G, et al. Differential signaling after beta1 integrin ligation is mediated through binding of CRKL to p120(CBL) and p110(HEF1). J Biol Chem. 1997;272(22):14320–14326. doi: 10.1074/jbc.272.22.14320. [DOI] [PubMed] [Google Scholar]
- 15.Salgia R, Quackenbush E, Lin J, et al. The BCR/ABL oncogene alters the chemotactic response to stromal-derived factor-1alpha. Blood. 1999;94(12):4233–4246. [PubMed] [Google Scholar]
- 16.Ptasznik A, Urbanowska E, Chinta S, et al. Crosstalk between BCR/ABL oncoprotein and CXCR4 signaling through a Src family kinase in human leukemia cells. J Exp Med. 2002;196(5):667–678. doi: 10.1084/jem.20020519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geay JF, Buet D, Zhang Y, et al. p210BCR-ABL inhibits SDF-1 chemotactic response via alteration of CXCR4 signaling and down-regulation of CXCR4 expression. Cancer Res. 2005;65(7):2676–2683. doi: 10.1158/0008-5472.CAN-04-2152. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Tabe Y, Konoplev S, et al. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7(1):48–58. doi: 10.1158/1535-7163.MCT-07-0042. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A, O'Hare T, Deininger MW. Novel Developments in Stem Cell Mobilization: Focus on CXCR4. New York, NY: Springer; 2012. CXCR4 antagonists for the treatment of CML. pp. 351–367. [Google Scholar]
- 20.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23(7):879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 21.Devine SM, Flomenberg N, Vesole DH, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J Clin Oncol. 2004;22(6):1095–1102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 22.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 23.DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113(23):5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 24.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nervi B, Ramirez P, Rettig MP, et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113(24):6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavor S, Eisenbach M, Jacob-Hirsch J, et al. The CXCR4 antagonist AMD3100 impairs survival of human AML cells and induces their differentiation. Leukemia. 2008;22(12):2151–2158. doi: 10.1038/leu.2008.238. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal A, Bumm TG, Corbin AS, et al. Absence of SKP2 expression attenuates BCR-ABL-induced myeloproliferative disease. Blood. 2008;112(5):1960–1970. doi: 10.1182/blood-2007-09-113860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo FR, Yang Z, Camuso A, et al. Dasatinib (BMS-354825) pharmacokinetics and pharmacodynamic biomarkers in animal models predict optimal clinical exposure. Clin Cancer Res. 2006;12(23):7180–7186. doi: 10.1158/1078-0432.CCR-06-1112. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix CW, Flexner C, MacFarland RT, et al. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother. 2000;44(6):1667–1673. doi: 10.1128/aac.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juarez J, Dela Pena A, Baraz R, et al. CXCR4 antagonists mobilize childhood acute lymphoblastic leukemia cells into the peripheral blood and inhibit engraftment. Leukemia. 2007;21(6):1249–1257. doi: 10.1038/sj.leu.2404684. [DOI] [PubMed] [Google Scholar]
- 32.Weisberg E, Azab AK, Manley PW, et al. Inhibition of CXCR4 in CML cells disrupts their interaction with the bone marrow microenvironment and sensitizes them to nilotinib. Leukemia. 2012;26(5):985–990. doi: 10.1038/leu.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolff NC, Ilaria RL., Jr Establishment of a murine model for therapy-treated chronic myelogenous leukemia using the tyrosine kinase inhibitor STI571. Blood. 2001;98(9):2808–2816. doi: 10.1182/blood.v98.9.2808. [DOI] [PubMed] [Google Scholar]
- 34.de Jong R, Haataja L, Voncken JW, Heisterkamp N, Groffen J. Tyrosine phosphorylation of murine Crkl. Oncogene. 1995;11(8):1469–1474. [PubMed] [Google Scholar]
- 35.Chien SZX, Papayannopoulou T, Appelbaum FR, Becker PS. The CXCR4 inhibitor plerixafor effectively mobilizes primary AML in NODscid IL2R c−/− xenografts and markedly reduces but does not eradicate leukemia in combination with cytarabine +/− clofarabine chemotherapy. Am Soc Hematol. 2011:1432. [Google Scholar]
- 36.Wolff NC, Richardson JA, Egorin M, Ilaria RL., Jr The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101(12):5010–5013. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- 37.Burger JA, Burkle A. The CXCR4 chemokine receptor in acute and chronic leukaemia: a marrow homing receptor and potential therapeutic target. Br J Haematol. 2007;137(4):288–296. doi: 10.1111/j.1365-2141.2007.06590.x. [DOI] [PubMed] [Google Scholar]
- 38.Parameswaran R, Yu M, Lim M, Groffen J, Heisterkamp N. Combination of drug therapy in acute lymphoblastic leukemia with a CXCR4 antagonist. Leukemia. 2011;25(8):1314–1323. doi: 10.1038/leu.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fei F, Stoddart S, Muschen M, Kim YM, Groffen J, Heisterkamp N. Development of resistance to dasatinib in Bcr/Abl-positive acute lymphoblastic leukemia. Leukemia. 2010;24(4):813–820. doi: 10.1038/leu.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Porkka K, Koskenvesa P, Lundan T, et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood. 2008;112(4):1005–1012. doi: 10.1182/blood-2008-02-140665. [DOI] [PubMed] [Google Scholar]
- 41.Weisberg E, Wright RD, McMillin DW, et al. Stromal-mediated protection of tyrosine kinase inhibitor-treated BCR-ABL-expressing leukemia cells. Mol Cancer Ther. 2008;7(5):1121–1129. doi: 10.1158/1535-7163.MCT-07-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burrell K, Jelveh S, Jalali S, Hill R, Zaden G. Role of bone marrow derived cells in response to ionizing radiation in normal brain [abstract].. Presented at AACR 2011 Annual Meeting; April 3, 2011; Orlando, FL. Abstract 566. [Google Scholar]
- 43.Genzyme. Mozobil (plerixafor injection) product monograph. Cambridge, MA: Genzyme; 2009. [Google Scholar]
- 44.Uy GL, Rettig MP, Motabi IH, et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. Apr 26;119(17):3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]