Abstract
Intracellular mechanism(s) that contribute to promiscuous signaling via oncogenic KIT in systemic mastocytosis and acute myelogenous leukemia are poorly understood. We show that SHP2 phosphatase is essential for oncogenic KIT-induced growth and survival in vitro and myeloproliferative disease (MPD) in vivo. Genetic disruption of SHP2 or treatment of oncogene-bearing cells with a novel SHP2 inhibitor alone or in combination with the PI3K inhibitor corrects MPD by disrupting a protein complex involving p85α, SHP2, and Gab2. Importantly, a single tyrosine at position 719 in oncogenic KIT is sufficient to develop MPD by recruiting p85α, SHP2, and Gab2 complex to oncogenic KIT. Our results demonstrate that SHP2 phosphatase is a druggable target that cooperates with lipid kinases in inducing MPD.
Introduction
Gain-of-function mutations in KIT receptor in humans are associated with gastrointestinal stromal tumors (GIST), systemic mastocytosis (SM), and acute myelogenous leukemia (AML).1–4 An activating KIT receptor mutation of aspartic acid to valine at codon 814 in mice (KITD814V) or codon 816 in humans (KITD816V) results in altered substrate recognition and constitutive tyrosine autophosphorylation leading to promiscuous signaling.5,6 Consequently, cell lines and primary BM cells that express the oncogenic KITD814V demonstrate ligand-independent proliferation in vitro and myeloproliferative disease (MPD) in vivo.5–9 However, the intracellular signals that contribute to KITD814V-induced MPD are not known. Although activating mutations of KIT involving the juxtamembrane domain found in GIST are highly sensitive to inhibition by imatinib mesylate (ie, Gleevec), KIT mutations within tyrosine kinase domain found in SM and AML, including KITD816V, are relatively resistant to imatinib treatment.10–12 Thus, it is vital to identify novel drug targets for diseases involving KITD816V mutation.
Emerging data suggest an essential role for SHP2 in MPD. SHP2 is a protein tyrosine phosphatase that is encoded by PTPN11 gene and has been implicated in diverse signaling pathways induced by a number of stimuli, including growth factors, cytokines, extracellular matrix, and even cellular stress.13–15 Given that activating mutations in SHP2 have been found in leukemias and solid tumors,16,17 efforts are ongoing to define the potential efficacy of SHP2 phosphatase inhibition in diseases bearing SHP2 hyperactivation, either because of activating SHP2 mutations or those in which SHP2 collaborates with other oncogenes. Using genetic approaches, including primary BM cells derived from SHP2−/− and Gab2−/− mice and a novel SHP2 inhibitor, II-B08, identified from a focused library of indole-based salicylic acid derivatives,18 we demonstrate that SHP2 is essential for KITD814V-induced MPD. We further demonstrate that SHP2 constitutively binds to p85α and Gab2 in KITD814V-bearing cells, which can be disrupted by II-B08 resulting in impaired ligand-independent growth in vitro and MPD in vivo. Importantly, tyrosine residue at 719 within the intracellular KIT domain plays a unique role in regulating KITD814V-induced proliferation and survival in vitro, and MPD in vivo through recruitment of p85α, SHP2, and Gab2 protein complex to KITD814V and activation of PI3K. Consistently, the SHP2 inhibitor, II-B08, enhances the efficacy of PI3K inhibitor in treating KITD814V-induced MPD in vivo. We define SHP2 as a novel therapeutic target for hematologic malignancies involving oncogenic KIT and suggest that treating KITD814V-bearing cells with a combination of a tyrosine phosphatase and a lipid kinase inhibitor may be a prudent and novel approach for treating diseases involving oncogenic forms of KIT.
Methods
SHP2 inhibitor II-B08
The discovery and characterization of SHP2 inhibitor II-B08 have been described previously.18
Antibodies and cytokines
Antibodies and cytokines used in this study are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Mice
C57BL/6 and C3H/HeJ mice were purchased from The Jackson Laboratory. Mice bearing 2 floxed alleles of Ptpn11 (SHP2flox/flox) were crossed with transgenic mice expressing Cre directed by the Mx1 promoter.19,20 Six- to 8-week-old SHP2flox/flox/Cre mice were treated with 3 intraperitoneal injections of 300 μg polyriboinosinic acid/polyribocytidylic acid (poly I:C; Sigma-Aldrich) on alternative days to induce Cre expression. SHP2flox/flox mice were crossed with TgCreER transgenic mice to generate congenic littermates that were of the genotype, SHP2flox/flox (control) or SHP2flox/flox:TgCreER.21 Gab2−/− mice and mast cell-deficient KitW-sh/W-sh mice were previously described.22,23 All mice used in this study were between 6 and 12 weeks of age. These mice were maintained under specific pathogen-free conditions at the Indiana University Laboratory Animal Research Center (Indianapolis, IN), and the study was approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
Construction of WT and mutant KIT receptors
The wild-type (WT) KIT and KITD814V were inserted into the bicistronic retroviral vector, MIEG3, upstream of the internal ribosome entry site and the enhanced green fluorescent protein (EGFP) gene as previously described.8 Chimeric KIT receptors were generated as previously described.24 Using these chimeric KIT receptors as a template, we generated the mutant KITD814V and KITD814V with none (KITD814V-F7) or single intracellular tyrosine add-back mutant at codon 719 (KITD814V-Y719) using the Quick change Site-Directed Mutagenesis kit (Stratagene) and the following primer pair (forward: 5′-GGG CTA GCC AGA GTC ATC AGG AAT GAT TCG-3′; and reverse: 5′-CGA ATC ATT CCT GAT GAC TCT GGC TAG CCC-3′). All these mutated KITD814V receptors were verified by direct sequencing.
Expression of WT and mutant KIT receptors in 32D cells and primary HSC/Ps
The 32D cells and primary low density mononuclear cells were transduced with WT or chimeric KIT receptors as described previously and in detail in supplemental Methods.8,25
Murine BM transplantation
A total of 1 × 106 transduced cells and 1 × 105 supporting fresh splenocytes from C57BL/6 mice were intravenously injected through tail vein into lethally irradiated (1100 cGy, split dose) recipient mice as described previously.8,25
Proliferation
Proliferation was assessed by conducting a thymidine incorporation assay as previously described.8,25
Apoptosis
Cells were subjected to apoptosis assay with annexin V/PE Apoptosis Detection Kit I (BD Biosciences PharMingen) according to the manufacturer's protocol as described previously.25
Immunoprecipitation and Western bloting
Immunoprecipitation and Western blot analysis were performed as previously reported.24
Pharmacokinetic studies
For pharmacokinetic studies, II-B08 levels were quantified in the mouse serum by liquid-liquid extraction and HPLC-MS/MS (API 4000) using compound II-B0518 (inactive II-B08 analog) as the internal standard. The Q1/Q3's for II-B08 and II-B05 were 557/304 and 480/304, respectively, with a lower limit of quantification for the assay of 3 ng/mL using 20 mL of serum. Assay development and execution were performed by Dr David Jones (Indiana University Clinical Pharmacology Analytical Core). Mice received 100 mg/kg II-B08 intraperitoneally each day by 7 days followed by collection of blood at 30 minutes, 1 hour, 4 hours, and 24 hours after the first injection. A serum sample was also collected 24 hours after the final (seventh) injection (168 hours after the first injection). We determined a Cmax of 155 ± 24.7μM. Twenty-four hours after first dose, a trough concentration of 0.14 ± 0.002μM was found (supplemental Figure 1), which is ∼ 100-fold lower than effective concentrations of II-B08 in vitro (∼ 10μM). However, after a cumulative dose of 700 mg/kg (7 doses of 100 mg/kg over 7 days), we found a steady-state concentration of 6.4 ± 6.3μM (supplemental Figure 1).
In vivo treatment of leukemic mice
To investigate the antileukemic effect of SHP2 inhibitor (II-B08) on KITD814V-induced MPD in vivo, a syngeneic transplantation mice model was used. The 32D cells expressing KITD814V (1 × 106 cells) were intravenously injected into syngeneic C3H/HeJ mice by tail vein injection. After 48 hours of transplantation, mice received either vehicle (10% DMSO in PBS) or 100 mg/kg II-B08 intraperitoneally each day for 14 days. In another study to investigate the cooperation between SHP2 inhibitor (II-B08) and PI3K inhibitor (LY294002) on KITD814V-induced MPD in vivo, mice after 48 hours of transplantation with KITD814V-bearing cells were injected with vehicle (10% DMSO in PBS) or inhibitor [II-B08 (50 mg/kg body weight) or LY294002 (10 mg/kg body weight) or II-B08 + LY294002 (50 + 10 mg/kg body weight)] at a 24-hour interval for 21 days. Mice were closely monitored for MPD and survival. Mice were harvested at moribund; tissues, including BM, spleen, liver, and lungs, were fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin for histopathologic analysis.
Statistics
All graphical data were evaluated by paired Student t test, and results were considered significantly different with P < .05. All data are represented as mean ± SD. Survival probability of transplanted mice cohorts was compared using a Kaplan-Meier survival analysis in which statistical significance was determined as P < .05 by log rank test.
Results
Constitutive activation of SHP2 in cells bearing KITD814V
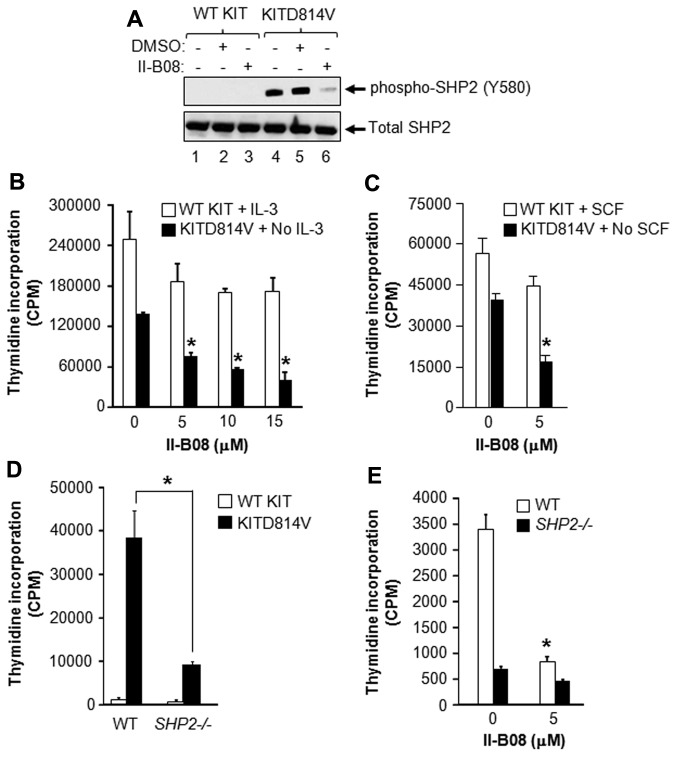
To determine the role of SHP2 in KITD814V-induced MPD, we examined whether SHP2 is hyperactivated in cells bearing KITD814V and whether SHP2 phosphatase inhibition using a novel SHP2 inhibitor, II-B08, normalizes SHP2 hyperactivation.18 We have recently described the generation and synthesis of II-B08.18 As seen in Figure 1A (lane 1), cells bearing WT KIT showed no constitutive phosphorylation of SHP2. In contrast, KITD814V-bearing cells showed constitutive phosphorylation of SHP2 (lane 4), which was significantly inhibited in the presence of II-B08 (lane 6), but not in the presence of DMSO (lane 5).
Figure 1.
SHP2 is essential for constitutive growth of cells bearing oncogenic KITD814V. (A) The 32D cells were transduced with retrovirus bearing WT KIT or KITD814V as described in “Expression of WT and mutant KIT receptors in 32D cells and primary HSC/Ps.” Cells bearing WT KIT or KITD814V were starved of serum and growth factors for 6 hours followed by treatment with SHP2 inhibitor, II-B08 (20μM), for 1 hour, after which cells were lysed and equal amount of protein lysates were subjected to Western blot analysis using an anti–phospho-SHP2 (Y580) or total SHP2 antibody. Similar results were observed in 3 independent experiments. (B) The 32D cells bearing WT KIT or KITD814V were starved of serum and growth factors for 6 hours and subjected to proliferation assay in the presence or absence of indicated concentration of II-B08. Assay was performed in the presence of IL-3 (10 ng/mL) for cells bearing WT KIT and in the absence of growth factors for cells bearing oncogenic KITD814V. Bars represent the mean thymidine incorporation (CPM ± SD) from 1 of the 3 independent experiments performed in quadruplicate. *P < .005. (C) Primary BM-derived WT KIT or KITD814V-expressing cells from WT mice were starved for 6 hours and subjected to thymidine incorporation assay in the presence or absence of indicated concentrations of II-B08. Assays were performed in the presence of murine SCF (50 ng/mL) for cells bearing WT KIT and in the absence of growth factors for cells bearing oncogenic KITD814V. Bars represent the mean thymidine incorporation (CPM ± SD) from 1 of 3 independent experiments performed in quadruplicate. *P < .001. (D) Primary BM-derived cells expressing WT KIT or KITD814V from WT or SHP2−/− mice were starved and subjected to proliferation assay in the absence of growth factors by thymidine incorporation. Bars represent the mean thymidine incorporation (mean ± SD) from 1 of 3 independent experiments performed in quadruplicate. *P < .01, WT-KITD814V vs SHP2−/−-KITD814V. (E) Primary BM-derived cells expressing KITD814V from WT or SHP2−/− mice were starved and subjected to proliferation assay in the presence or absence of II-B08 (5μM) by thymidine incorporation. Bars represent the mean thymidine incorporation (mean ± SD) from 1 of 2 independent experiments performed in triplicate. *P < .05, WT-KITD814V-0μM vs WT-KITD814V-5μM.
Constitutive phosphorylation of SHP2 is associated with ligand-independent growth and survival of KITD814V-bearing cells
Because constitutive phosphorylation of SHP2 was observed in cells bearing KITD814V, we tested whether inhibition of constitutive SHP2 phosphorylation using II-B08 would suppress KITD814V-induced ligand-independent growth in vitro. As seen in Figure 1B, II-B08 treatment resulted in only modest repression in the growth of cells bearing WT KIT. In contrast, a significant dose-dependent repression in ligand-independent growth of 32D cells bearing KITD814V was observed in the presence of II-B08 (Figure 1B). Similar results were obtained using primary HSC/Ps bearing WT KIT or KITD814V in the presence of II-B08 (Figure 1C).
SHP2 is functionally essential for KITD814V-induced ligand-independent growth
We further examined the functional contribution of SHP2 in KITD814V-induced ligand-independent growth. Since SHP2 deficiency in mice results in embryonic lethality, we therefore generated an inducible SHP2 knockout line in which Cre expression is induced by Poly-I:C.20 To induce SHP2 deletion, 3 doses of poly I:C were injected on alternative days. Two weeks after final injection, BM was harvested. Consistent with previous studies, efficient deletion of SHP2 was observed in hematopoietic cells after poly I:C treatment (supplemental Figure 2A-B).20 SHP2flox/flox/Cre- mice are referred as WT mice, whereas SHP2flox/flox/Cre+ mice are referred as SHP2−/− mice after poly I:C treatment. HSC/Ps from WT or SHP2−/− mice were transduced with retrovirus bearing WT KIT or KITD814V, and transduced cells were sorted to homogeneity based on EGFP expression. Supplemental Figure 2C shows similar expression of WT KIT or KITD814V in WT or SHP2−/− cells. As expected, WT HSC/Ps bearing KITD814V showed ligand-independent growth, but not WT KIT-bearing cells (Figure 1D). Deficiency of SHP2 resulted in significant repression (∼ 75%) in ligand-independent growth of cells bearing KITD814V. It is important to point out that loss of SHP2 also impairs the proliferation of WT KIT-expressing BM cells in response to SCF stimulation; however, the reduction in proliferation under these conditions is modest relative to the inhibition observed in SHP2-deficient BM cells bearing the KITD814V receptor (supplemental Figure 2D). In addition, partial loss of SHP2 protein in the context of KITD814V only results in 30% growth inhibition, indicating that the amount of SHP2 protein status to a large extent determines functional outcomes in normal versus KITD814V-bearing cells (supplemental Figure 2E). Furthermore, chemical epistasis experiments using SHP2−/− BM cells expressing WT KIT or KITD814V did not show a further reduction in ligand-independent growth in the presence of II-B08, suggesting that II-B08 is highly specific for SHP2 under test conditions (Figure 1E; supplemental Figure 3). However, we cannot completely rule out the possibility of off-target effects of II-B08 at this point.
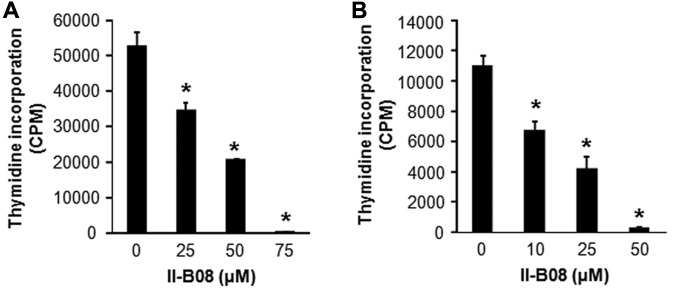
SHP2 inhibitor II-B08 inhibits the constitutive growth of human leukemic mast cell line HMC1.2 and primary human CD34+ cells bearing KITD816V
We next performed studies in cells derived from mastocytosis patient (HMC1.2 cells) bearing the activating KIT mutation.26 II-B08 treatment of these cells showed a dose-dependent reduction in growth (Figure 2A). To further confirm the role of SHP2 in activating KIT induced MPD, we isolated CD34+ cells from human cord blood cells, transduced them with a retrovirus expressing KITD816V, and analyzed constitutive growth in the presence of increasing doses of II-B08. As seen in Figure 2B, II-B08 treatment resulted in a dose-dependent repression in constitutive growth of primary human CD34+ cells bearing KITD816V.
Figure 2.
SHP2 inhibitor II-B08 inhibits the constitutive growth of human leukemic mast cell line HMC1.2 and human CD34+ cells bearing KITD816V as well as primary BM-derived AML blasts. (A) Human leukemic mast cell line HMC 1.2 or (B) human CD34+ cells transduced with KITD816V were starved for 6 hours in serum- and cytokine-free media and treated with indicated amounts of SHP2 inhibitor II-B08. After 48 hours, proliferation was evaluated by [3H] thymidine incorporation. Bars represent the mean thymidine incorporation (CPM ± SD) from 1 independent experiment performed in quadruplicate. *P < .001.
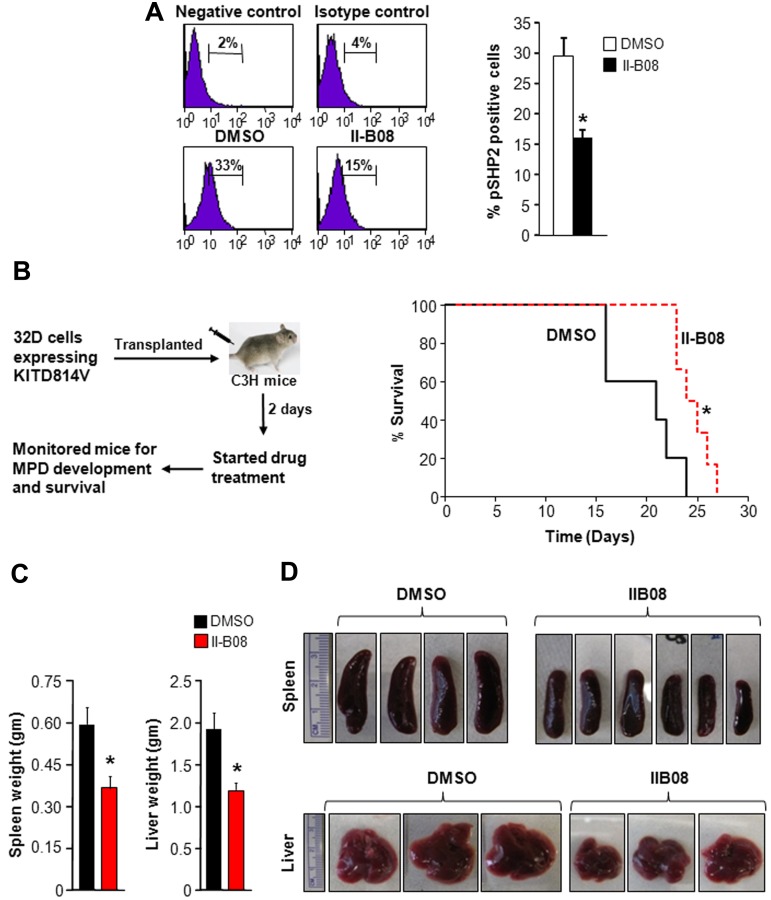
SHP2 inhibitor, II-B08, prolongs the survival of mice transplanted with cells bearing KITD814V
To further determine the in vivo antileukemic effect of II-B08, syngeneic C3H/HeJ mice transplanted with myeloid cells bearing oncogenic KITD814V were treated with either DMSO or II-B08 (100 mg/kg body weight per day) for 14 days. In normal control mice, this dose of II-B08 was well tolerated, and no drug-related tissue toxicity was observed (supplemental Figures 1, 4, and 5). The drug dose used in these studies resulted in 50% inhibition of SHP2 phosphorylation in splenocytes (Figure 3A). As seen in Figure 3B, mice treated with II-B08 survived significantly longer compared with vehicle (DMSO)–treated group (P < .05). In addition, spleen and liver weight of II-B08 treated mice was significantly reduced compared with the DMSO-treated group (Figure 3C-D).
Figure 3.
In vivo SHP2 inhibitor treatment of KITD814V-bearing mice enhances their survival and modulates MPD. (A) Mice received 100 mg/kg II-B08 intraperitoneally each day for 7 days. Mice were harvested after 24 hours of final injection, and peripheral blood, BM, spleen, and thymus were analyzed. Cells from spleen were stained with anti–phospho-SHP2 antibody by intracellular staining and analyzed by flow cytometry. Left panel: representative phospho-SHP2 flow micrographs. Right panel: average percentage of phospho-SHP2-positive cells from 4 mice. A 50% reduction in the phosphorylation of SHP2 was observed in mice treated with II-B08 compared with DMSO. (B) Kaplan-Meier survival analysis of leukemic mice treated with DMSO or II-B08. The 32D cells bearing oncogenic KITD814V were injected into syngeneic C3H/HeJ mice through tail vein. Mice were treated with either vehicle DMSO (n = 5) or II-B08 (50 mg/kg body weight; n = 6) at 12-hour intervals for 14 days. Left panel: schematic of drug treatment model. Right panel: survival curve. Significantly prolonged survival of mice treated with II-B08 was observed compared with mice treated with DMSO. *P < .05. (C-D) Reduced splenomegaly and hepatomegaly in KITD814V-bearing mice treated with II-B08. Average weights (C) and pictures (D) of spleen and liver of mice transplanted with cells bearing oncogenic KITD814V and treated with DMSO or II-B08. Significant reduction in spleen and liver weights was observed in mice treated with II-B08 compared with mice treated with DMSO. n = 5 or 6. *P < .05.
To determine the in vivo specificity of II-B08 for SHP2, we treated WT and SHP2−/− mice with II-B08 for 7 days and analyzed blood parameters. Consistent with previously published data,20 SHP2−/− mice show significantly reduced WBC counts, BM cellularity, and splenic cellularity (supplemental Figure 6). We did not see any significant differences in WBC counts, BM cellularity, and splenic cellularity between SHP2−/− mice treated with vehicle (DMSO) or II-B08, suggesting that II-B08 is specific for SHP2 (supplemental Figure 6).
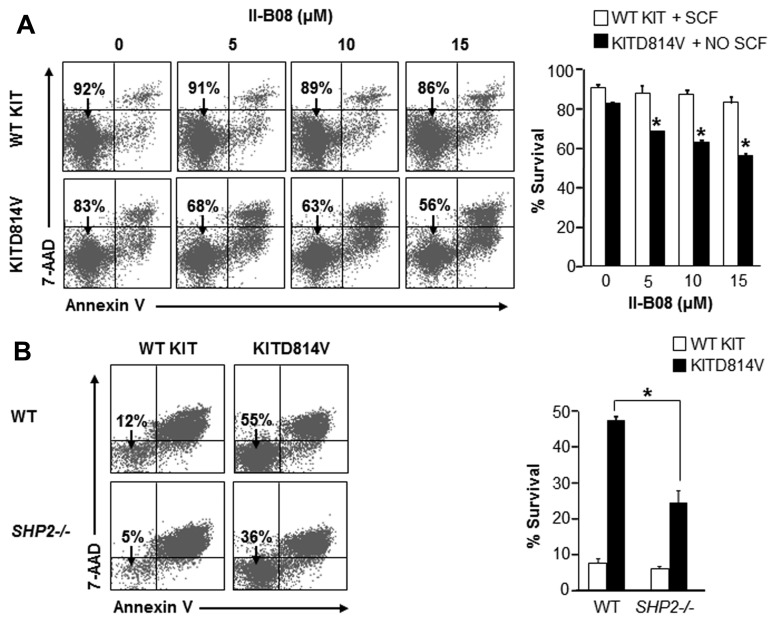
SHP2 is required for ligand-independent survival of KITD814V-bearing cells
To determine the mechanism behind the repression in ligand-independent growth of cells bearing KITD814V by II-B08, we examined the role of SHP2 in cell survival and cycling. II-B08 treatment resulted in a significant dose-dependent increase in apoptosis of cells bearing KITD814V compared with WT KIT-bearing cells (Figure 4A). To further confirm the role of SHP2 in KITD814V-induced cell survival and to establish IIB08 specificity for SHP2, primary HSC/Ps from WT or SHP2−/− mice were transduced with WT KIT or KITD814V and examined for apoptosis. As seen in Figure 4B, WT cells bearing KITD814V showed increased survival in the absence of growth factors compared with WT KIT-expressing cells. In contrast, deficiency of SHP2 resulted in a significant reduction in survival of cells bearing KITD814V. In addition, no significant difference in cycling of cells bearing WT KIT or KITD814V was observed in the presence of II-B08 (supplemental Figure 7). These results suggest that the reduced growth of cells bearing KITD814V in the presence of II-B08 is mainly because of cell survival, but not cycling.
Figure 4.
SHP2 is essential for oncogenic KITD814V-induced ligand-independent survival. (A) Primary BM-derived WT KIT or KITD814V-expressing cells were starved and treated with indicated concentrations of II-B08 for 48 hours. Assays were performed in the presence of SCF (50 ng/mL) for cells bearing WT KIT and in the absence of growth factors for cells bearing KITD814V. Cells were harvested and stained with PE-conjugated anti–annexin V antibody and 7-amino-actinomycin D followed by flow cytometric analysis. Left panel: representative flow micrographs. Right panel: bars represent the mean percent of surviving cells from 2 independent experiments. *P < .05. (B) Primary BM cells bearing WT KIT or KITD814V from WT or SHP2−/− mice were starved of serum and growth factors for 6 hours and cultured in media containing serum for 48 hours in the absence of growth factors. After 48 hours, cells were harvested and stained with PE-conjugated anti–annexin V antibody and 7-amino-actinomycin D followed by flow cytometric analysis. Left panel: representative flow micrographs. Right panel: bars represent the mean percent of surviving cells from 1 of 3 independent experiments. *P < .01.
Constitutive phosphorylation of SHP2 is associated with binding of SHP2 with PI3K regulatory subunit p85α and/or Gab2 in oncogenic KITD814V-bearing cells
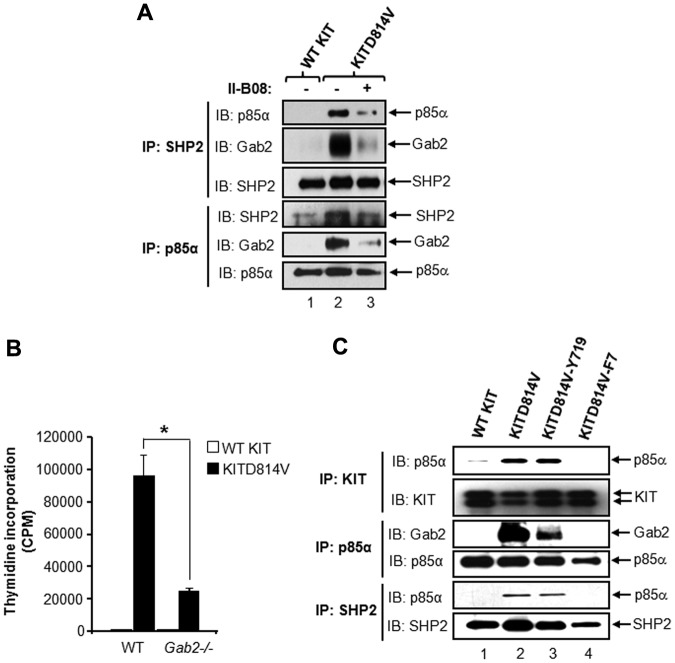
To further determine the signaling molecules that cooperate with SHP2 in KITD814V-induced MPD, we examined proteins that potentially interact with SHP2 in KITD814V-bearing cells and whether inhibition of SHP2 phosphorylation by II-B08 suppresses these cooperating protein interactions. Given that SHP2 and Gab2 have been shown to interact with p85α,27,28 we tested whether SHP2, Gab2, and p85α form a constitutive protein complex in KITD814V-bearing cells. As seen in Figure 5A, SHP2 constitutively bound to p85α and Gab2 in cells bearing KITD814V, but not in WT KIT-expressing cells. Treatment of these cells with II-B08 significantly inhibited the formation of this complex (Figure 5A). Similar results were observed in cells derived from mastocytosis patient (HMC1.2 cells) bearing the activating KIT mutation (supplemental Figure 8). These results suggest that phosphorylation of SHP2 is associated with formation of a protein complex involving p85α, SHP2, and/or Gab2 in KITD814V-bearing cells.
Figure 5.
Tyrosine 719 in KIT receptor is sufficient for recruiting protein complex involving p85α, SHP2, and Gab2 to KITD814V. (A) The 32D cells bearing WT KIT or KITD814V were starved for 8 hours in serum- and growth factor-free medium followed by incubation with or without II-B08 (20μM) for 1 hour. After incubation, equal amounts of protein lysates were subjected to immunoprecipitation with anti-SHP2 or anti-p85α antibodies followed by Western blot analysis using anti-p85α, anti-SHP2, or anti-Gab2 antibodies. Similar results were observed in 2 independent experiments. (B) Primary BM-derived cells expressing WT KIT or KITD814V from WT or Gab2−/− mice were starved and subjected to proliferation assay in the absence of growth factors by thymidine incorporation. Bars represent the mean (± SD) thymidine incorporation from 1 of 3 independent ex-eriments performed in quadruplicate. *P < .01, WT-KITD814V vs Gab2−/−-KITD814V. (C) The 32D cells bearing WT KIT, KITD814V, KITD814V-Y719, or KITD814V-F7 were starved of serum and growth factors for 8 hours, and equal amounts of protein lysates were subjected to immunoprecipitation with an anti-KIT antibody, anti-p85α antibody, or anti-SHP2 antibody followed by Western blot analysis using anti-KIT, anti-p85α, anti-SHP2, or anti-Gab2 antibodies as indicated. Similar results were observed in 2 or 3 independent experiments.
Involvement of Gab2 in oncogenic KITD814V-induced ligand-independent growth
Because recent studies have also shown that Gab2 is an essential mediator for the pathogenic effects of Ptpn11 mutations, we examined the contribution of Gab2 in KITD814V-induced ligand-independent growth.29 We transduced primary HSC/Ps from WT or Gab2−/− mice with retrovirus expressing WT KIT or KITD814V and assessed proliferation. As seen in Figure 5B, deficiency of Gab2 resulted in significant repression (∼ 75%) in ligand-independent growth of cells bearing KITD814V similar to SHP2−/− cells (Figure 1D). These results suggest that SHP2 and Gab2 are essential and cooperate in KITD814V-induced ligand-independent growth.
Intracellular tyrosine residue at 719 in KIT receptor is sufficient for recruiting protein complex involving p85α, SHP2, and Gab2 to KITD814V
To assess whether binding of p85α to SHP2, Gab2, and KITD814V is sufficient to induce MPD, we generated a KIT mutant receptor, KITD814V-F7, in which 7 tyrosine residues in KITD814V were converted to phenylalanine, and KITD814V-Y719, in which only tyrosine residue 719 (binding site for p85α) was added back to the KITD814V-F7 receptor. Supplemental Figure 9 shows a schematic of these mutant KITD814V receptors. Cells bearing WT KIT, KITD814V, KITD814V-Y719, and KITD814V-F7 were starved and subjected to immunoprecipitation assay with an anti-KIT antibody followed by Western blotting with an anti-p85α antibody. The results show constitutive binding of p85α subunit to KITD814V and KITD814V-Y719, but not to WT KIT or KITD814V-F7 (Figure 5C). We further confirmed these results by reverse immunoprecipitation in which immunoprecipitation was done with an anti-p85α antibody followed by Western blotting with an anti-KIT antibody (data not shown). In addition, constitutive binding of SHP2 and Gab2 to p85α was also observed in cells bearing KITD814V and KITD814V-Y719, but not in WT KIT or KITD814V-F7–bearing cells (Figure 5C). These results demonstrate that p85α recruits SHP2 and Gab2 to KITD814V at Y719, which might contribute to KITD814V-induced MPD.
Tyrosine 719-associated p85α, SHP2, and Gab2 complex is sufficient for KITD814V-induced ligand-independent growth in vitro and MPD in vivo
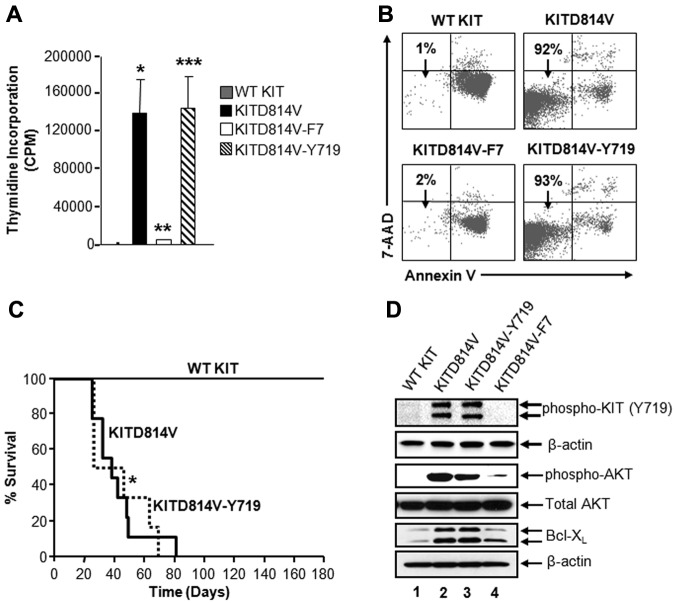
To further determine the contribution of Y719-associated p85α, SHP2, and Gab2 complex in KITD814V-induced ligand-independent growth in vitro and MPD in vivo, we transduced primary HSC/Ps from KitW-sh/W-sh mice, which lack endogenous KIT receptor, with retrovirus encoding KIT receptors and sorted to homogeneity. Similar expression of these receptors was confirmed by Western blot analysis (data not shown). As seen in Figure 6A, cells bearing WT KIT showed minimal thymidine incorporation in the absence of growth factors. In contrast, cells bearing oncogenic KITD814V showed constitutive growth in the absence of growth factors. However, conversion of all the 7 intracellular tyrosine residues in KITD814V to phenylalanine (KITD814V-F7) resulted in complete loss of ligand-independent growth (Figure 6A). Importantly, restoration of tyrosine residue at position 719 (KITD814V-Y719) was sufficient to induce ligand-independent growth to a level similar to cells bearing the KITD814V receptor. These data suggest that the tyrosine residue at position 719, which is the binding site for class IA PI3K regulatory subunit p85α, is sufficient to rescue ligand-independent proliferation in vitro to KITD814V levels by recruiting a complex involving SHP2.
Figure 6.
Tyrosine 719 associated p85α, SHP2, and Gab2 complex is sufficient for KITD814V-induced ligand-independent growth and survival in vitro and MPD in vivo. (A) Primary HSC/Ps derived from Wsh/sh mice, which lack endogenous expression of KIT receptor, were transduced with the indicated chimeric KIT receptors and sorted to homogeneity based on EGFP expression. Cells were starved in serum- and growth factor-free media for 6 hours and subjected to proliferation assay in the absence of growth factors by thymidine incorporation. Bars represent the mean (CPM ± SD) thymidine incorporation from 1 of the 3 independent experiments performed in quadruplicate. *P < .05, WT KIT vs KITD814V. **P < .05, KITD814V vs KITD814V-F7. ***P < .05, KITD814V-F7 vs KITD814V-Y719. (B) The 32D cells bearing the indicated chimeric receptors were starved of serum and growth factors and cultured for 48 hours in the absence of growth factors. Cells were harvested and stained with anti–annexin V antibody and 7-amino-actinomycin D followed by flow cytometric analysis. Double-negative cells in the lower left quadrant are indicated as surviving cells. Representative dot blots are shown. (C) Kaplan-Meier survival analysis of mice transplanted with the primary HSC/Ps bearing indicated KIT receptors (n = 8-18 per group). Results show that restoration of Y719 alone is sufficient to induce transformation in vivo (median survival, 55 days; n = 15). (D) The 32D cells bearing the indicated chimeric KIT receptors were starved in serum- and growth factor-free medium for 8 hours. Starved cells were lysed, and equal amounts of protein lysates were subjected to Western blot analysis using an anti–phospho-KIT (Y719), anti–phospho-AKT, total AKT, Bcl-xL, and β-actin antibodies as indicated. Similar results were observed in 3 independent experiments.
To assess whether the differences in ligand-independent growth of cells bearing WT KIT, KITD814V, KITD814V-F7, and KITD814V-Y719 were the result of differences in cell survival or cycling, myeloid cells bearing these KITD814V receptors were subjected to apoptosis and cell cycle analysis. As seen in Figure 6B, cells bearing KITD814V showed significantly increased survival compared with WT KIT-bearing cells in the absence of growth factors. Loss of intracellular tyrosine residues in KITD814V (KITD814V-F7) abrogated ligand-independent survival, and restoration of tyrosine residue at 719, KITD814V-Y719, was sufficient to completely maintain the survival of these cells to the levels seen in KITD814V-bearing cells. These results demonstrate that Y719 is sufficient not only to restore the binding of SHP2 and Gab2 to p85α, but also sufficient to completely restore KITD814V-induced ligand-independent growth and survival.
To determine the physiologic consequences of SHP2 and Gab2 binding to p85α at Y719 in KITD814V-induced MPD in vivo, we transduced primary HSC/Ps from 5-fluorouracil–treated C57BL/6 mice with WT KIT, KITD814V, or KITD814V-Y719 receptors. Transduced cells showing similar transduction efficiencies were sorted to homogeneity based on EGFP expression and transplanted into lethally irradiated syngeneic C57BL/6 recipient mice. Mice were monitored for MPD development and survival. As seen in Figure 6C, mice transplanted with cells bearing WT KIT (black line) showed no signs of disease and survived for the entire course of the study. In contrast, mice transplanted with cells bearing KITD814V (black line) died within 90 days of transplantation and developed a fatal MPD (Figure 6C). Importantly and consistent with the in vitro proliferation and survival observations, recipient mice expressing KITD814V-Y719 (dotted line) showed similar disease progression and survival as the KITD814V-bearing mice (Figure 6C). The median time of survival in these 2 groups was 59 days for KITD814V versus 55 days for KITD814V-Y719 (Figure 6C). These data suggest that the intracellular tyrosine residue at position 719, which recruits a complex of p85α, SHP2, and Gab2, is sufficient for KITD814V-induced MPD.
Tyrosine 719-associated p85α, SHP2, and Gab2 complex is sufficient for activation of PI3K and its downstream target Bcl-xL in cells bearing KITD814V
To further assess the biochemical basis for the role of tyrosine 719-associated p85α, SHP2, and Gab2 complex in KITD814V-induced MPD, activation of AKT and its downstream target Bcl-xL was analyzed. As seen in Figure 6D, constitutive phosphorylation of KIT and AKT and enhanced expression of Bcl-xL were observed in cells bearing KITD814V (lane 2), but not in WT KIT-expressing cells (lane 1). Interestingly, loss of the 7 intracellular tyrosine residues in KITD814V resulted in complete abrogation of the constitutive activation of KIT and AKT and reduced expression of Bcl-xL (Figure 6D lane 4). Importantly, restoring tyrosine 719 completely rescued the robust constitutive phosphorylation of KIT and AKT and expression of Bcl-xL to the levels observed in KITD814V-expressing cells (Figure 6D lane 3).
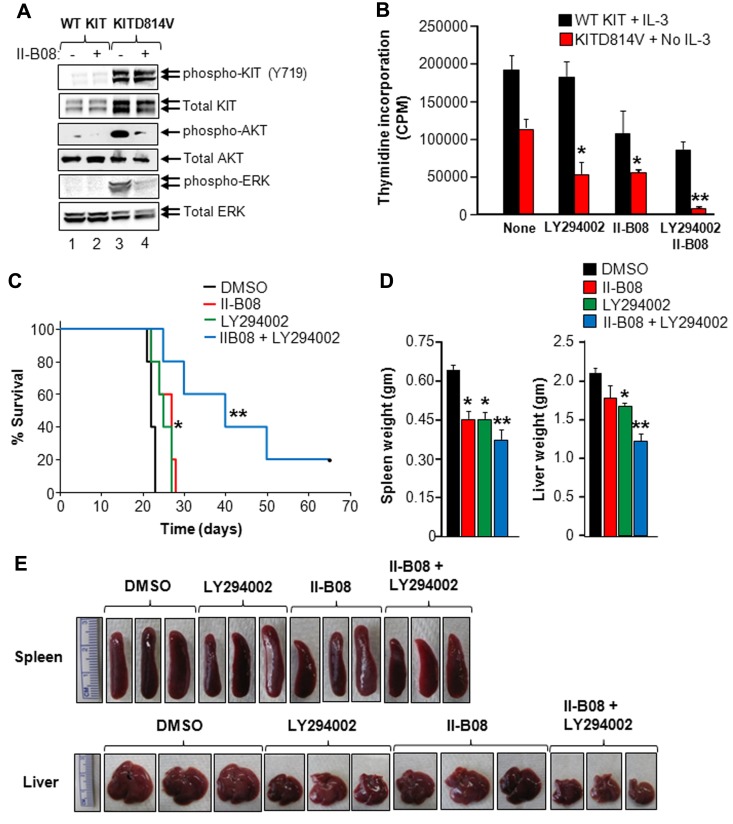
SHP2 inhibitor, II-B08, inhibits the activation of PI3K and ERK MAP kinase in oncogenic KITD814V-bearing cells
Because deficiency of SHP2 or inhibition of SHP2 phosphorylation results in suppression of constitutive growth and survival of cells bearing oncogenic KITD814V, as well as disruption of the p85α/SHP2/Gab2 complex, we examined whether disruption of this complex would modulate AKT and ERK activation in oncogenic KITD814V-bearing cells. For this, we starved cells bearing WT KIT or KITD814V for 6 hours and treated them with II-B08 for 1 hour. Equal amounts of protein lysates were subjected to Western blotting with an anti–phospho-KIT, anti–phospho-AKT, or anti–phospho-ERK antibody. Constitutive activation of KIT, AKT, and ERK was observed in cells bearing KITD814V, but not WT KIT (Figure 7A). Although treatment of these cells with II-B08 significantly reduced the phosphorylation of AKT and ERK in KITD814V-bearing cells, no significant effect was observed on KIT phosphorylation in KITD814V-bearing cells (Figure 7A). Similar results were observed in HMC1.2 cells derived from human mastocytosis patients (supplemental Figure 10A). Likewise, splenocytes derived from KITD814V-bearing mice treated with II-B08 showed significantly reduced phosphorylation of ERK compared with splenocytes derived from KITD814V-bearing mice treated with vehicle (DMSO; supplemental Figure 10B). These results suggest that SHP2 regulates ligand-independent growth and survival of KITD814V-bearing cells in part by regulating the activation of AKT and ERK, but not KIT tyrosine kinase activity.
Figure 7.
SHP2 inhibitor II-B08 enhances the efficacy of PI3K inhibitor in repressing oncogenic KITD814V-induced growth. (A) The 32D cells bearing WT KIT or KITD814V were starved for 6 hours followed by treatment with or without II-B08 (20μM) for 1 hour. After incubation, cells were lysed and equal amounts of protein lysates were subjected to Western blot analysis using an anti–phospho-KIT (Y719), total KIT, anti–phospho-AKT, total AKT, anti–phospho-ERK, or total ERK antibody as indicated. Similar results were observed in 3 independent experiments. (B) The 32D cells bearing WT KIT or KITD814V were starved of serum and growth factors for 6 hours and subjected to proliferation assay in the presence or absence of PI-3K inhibitor (LY294002, 2μM) and SHP2 inhibitor (II-B08, 5μM) alone or in combination. Bars represent the mean thymidine incorporation (CPM ± SD) from 1 of 3 independent experiments performed in quadruplicate. *P < .05, no GF vs LY294002 or II-B08. **P < .05, LY294002 or II-B08 vs LY294002 and II-B08. (C) Kaplan-Meier survival analysis of MPD mice treated with II-B08 or LY294002 alone or in combination. The 32D cells bearing oncogenic KITD814V were injected into syngeneic C3H/HeJ mice through tail vein. After 48 hours, mice were treated with either vehicle DMSO or II-B08 (50 mg/kg body weight) or LY294002 (10 mg/kg body weight) or a combination of II-B08 and LY294002 (50 + 10 mg/kg body weight, respectively) at 24-hour intervals for 21 days (n = 5). Significantly prolonged survival of mice treated with II-B08 or LY294002 alone or in combination was observed compared with mice treated with DMSO. *P < .05, DMSO vs II-B08 or LY294002. **P < .05, II-B08 or LY294002 vs II-B08 and LY294002. (D-E) Reduced splenomegaly and hepatomegaly in mice treated with II-B08 or LY294002 or combination. Average weights (D) and pictures (E) of spleens and livers from mice transplanted with cells bearing oncogenic KITD814V and treated with DMSO or II-B08 or LY294002 or combination of II-B08 and LY294002. Significant reduction in spleen and liver weights was observed in mice treated with II-B08 or LY294002 or combination of II-B08 and LY294002 compared with mice treated with DMSO. n = 4. *P < .05, DMSO vs II-B08 or LY294002. **P < .05, II-B08 or LY294002 vs II-B08 and LY294002.
SHP2 inhibitor, II-B08, enhances the efficacy of PI3K inhibitor LY294002 in suppressing KITD814V-induced ligand-independent growth in vitro and MPD in vivo
To further determine whether a lipid kinase (PI3K) and a tyrosine phosphatase (SHP2) cooperate in KITD814V-induced ligand-independent growth, we performed proliferation assay in the presence of PI3K inhibitor LY294002 or SHP2 inhibitor II-B08 alone or in combination. As seen in Figure 7B, low doses of LY294002 and II-B08 alone showed 50% reduction in ligand-independent growth of cells bearing KITD814V. Importantly, treatment of these cells with a combination of LY294002 and II-B08 completely suppressed ligand-independent growth (Figure 7B).
We next examined the in vivo consequence of KITD814V-bearing mice with a combination of LY294002 and IIB08. For this, mice transplanted with cells bearing KITD814V were treated with either DMSO or II-B08 (50 mg/kg body weight) or PI3K inhibitor LY294002 (10 mg/kg body weight) or a combination of II-B08 and LY294002 (50 + 10 mg/kg body weight, respectively) at 24-hour intervals for 21 days. Mice were monitored for MPD development and survival. As seen in Figure 7C, mice treated with low doses of II-B08 or LY294002 survived significantly longer compared with the DMSO-treated group. Importantly, mice treated with a combination of II-B08 and LY294002 survived significantly longer compared with mice treated either with II-B08 or LY294002 alone (Figure 7C). Spleen size and weight of II-B08 or LY294002 or II-B08 and LY294002 treated mice were significantly reduced compared with the DMSO-treated group (Figure 7D-E). A similar reduction in liver size and weight was observed in mice treated with II-B08 or LY294002 or II-B08 and LY294002 compared with DMSO (Figure 7D-E).
Discussion
SHP2 can be phosphorylated at 2 C-terminal tyrosyl residues by receptor tyrosine kinases, including KIT as well as cytosolic tyrosine kinases, including Src and Abl.30–33 The level of tyrosyl phosphorylation of SHP2 has been associated with its recruitment to the receptor. Although it is not entirely clear how SHP2 phosphorylation is regulated, studies have shown that the phosphatase activity of SHP2 is associated with its own tyrosine phosphorylation.34,35 Thus, pharmacologic inhibition of SHP2 phosphatase function might permit SHP2 to return to its inactive conformation resulting in reduced tyrosine phosphorylation. Consistent with these findings, we demonstrate that pharmacologic inhibition of SHP2 phosphatase function using a novel SHP2 inhibitor II-B08 results in reduced SHP2 constitutive phosphorylation in cells bearing KITD814V. In addition, inhibition of SHP2 phosphorylation by II-B08 resulted in significant repression in ligand-independent growth and survival of cells bearing KITD814V and primary human CD34+ cells bearing KITD816V. Consistent with pharmacologic findings using IIB08, deficiency of SHP2 in HSC/Ps expressing KITD814V also resulted in inhibition in ligand-independent growth and survival. Collectively, these findings support the notion that SHP2 plays a significant role in KITD814V- and KITD816V-induced ligand-independent growth and MPD.
Emerging data suggest that scaffolding protein Gab1 and Gab2 form a stable complex with SHP2 and regulate PI3K/AKT pathway.36,37 In addition, gain-of-function SHP2 mutants have enhanced tyrosine phosphorylation and interaction with Gab1, Gab2, Grb2, and p85, leading to enhanced PI3K pathway.35,38 Furthermore, studies have shown that deficiency of Gab2 rescues the oncogenic SHP2 mutant-induced MPD.29 Our results in KITD814V cells show that deficiency of SHP2 or Gab2 results in reduced constitutive growth and survival of cells bearing oncogenic KITD814V. In addition, treatment with pharmacologic inhibitor, II-B08, also results in suppression in constitutive growth and survival of cells bearing oncogenic KITD814V. Further, we observed constitutive binding of SHP2 to p85α and Gab2 in oncogenic KITD814V-bearing cells, but not in WT-bearing cells. Pharmacologic inhibition of SHP2 using II-B08 resulted in reduced tyrosine phosphorylation and reduced binding to p85α and Gab2. Likewise, inhibition of SHP2 phosphorylation using II-B08 resulted in reduced activation of AKT in cells bearing oncogenic KITD814V. These results suggest that oncogenic KITD814V induced constitutive phosphorylation of SHP2, leading to enhanced recruitment to p85α and Gab2 complex results in prolonged activation of AKT and MPD.
Inhibition of SHP2 phosphatase has been speculated to be potentially relevant in diseases that bear activating mutations of SHP2, such as juvenile myelomonocytic leukemia, as well as in diseases in which SHP2 collaborates with other oncogenes to induce carcinogenesis, such as KIT in systemic mastocytosis and AML. Currently, no clinically relevant inhibitors of SHP2 phosphatase activity exist. The first published example of an SHP2 inhibitor was NSC-87877; however, this compound demonstrates no selectivity for SHP2 over the related protein tyrosine phosphatase, SHP1.39 The inhibitor, phenylhydrazonopyrazolone sulfonate (PHPS1), demonstrates improved specificity for SHP2 over SHP1; however, functional data using this compound are limited to immortalized or transformed cell lines without clear disease indication.40 An additional study identified some SHP2-specific compounds; however, these compounds were only effective at inhibiting cellular proliferation at relatively high concentrations (100μM).41 The SHP2 phosphatase inhibitor, II-B08, demonstrates selectivity for SHP2 over the related phosphatases, SHP1 and PTP1B, and, importantly, is able to block SHP2 activity within the cells, demonstrated by its highly efficacious effects in cell-based assays.18 We also provide PK/PD data to suggest that II-B08 is well tolerated in vivo (see “Pharmacokinetic studies” for pharmacokinetic studies). The dose of II-B08 we used in our studies results in 50% SHP2 inhibition in normal splenocytes (Figure 3A); this degree of SHP2 inhibition does not impact normal hematopoiesis, including WBC counts, BM cellularity, and spleen cellularity as well as progenitor cell frequency, although it does prolong the survival of MPD mice and significantly modulates hepatosplenomegaly in KITD814V-bearing mice. The findings related to the lack of impact of partial SHP2 inhibition by II-B08 on normal hematopoiesis is consistent with our previous studies demonstrating that SHP2+/− mice under steady-state conditions demonstrate no hematopoietic defects, whereas complete loss of SHP2 significantly modulates normal hematopoiesis.20,42 Consistently, in vitro, treatment of WT KIT expressing BM cells with SCF in the presence of II-B08 only modestly impacts the growth and survival; however, under identical conditions, KITD814V-bearing cells are significantly more sensitive. Furthermore, although complete SHP2 deletion in BM cells reduces ligand-independent growth in KITD814V-expressing cells by 70%-80%, partial loss of SHP2 protein in the context of KITD814V only results in 30% growth inhibition (supplemental Figure 2B). Thus, the amount of SHP2 protein and its phosphorylation/activation status, to a large extent, determines functional outcomes in normal versus leukemic cells, including in KITD814V-bearing cells. It is also conceivable that II-B08 treatment, while impairing SHP2 phosphorylation, may not impact the adaptor function of SHP2. In contrast, loss of SHP2 protein essentially removes all of the functions associated with SHP2 and hence results in a more severe hematopoietic phenotype.20,42 These results clearly suggest the possibility of a therapeutic window that can be exploited to treat MPD. In this scenario, the major impact of SHP2 repression would be observed on leukemic cells, with only a minimal effect on normal hematopoiesis.
In conclusion, we propose that targeting multiple cooperating signaling pathways is important in treating hematologic malignancies involving oncogenic receptor tyrosine kinases. Consistent with this notion, treatment of leukemic mice with PI3K inhibitor LY294002 and SHP2 inhibitor II-B08 significantly prolonged the survival of mice compared with mice treated with either inhibitor alone in vivo. Taken together, our results suggest that SHP2 is a novel drug target for treating hematologic malignancies involving oncogenic KIT and shows cooperation with the PI3K pathway. To our knowledge, these are first studies to describe the in vivo effectiveness of combined targeting of a lipid kinase and a tyrosine phosphatase for potential treatment of leukemia and/or MPD.
Supplementary Material
Acknowledgments
The authors thank Marilyn Wales for administrative support.
This work was supported in part by the National Institutes of Health (grants R01 HL077177 and R01 HL08111, R.K.; and grant CA152194, Z.-Y.Z.).
Footnotes
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.S.M. performed research, analyzed data, and wrote the paper; P.M., L.-F.Z., H.M., B.R., Y.H., E.S., S.N., J.G., N.S., S.L., A.W.C., and G.S. performed research and analyzed data; A.W.C., K.D.B., G.-S.F., R.J.C., and Z.-Y.Z. provided reagents and edited the paper; and R.K. designed research and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Reuben Kapur, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St, Room W168, Indianapolis, IN 46202; e-mail: rkapur@iupui.edu.
References
- 1.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Longley BJ, Reguera MJ, Ma Y. Classes of c-KIT activating mutations: proposed mechanisms of action and implications for disease classification and therapy. Leuk Res. 2001;25(7):571–576. doi: 10.1016/s0145-2126(01)00028-5. [DOI] [PubMed] [Google Scholar]
- 3.Beghini A, Peterlongo P, Ripamonti CB, et al. C-kit mutations in core binding factor leukemias. Blood. 2000;95(2):726–727. [PubMed] [Google Scholar]
- 4.Nagata H, Worobec AS, Oh CK, et al. Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci U S A. 1995;92(23):10560–10564. doi: 10.1073/pnas.92.23.10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitayama H, Kanakura Y, Furitsu T, et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood. 1995;85(3):790–798. [PubMed] [Google Scholar]
- 6.Piao X, Bernstein A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood. 1996;87(8):3117–3123. [PubMed] [Google Scholar]
- 7.Hashimoto K, Tsujimura T, Moriyama Y, et al. Transforming and differentiation-inducing potential of constitutively activated c-kit mutant genes in the IC-2 murine interleukin-3-dependent mast cell line. Am J Pathol. 1996;148(1):189–200. [PMC free article] [PubMed] [Google Scholar]
- 8.Munugalavadla V, Sims EC, Borneo J, Chan RJ, Kapur R. Genetic and pharmacologic evidence implicating the p85 alpha, but not p85 beta, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood. 2007;110(5):1612–1620. doi: 10.1182/blood-2006-10-053058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujimura T, Furitsu T, Morimoto M, et al. Ligand-independent activation of c-kit receptor tyrosine kinase in a murine mastocytoma cell line P-815 generated by a point mutation. Blood. 1994;83(9):2619–2626. [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 11.Frost MJ, Ferrao PT, Hughes TP, Ashman LK. Juxtamembrane mutant V560GKit is more sensitive to Imatinib (STI571) compared with wild-type c-kit whereas the kinase domain mutant D816VKit is resistant. Mol Cancer Ther. 2002;1(12):1115–1124. [PubMed] [Google Scholar]
- 12.Ma Y, Zeng S, Metcalfe DD, et al. The c-KIT mutation causing human mastocytosis is resistant to STI571 and other KIT kinase inhibitors; kinases with enzymatic site mutations show different inhibitor sensitivity profiles than wild-type kinases and those with regulatory-type mutations. Blood. 2002;99(5):1741–1744. doi: 10.1182/blood.v99.5.1741. [DOI] [PubMed] [Google Scholar]
- 13.Neel BG, Gu H, Pao L. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 14.Dance M, Montagner A, Salles JP, Yart A, Raynal P. The molecular functions of Shp2 in the Ras/mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal. 2008;20(3):453–459. doi: 10.1016/j.cellsig.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Chan RJ, Feng GS. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109(3):862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartaglia M, Niemeyer CM, Fragale A, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34(2):148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 17.Bentires-Alj M, Paez JG, David FS, et al. Activating mutations of the Noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64(24):8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, He Y, Liu S, et al. Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2). J Med Chem. 2010;53(6):2482–2493. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang EE, Chapeau E, Hagihara K, Feng GS. Neuronal Shp2 tyrosine phosphatase controls energy balance and metabolism. Proc Natl Acad Sci U S A. 2004;101(45):16064–16069. doi: 10.1073/pnas.0405041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu HH, Ji K, Alderson N, et al. Kit-Shp2-Kit signaling acts to maintain a functional hematopoietic stem and progenitor cell pool. Blood. 2011;117(20):5350–5361. doi: 10.1182/blood-2011-01-333476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson VA, Sharma N, Everingham S, et al. SH2 domain-containing phosphatase-2 protein-tyrosine phosphatase promotes Fc epsilon RI-induced activation of Fyn and Erk pathways leading to TNF alpha release from bone marrow-derived mast cells. J Immunol. 2009;183(8):4940–4947. doi: 10.4049/jimmunol.0900702. [DOI] [PubMed] [Google Scholar]
- 22.Nishida K, Wang L, Morii E, et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood. 2002;99(5):1866–1869. doi: 10.1182/blood.v99.5.1866. [DOI] [PubMed] [Google Scholar]
- 23.Duttlinger R, Manova K, Berrozpe G, et al. The Wsh and Ph mutations affect the c-kit expression profile: c-kit misexpression in embryogenesis impairs melanogenesis in Wsh and Ph mutant mice. Proc Natl Acad Sci U S A. 1995;92(9):3754–3758. doi: 10.1073/pnas.92.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan BL, Hong L, Munugalavadla V, Kapur R. Functional and biochemical consequences of abrogating the activation of multiple diverse early signaling pathways in Kit: role for Src kinase pathway in Kit-induced cooperation with erythropoietin receptor. J Biol Chem. 2003;278(13):11686–11695. doi: 10.1074/jbc.M207068200. [DOI] [PubMed] [Google Scholar]
- 25.Mali RS, Ramdas B, Ma P, et al. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell. 2011;20(3):357–369. doi: 10.1016/j.ccr.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butterfield JH, Weiler D, Dewald G, Gleich GJ. Establishment of an immature mast cell line from a patient with mast cell leukemia. Leuk Res. 1988;12(4):345–355. doi: 10.1016/0145-2126(88)90050-1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Broxmeyer HE. p85 subunit of PI3K does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells. Biochem Biophys Res Commun. 1999;254(2):440–445. doi: 10.1006/bbrc.1998.9959. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Broxmeyer HE. Flt3 ligand induces tyrosine phosphorylation of gab1 and gab2 and their association with shp-2, grb2, and PI3K. Biochem Biophys Res Commun. 2000;277(1):195–199. doi: 10.1006/bbrc.2000.3662. [DOI] [PubMed] [Google Scholar]
- 29.Xu D, Wang S, Yu WM, et al. A germline gain-of-function mutation in Ptpn11 (Shp-2) phosphatase induces myeloproliferative disease by aberrant activation of hematopoietic stem cells. Blood. 2010;116(18):3611–3621. doi: 10.1182/blood-2010-01-265652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel W, Lammers R, Huang J, Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259(5101):1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 31.Vogel W, Ullrich A. Multiple in vivo phosphorylated tyrosine phosphatase SHP-2 engages binding to Grb2 via tyrosine 584. Cell Growth Differ. 1996;7(12):1589–1597. [PubMed] [Google Scholar]
- 32.Araki T, Nawa H, Neel BG. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J Biol Chem. 2003;278(43):41677–41684. doi: 10.1074/jbc.M306461200. [DOI] [PubMed] [Google Scholar]
- 33.Mitra S, Beach C, Feng GS, Plattner R. SHP-2 is a novel target of Abl kinases during cell proliferation. J Cell Sci. 2008;121(20):3335–3346. doi: 10.1242/jcs.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein-Gerlach M, Kharitonenkov A, Vogel W, Ali S, Ullrich A. Protein-tyrosine phosphatase 1D modulates its own state of tyrosine phosphorylation. J Biol Chem. 1995;270(42):24635–24637. doi: 10.1074/jbc.270.42.24635. [DOI] [PubMed] [Google Scholar]
- 35.Fragale A, Tartaglia M, Wu J, Gelb BD. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat. 2004;23(3):267–277. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- 36.Gu H, Neel BG. The “Gab” in signal transduction. Trends Cell Biol. 2003;13(3):122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 37.Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94(12):1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu WM, Daino H, Chen J, Bunting KD, Qu CK. Effects of a leukemia-associated gain-of-function mutation of SHP-2 phosphatase on interleukin-3 signaling. J Biol Chem. 2006;281(9):5426–5434. doi: 10.1074/jbc.M507622200. [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Sung SS, Yip ML, et al. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70(2):562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 40.Hellmuth K, Grosskopf S, Lum CT, et al. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc Natl Acad Sci U S A. 2008;105(20):7275–7280. doi: 10.1073/pnas.0710468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu WM, Guvench O, Mackerell AD, Qu CK. Identification of small molecular weight inhibitors of Src homology 2 domain-containing tyrosine phosphatase 2 (SHP-2) via in silico database screening combined with experimental assay. J Med Chem. 2008;51(23):7396–7404. doi: 10.1021/jm800229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan G, Cheung LS, Yang W, et al. Essential role for Ptpn11 in survival of hematopoietic stem and progenitor cells. Blood. 2011;117(16):4253–4261. doi: 10.1182/blood-2010-11-319517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.