Abstract
Introduction
More than a million people acquire HIV infection annually. Pre-exposure prophylaxis (PrEP) using antiretrovirals is currently being investigated for HIV prevention. Oral and topical formulations of tenofovir have undergone pre-clinical and clinical testing to assess acceptability, safety and effectiveness in preventing HIV infection.
Areas covered
The tenofovir drug development pathway from compound discovery; pre-clinical animal model testing and human testing were reviewed for safety, tolerability, and efficacy. Tenofovir is well tolerated and safe when used both systemically or applied topically for HIV prevention. High drug concentrations at the site of HIV transmission and concomitant low systemic drug concentrations are achieved with vaginal application. Coitally applied gel may be the favoured prevention option for women compared to the tablets which may be more suitable for prevention in men and sero-discordant couples. However, recent contradictory effectiveness outcomes in women need to be better understood.
Expert opinion
Emerging evidence has brought new hope that antiretrovirals can potentially change the course of the HIV epidemic when used as early treatment for prevention, as topical or oral PrEP. Although some trial results appear conflicting, behavioral factors, adherence to dosing, and pharmacokinetic properties of the different tenofovir formulations and dosing approaches offer plausible explanations for most of the variations in effectiveness observed in different trials.
Keywords: ART, NtRTI, tenofovir, tenofovir disoproxil fumarate, HIV prevention, microbicide, PMPA – [9-R-(2-Phosphonomethoxypropyl) adenine]
1. Introduction
Thirty years after the discovery of the Human Immunodeficiency Virus (HIV), the HIV pandemic continues to spread [1]. UNAIDS estimates indicate 2.7 million (2.4-2.9 million) newly infected people at the end of 2010, whilst 34 million (31.6-35.2 million) people are living with HIV globally [2]. More than 6.6 million of the approximately 14.2 million people, living with HIV and eligible for treatment, are currently actually receiving HIV treatment. Sub-Saharan Africa contributes 70% of all infections primarily via heterosexual transmission. Women are disproportionally burdened with disease and contribute 60% of all HIV infections in the sub-Saharan African generalised epidemic [2]. Hyper- vulnerability of women in fuelling the epidemic is a result of complex convergences of social and biological factors [3]. In this context, women's inability to negotiate mutual monogamy and consistent condom use translates to limited control of existing HIV prevention methods. Therefore, in the absence of an effective vaccine the search for HIV biomedical prevention modalities that can stem the tide of new infections - the use of which can be controlled by women themselves - is a public health priority [4]
The concept of using orally administered anti-retroviral (ARV) drugs to prevent HIV infection is well established and has been successfully used either as a single drug [5] or in a drug combination [6] for prevention of mother to child-transmission (PMTCT) and for Post-Exposure Prophylaxis (PEP) following occupational exposure to HIV or sexual assault [7]. Pre-exposure prophylaxis (PrEP) using daily or intermittent oral or topical agents (microbicides) is currently being tested in randomised clinical trials in HIV-uninfected people to assess safety and efficacy in preventing HIV acquisition[8]. Microbicides are products formulated for application on the vaginal or rectal mucosa and contain active ingredients’ that potentially block HIV and possibly other sexually transmitted infections [9].
Tenofovir, (PMPA, (R)-9-[2-(phosphonomethoxy)propyl]adenine monohydrate) is a adenosine nucleoside monophosphate (nucleotide) analogue with potent activity against retroviruses[10]. Tenofovir is phosphorlyated intracellularly to tenofovir diphosphate,its active metabolite and competitively inhibits HIV reverse transcriptase resulting in DNA chain termination, thereby preventing further replication [11]. An orally bioavailable form of tenofovir (tenofovir disoproxil fumarate, TDF) has been approved for the treatment of HIV since 2001. With tenofovirs’ efficient intracellular activation, pro-longed intracellular half-life and activity in both active and resting cells and high barrier to resistance this agent is a safe and potent antiretroviral with a proven track record for effective treatment of HIV-1 infection. When used daily for HIV prevention, TDF has shown significant activity in sero-discordant couples [12] and when used in combination with emtricitabine (FTC) was effective in protecting men who have sex with men (MSM) [13] as well as in heterosexual men and women[14]. Over the last 20 years of microbicide research, of the 12 effectiveness trials of seven candidate products only one candidate product demonstrated proof of concept for significant protection against HIV infection. The CAPRISA 004 phase IIb safety and effectiveness trial showed that 1% tenofovir, vaginally administered gel (TFV), reduced HIV acquisition in 18-40 year old women, at high risk for HIV infection, by 39% overall and by 54% in women who used the gel consistently [15].
In this drug evaluation we review available pre-clinical and clinical data on the tenofovir compound, in both its gel and oral formulations to assess its HIV infection prevention potential for PrEP.
2. Introduction to the PMPA compound and history of the development of tenofovir
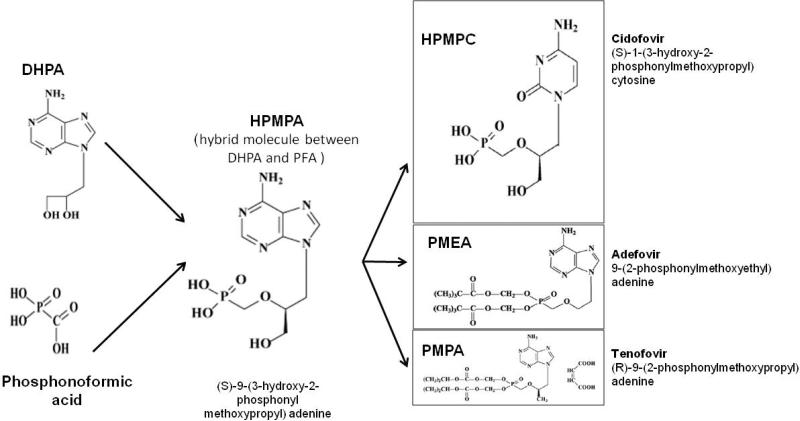
Nucleoside derivatives were identified in the early 1960's as molecules that played an important role in drug development for the treatment of cancers. Being anti-metabolites that would interfere with the growth of tumour cells, they were invariably accompanied by host toxicity. However, extensive complex modifications of the nucleosides subsequently enhanced the therapeutic effect of the drugs. Further development of nucleoside derivatives, namely the acyclic nucleoside phosphonates began in 1976, following a symposium on synthetic nucleosides, nucleotides and polynucleotides at the Max-Planck-Institute. The collaboration between the two research groups of Erik De Clercq and Antonin Holy two years later resulted in the identification of the acyclic nucleoside analogue (S)-9-(2,3-dihydroxypropyl) adenine (DHPA) as a broad-spectrum antiviral agent [16-18].
DHPA, an acyclic nucleoside analogue was clearly distinct from the acyclic guanosine analogue – acyclovir- as a selective anti-herpes simplex virus (HSV) agent, with acyclovir owing its selectivity to the specific phosphorylation by the HSV-induced thymidine kinase (TK). In spite of the broad spectrum of antiviral activity as well as its mode of action, DHPA had a limited appearance on the market as a gel formulation for the treatment of cold sores compared to acyclovir [19, 20]. Thus, acyclovir became the ‘gold standard’ for the treatment of HSV types 1 and 2 infections. A clear disadvantage of DHPA was that it is not metabolized [21], but served as an excellent tool for various subsequent molecular and biological investigations. DHPA behaves as an aliphatic nucleoside analogue by occupying the adenosine-binding site of S-adenosyl-l-homocysteine hydrolase, an important regulatory enzyme in S-adenosyl-l-methionine-mediated methylations [21] with the sugar moieties replaced by the aliphatic chains. DHPA is representative of unusual acyclic nucleoside analogues that have an alkyl group linked to N1 (in pyrimidines) or N9 (in purines) that bear hydroxyl(s) necessary for activation by phosphorylation. Such compounds can readily adopt a conformation appropriate for forming a complex with the active site of the enzyme. Thus, investigations of DHPA as a phosphonate derivative of bioactive nucleoside were aimed at creating catabolically stable, isopolar and, possibly, isosteric, nucleotide analogues. The polar nature of nucleotides precludes their crossing the cellular membrane and the phosphonate derivatives of bioactive nucleosides in which the oxygen atom is placed in the nearest position adjacent to the α-carbon to transform the phosphoric ester grouping (=P-O-C-) to its isomeric phosphonomethyl ether (=P-C-O-) are therefore catabolically stable [22]. The rationale for the underlying development of acyclic nucleoside phosphonates is that nucleoside analogues generally need to be converted by three phosphorylation steps to their 5′-triphosphate metabolites to show activity. The first phosphorylation step, which affords the 5′-monophosphate, is often the bottleneck in this transformation, and so agents could show poor activity owing to lack of transformation to the monophosphate form by nucleoside kinases. This problem can be circumvented by using phosphorus-modified nucleotide analogues, such as phosphonates [23]. It is important that the linkage is resistant to cleavage by cellular enzymes
2.1 Preclinical data on the effects of the precursors of tenofovir against viruses
Early experiments provided promising data on the marked antiviral activity of DHPA against several viruses in cell cultures. Cell lines, following inoculation with viruses were exposed to differing concentrations of DHPA to determine the dose required to suppress viral cytopathogenicity by 50 percent. DHPA in human skin fibroblast cells demonstrated the inhibition of in vitro replication of several DNA and RNA viruses, including vaccinia, HSV 1 and 2, measles, and vesicular stomatitis virus (VSV) at concentrations at which cellular DNA and RNA synthesis were not affected. DHPA at a concentration of 100 ug/ml caused a dramatic decrease of virus titre and this reduction amounted to approximately 4 log10 for the virus yields measured at 24 and 48 hours after infection [16]. A key finding was that only the (S)-enantiomer of DHPA proved active, whilst the (R)-enantiomer was not and the racemic mixture of (R, S)-DHPA, was also as effective as the (S)-enantiomer. Primary rabbit kidney cells with VSV, vaccinia, and herpes simplex 1 (strain KOS), (S)-DHPA inhibited the cytopathogenic effects at a concentration of about 10 to 20 ug/ml. However, viruses such as poliovirus, Coxsackievirus, and Sindbis were not inhibited by DHPA. DHPA did not exhibit any antibacterial or antifungal activity. Streptococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus vulgaris, and Escherichia coli were found not to be inhibited at concentrations up to 100ug/ml, whilst for Mycobacterium tuberculosis, Trichophyton mentagrophytes, Candida albicans, and Aspergillus niger the minimum inhibitory dose was 50ug/ml[16]. An added advantage was that DHPA even at concentrations as high as 200 ug/ml did not significantly reduce DNA or RNA synthesis as monitored in HeLa, Vero, and primary rabbit kidney cells. Similarly, DHPA did not inhibit protein synthesis when applied to primary rabbit kidney cells at a concentration of 400ug/ml maintaining the viability of the uninfected host cells unless extremely high concentrations were employed.
Following the development of the hybrid molecule HPMPA, similar to DHPA, (S)-HPMPA was shown to exhibit activity against a range of DNA viruses including vaccinia, HSV-1 and HSV-2, adenovirus and Maloney murine sarcoma retrovirus. Later work also found it to be effective against Hepatitis B viruses. The inhibitory effect of the derivatives of adenine (PMPA) and 2,6- diaminopurine (PMPDAP) on HIV replication in several human cell systems, including natural peripheral blood lymphocytes (PBL) and freshly isolated monocyte/ macrophages (M/M) demonstrated the (R) - enantiomers of PMPDAP and PMPA to be ~10- to 100-fold more effective compared to the (S) - enantiomeric complement [24, 25]. The antiviral efficacy of (R)-PMPA was comparable to that of the prototype acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl) adenine (PMEA). Thus, (R)-PMPA and (R)-PMPDAP displayed superior inhibitory effects against HIV-1 when the virus was exposed to those cells (i.e. peripheral blood lymphocytes and monocyte/macrophages) that in nature are the most important target cells for virus infection. These observations provided the potential of PMPDAP and (R)-PMPA as candidate drugs for the treatment of HIV infection in humans. The virtual lack of toxicity of (R)-PMPA and (R)-PMPDAP for proliferating and non-proliferating cells when administered daily for 4 weeks at 20 to 30 mg/kg further indicated their potential therapeutic usefulness[25].
2.2 Pre-clinical animal safety data of the precursors of tenofovir
The potential activity of DHPA in vivo was assessed in mice inoculated intra-nasally with VSV. The experimental infection resembled natural transmission, infection and spread from a respiratory tract site in humans. The intranasal VSV model showed that repeated doses of DHPA (2 mg per mouse or - 135 mg/kg) injected intraperitoneally 1 hour and 1, 2, 3, and 4 days after VSV challenge brought about a significant increase in the final number of surviving mice (DHPA = 67% versus control group =37,5%; P <0 .05) with sustained significant protection 9 days post infection. Repeated doses of lower concentrations of DHPA at 0.08 mg per mouse (~ 5.4 mg/kg) did not confer protection, whereas repeated doses at 0.4 mg per mouse (~ 27 mg/kg) gave slightly better protection. No signs of toxicity were noted in the mice injected with DHPA[16].
The hybrid molecule between DHPA and PFA (phosphonoformic acid) (Figure. 1) [22, 23] saw the development of (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl) adenine (HPMPA) and a totally new concept of antimicrobials emerged. HPMPA was subsequently shown to exhibit broad-spectrum activity against a range of DNA viruses, including those that did not induce a specific viral TK such as human cytomegalovirus or strains becoming resistant to acyclovir by a deficiency in their TK, such as the TK- HSV strains [17, 26]. Apart from its antiviral activity, HPMPA demonstrated anti-parasitic activity as well. Although HPMPA itself was not further developed as an antiviral drug, it also served as the prototype compound for a series of acyclic nucleoside phosphonates now used for the treatment of viral infections, some in their prodrug form to improve their oral bioavailability. The agents are (S)-1-[3-hydroxy-2-(phosphonomethoxy)propyl]cytosine, HPMPC (cidofovir); 9-[2-(phosphonomethoxy)ethyl]adenine, PMEA (adefovir) and (R)-9-[2-(phosphonomethoxy)propyl]adenine monohydrate, PMPA (tenofovir) – the featured drug for this review (Figure 1) [23].
Figure 1.
Development of acyclic nucleoside phosphonate antiviral agents (adapted from De Clercq, 2007[22]
3. Non-human primate data on tenofovir (PMPA) in preventing HIV infection
Multiple non-human primate infection models have demonstrated the effectiveness of subcutaneous, gel, and oral formulations of PMPA in preventing transmission of Simian Immunodeficiency Virus (SIV) or Simian-Human Immunodeficiency Virus (SHIV) (Table 1), even when applied several hours after viral challenge. The data are summarised by route of tenofovir administration.
Table 1.
Summary of studies of non-human primate models of infection using tenofovir as Pre-Exposure Prophylaxis against Simian Immunodeficiency Virus infection
| Study* | Number of Exposures | Inoculating virus | Treatment | Time of Administration | Number Infected | Protection (%) |
|---|---|---|---|---|---|---|
| Tenofovir administered subcutaneously | ||||||
| Tsai et al[27] 1995 | 1 | SIV Intravenous (IV) inoculation | PMPA (20mg/kg) PMPA (30mg/kg) PMPA (30mg/kg) PMPA (30mg/kg) vehicle |
-48 h - 48 h 4 h 24 h n/a |
0 of 5 0 of 10 0 of 5 0 of 5 10 of 10 |
100 100 100 100 0 |
| Tsai et al [30] 1998 | 1 | SIV IV inoculation | PMPA (30mg/mL) PMPA (30mg/mL) PMPA (30mg/mL) PMPA (30mg/mL) PMPA (30mg/mL) Untreated control |
24h, daily for 28 days 48h, daily for 28 days 72h, daily for 28 days 24h, daily for 10 days 24h, daily for 3 days n/a |
0 of 4 2 of 4 2 of 4 2 of 4 4 of 4 4 of 4 |
100 50 50 50 0 0 |
| Van Rompay et al [28] 1998 | 1 | SIVmac oral inoculation | PMPA SC Untreated controls |
-4h, 24h N/a |
0 of 4 4 of 4 |
100 0 |
| *Van Rompay et al [94] 1998 | 2 | SIVmac251 oral inoculation and SHIV-SF33 IV | PMPA SC Untreated controls |
0h for 14 days n/a |
1 of 4 12 of 12 |
75 0 |
| Van Rompay et al [29] 2000 | 1 | SIVmac055∞ | PMPA Untreated control |
-24h for 4 weeks n/a |
3 of 5 3 of 3 |
40 0 |
| Otten et al [31] 2000 | 1 | HIV-2GB122 inoculated intravaginally | PMPA SC PMPA SC PMPA SC Untreated control |
12h 36h 72h n/a |
0 of 4 0of 4 1of 4 4 of 4 |
100 100 75 0 |
| Van Rompay et al [95] 2001 | 1 | SIVmac251 oral inoculation | PMPA SC (4mg/kg) PMPA SC (4mg/kg) PMPA SC (30mg/kg) Untreated control |
-4h, 20h 1h, 25h 1h n/a |
1of 4 2of 4 0 of 1 4 of 4 |
75 50 100 0 |
| Subbarao et al [39] 2006 | 14 | SHIVSF162P3 | Tenofovir orally Tenofovir orally Untreated controls |
-2, daily for 36 weeks -2, weekly for 36 weeks n/a |
4 of 4 by 6 weeks 4 of 4 by 7 weeks 4 of 4 by 1.5 weeks |
0 0 0 |
| TFV gel administered intravaginally | ||||||
| NIH/CDC study 1[32] | 2 | SIVmac251 inoculated intravaginally | 10% TFV gel 1 mL vehicle |
-24h, 0h, 24h, 48h -24h, 0h, 24h, 48h |
0 of 4 2 of 2 |
100 0 |
| NIH/CDC study 2[32] | 1 | SIVmac251 inoculated intravaginally | 10% TFV gel 1% TFV gel 1% TFV gel untreated control |
-24h, -15m, -24h -24h, -15m, -24h -15m N/A |
1 of 5 1of 5 2of 5 5 of 5 |
80 80 60 0 |
| NIH/CDC study 3[32] | 1 | SIVmac251 inoculated intravaginally | 1% TFV gel 1% TFV gel 1% TFV gel vehicle untreated control |
-15m -2h -8h -15m N/A |
1 of 5 3 of 5 1 of 5 1 of 5 2 of 5 |
80 40 80 80 60 |
| NIH/CDC study 4[32] | 1 | SIVmac251 inoculated intravaginally | 1% TFV gel 1% TFV gel 1% TFV gel vehicle untreated control |
-15m -2h -8h -15 m N/A |
1 of 5 1of 5 2of 5 2 of 5 4 of 5 |
80 80 60 60 20 |
| NIH/CDC study 5[32] | 1 | SIVmac251 inoculated intravaginally | 1% TFV gel vehicle untreated control |
-2h -2h N/A |
0 of 5 2 of 5 2 of 5 |
100 60 60 |
| NIH/CDC study 6[32] | 1 | SIVmac251 inoculated intravaginally | 1% TFV gel 1% TFV gel 1% TFV gel vehicle vehicle untreated control |
-12h -24h -72h, -48h, -24h -12h -24h N/A |
5of 8 8 of 8 6of 8 8 of 8 8 of 8 8 of 8 |
38 0 25 0 0 0 |
| *Parikh et al [33] 2009 | 20 | SHIVSF162p3 | 5%FTC and 1% TFV gel Vehicle Untreated control 1% TFV gel vehicle |
- 30 min | 0 of 6 5 of 6 2of 2 0 of 6 3of 3 |
100 17 0 100 0 |
| Miller et al [96] 1996 | 1 | SIVmac251 | 10% TFV gel vehicle |
-24, 0h, 24h, 48h -24, 0h, 24h, 48h |
0 of 5 5 of 5 |
100 0 |
| Dobard et al 2011 | 2 | SHIVSF162P3 | 1% TFV gel 2% HEC placebo gel |
20m,72h | 4of 6 9 of 10 |
74 |
| TFV gel administered rectally | ||||||
| Cranage et al [97]2008 | 1 | SIVmac251/32H | TFV gel rectally TFV gel rectally Vehicle Untreated control |
-2h 2h -2 n/a |
3 of 9 1 of 3 3 of4 4of 4 |
67 67 25 0 |
| Tenofovir administered orally | ||||||
| Garcia-Lerma et al [98] 2008 | 14 | SHIV rectal inoculation | FTC SC TDF (20mg/kg) and FTC orally TDF (22mg/kg) and FTC SC Intermittent FTC and TDF (22mg/kg) SC Untreated controls |
-7-9 days, daily for 28 days -7-9 days, daily for 28 days -7-9 days, daily for 28 days -2h, 24h n/a |
4 of 6 2 of 6 0 of 6 0 of 6 17 of 18 |
33 66 100 100 5 |
| Van Rompay[37] et al 2002 | 15 | SIVmac251 oral inoculation 3 times/day | 5mL Tenofovir orally 2.5mL Tenofovir orally vehicle |
daily daily daily |
3 of 4 2of 4 3of 6 |
50 50 50 |
| Van Rompay et al[99] | 30 | SIVmac251 | 10mg/kg Tenofovir orally Topical GS-7340 Untreated controls |
Once daily for 7 days 3 times daily for 7 days n/a |
8 of 12 4 of 5 29 of 31 |
33 20 6 |
macaques pretreated with 30mg depo-provera 30 days prior to vaginal challenge, ∞virulent isolate that has a five-fold reduced susceptibility to tenofovir, TDF - Tenofovir Disoproxil Fumarate, FTC – emtricitabine, PMPA – [9-R-(2-Phosphonomethoxypropyl)adenine] (tenofovir), SC – subcutaneous, IV – intravenously
3.1 Tenofovir administered subcutaneously
One of the first pre-clinical studies demonstrating tenofovir's prevention potential was an investigation of subcutaneous injection of tenofovir daily for 4 weeks in macaques who had been intravenously inoculated with SIV. All 25 of the tenofovir-treated macaques were protected from SIV infection without toxicity whether administration began 48 hours prior to intravenous inoculation (dose of tenofovir, 20 mg/kg in five animals, 30 mg/kg in 10 animals), 4 hours after inoculation (dose of tenofovir 30 mg/kg in five animals), or 24 hours post-inoculation (dose of tenofovir, 30 mg/kg in five animals). Evidence of SIV infection was not present in any of the treated animals monitored for up to 52 weeks, including viral load in plasma and peripheral blood mononucleocytes (PBMCs), SIV DNA in PBMCs, SIV-specific antibody, and lymph node biopsies. In contrast, each of 10 animals receiving placebo 48 hours prior to inoculation became infected [27].
Complete protection against SIV infection was also observed in a study among infant macaques that received just two subcutaneous doses of tenofovir; one 4 hours prior and the second, 24 hours post oral SIV inoculation [28]. Even macaques inoculated with the virulent SIVmac055 strain, which has a five-fold reduced susceptibility to tenofovir, were partially protected when they received subcutaneous tenofovir 24 hours prior to viral challenge followed by administration for 4 weeks compared to the untreated controls that all became infected[29].
The ability of tenofovir to prevent the establishment of persistent infection was investigated in a study on cynomolgus macaques (Macaca fascicularis). Daily subcutaneous dosing with tenofovir was initiated at varying times, as well as different durations of treatment, following intravenous inoculation with SIV. Twenty-four macaques were studied for 46 weeks after inoculation with SIV. All mock-treated control macaques showed evidence of infection within 2 weeks post-inoculation. All macaques that were treated with tenofovir for 28 days beginning 24 hours post-inoculation showed no evidence of viral replication following discontinuation of tenofovir treatment. However, extending the time to initiation of treatment from 24 to 48 or 72 hours post-inoculation or decreasing the duration of treatment, reduced effectiveness in preventing establishment of persistent infection. Only half of the macaques treated for 10 days, and none of those treated for 3 days, were completely protected when treatment was initiated at 24 hours. Despite the reduced efficacy of delayed and shortened treatment, all tenofovir-treated macaques that were not protected showed delays in the onset of cell-associated and plasma viremia and antibody responses compared with mock controls[30].
Subcutaneous administration of tenofovir has also been shown to be completely protective against HIV-2 infection when given up to 36 hours post-inoculation[31]. However, breakthrough infection in one animal in the 72 hour post HIV exposure group was detected at 16 weeks [31].
Such in vivo activity of tenofovir made it a promising agent for prevention of HIV infection.
3.2 TFV gel administered intravaginally
Nine studies, conducted by the NIAID Division of AIDS and the Centers for Disease Control (CDC), have shown that intravaginal administration of TFV gel can prevent the transmission of SIV /SHIV. The studies explored the effectiveness of tenofovir provided at different concentrations and durations. While interpretation of some of these studies remains limited because some control animals remained uninfected and sample sizes were small, they provided proof-of-concept in animals that TFV gel could prevent the transmission of SIV/SHIV when vaginal dosing mimics coital usage of the gel. In Study 1, a TFV gel dose given 24 hours prior to exposure, at exposure, 24 hours after, and 48 hours after exposure, provided complete protection from SIV infection[32]. A repeat-challenge macaque model with a low dose SHIV inoculum containing an CCR5-tropic HIV-1 envelope similar to naturally transmitted human viruses (10 TCID50) showed that application of 1% TFV gel just 30 minutes prior to viral challenge consistently protected from vaginal SHIV infection[33]. Higher doses of TFV gel (10% weight per weight) administered intravaginally at four timepoints; 24 hours before, 0 hours, 24 hours after and 48 hours post-inoculation, was also fully effective in preventing intravaginal SIV transmission after repeated viral challenge[34]. Even a single dose given 15 minutes before viral inoculation provided partial protection[32]. Most recently, in delayed challenge experiments four out of six macaques were protected from SHIV exposure occurring 30 minutes and 3 days after 1% TFV gel application, compared to 10 placebo treated animals [35]. Together, these studies provided a scientific rationale for clinical studies of vaginally applied TFV gel in humans.
3.3 TFV gel administered rectally
TFV gel has also been tested in a rectal SIV challenge model. Mucosally-applied TFV gel was given rectally as a single dose 15 minutes or 2 hours prior to, or 2 hours after intrarectal challenge. In the 2 control groups of macaques, 4 of 4 untreated macaques and 3 of 4 macaques given placebo gel became infected. Virus was recovered from only 1 of 6 animals receiving TFV gel 15 minutes prior to virus challenge. In one other animal in this group, virus was recovered only at weeks 2 and 6. Two of 3 animals receiving the drug 2 hours prior to virus challenge showed no evidence of circulating virus and in the third animal virus isolation was delayed until week 12. In the third intervention group where gel was administered 2 hours after virus challenge, 2 out of the 3 animals became infected. Interestingly, gag-specific interferon-gamma secreting T cells were detected by ELISpot in 4 of 7 animals in which virus were unrecoverable from PBMC. These T-cell responses confirm exposure to challenge virus antigens and suggest that infection did not become established despite the virus having triggered an immune response[36].
3.4 Oral tenofovir administration
Several repeated challenge macaque models have investigated the use of oral TDF in preventing SIV/SHIV. Two studies, mimicking viral exposure through breastfeeding, showed that oral tenofovir given daily was partially protective in infant macaques who received 15[37] or 30[38] low doses of SIV. In another study, tenofovir given daily for 36 weeks or weekly for 36 weeks to Chinese rhesus macaques - inoculated intrarectally with a high dose SHIVSF162P3 once weekly for 14 weeks or until a macaque became infected - provided only partial protection against SHIV infection[39]. A repeat-exposure macaque model with 14 weekly rectal virus challenges was used to compare the effectiveness of daily versus intermittent PrEP. The four treatment groups of 6 macaques each received either: a daily subcutaneous dose of FTC (group 1) for 28 day, or a combination of oral FTC and TDF (group 2) for 28 days, or a subcutaneous dose of FTC and in combination with a higher dose of TDF (group 3) for 28 days, or a regimen similar to group 3 but only 2 h before and 24 h after each weekly virus challenge (group 4). Results of these groups were compared to 18 control macaques that did not receive any drug treatment. The risk of infection in macaques treated in groups 1 and 2 was 3.8- and 7.8-fold lower than in untreated macaques (p = 0.02 and p = 0.008, respectively). All six macaques in group 3 were protected and all six animals in group 4 that received intermittent PrEP were protected. These results showed that short but potent intermittent PrEP could provide protection comparable to that of daily PrEP [40].
4. Pharmacokinetics and metabolism of tenofovir in humans
The pharmacokinetics of oral TDF has been evaluated in healthy volunteers and HIV-1 infected individuals and was found to be similar. TDF is a water soluble diester prodrug of the active ingredient tenofovir. Following oral administration of a single dose of TDF 300 mg to HIV infected fasting subjects , maximum serum concentrations (Cmax) are achieved in 1.0 ± 0.4 hrs. Cmax and area under the curve (AUC) values are 0.30 ± 0.09 μg/mL and 2.29 ± 0.69 μg·hr/mL, respectively[41]. The volume of distribution at steady-state is 1.3 ± 0.6 L/kg and 1.2 ± 0.4 L/kg, following intravenous administration of tenofovir 1.0 mg/kg and 3.0 mg/kg [41] and 7.2% of the drug is plasma protein bound. Tenofovir is not a substrate of cytochrome P450 enzymes and following intravenous administration approximately 70–80% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Following a single oral dose, the elimination half-life of tenofovir is approximately 17 hours and intracellular half-life is >60 hours [42]. After multiple oral doses of TDF 300 mg once daily (under fed conditions), 32 ± 10% of the administered dose is recovered in urine over 24 hours. Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion[41].
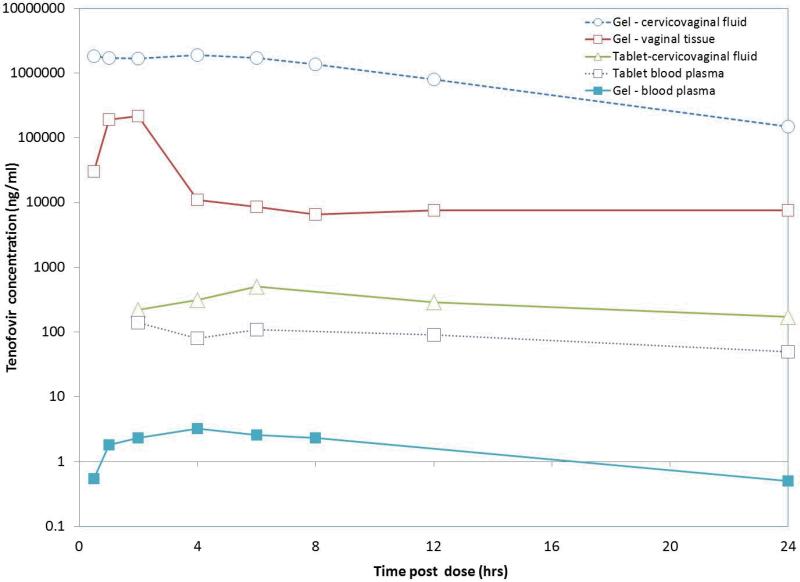
Figure 2 is adapted from published data to illustrate the cervicovaginal fluid, vaginal tissue and blood plasma distribution of tenofovir over a 24 hour interval following single dose oral and vaginal tenofovir administration.
Figure 2.
Tenofovir concentration distribution post single dose oral and topical vaginal administration. Adapted from Dumond et al, 2007 [43], Schwartz et al, 2011 [45]
Tenofovir concentrations in the genital tract and in rectal tissues have been assayed in phase 1 and PK studies. Following oral TDF dosing, female genital tract (cervicovaginal fluid) concentrations of tenofovir relative to blood plasma at first dose, achieved a median of 135%, and 75% of that of blood plasma concentrations at steady state[43]. More recently, tenofovir concentrations in genital secretions was found to be 2.5 fold greater than blood plasma concentrations[44]. The median cervicovaginal fluid concentrations of tenofovir at the end of a 24h dosing interval at steady state was 68 ng/mL [43] Vaginal tissue concentrations at 24 hours are 6.8 ng/g and 50 ng/g in the vaginal and cervical tissue respectively [44]. Rectal tissue exposure of oral tenofovir (C24 = 1877 ng/g) is a 100 fold higher than vaginal and cervical tissue content [44].
Following vaginal use of TFV gel, the median Cmax in cervicovaginal fluid was 1,9 × 106 ng/ml (range: 1.2 × 104 – 9.9 × 106 ng/ml) after a single dose and 1.4 × 106 ng/ml (range 8.4 × 104 – 5.8 × 106 ng/ml) after multiple doses. Blood plasma concentrations were low; Cmax: 4.0 ng/ml and 3.4 ng/ml after single and multiple doses respectively. Tenofovir vaginal tissue concentrations (assayed from biopsies) and assessed by pooling samples from 1 to 24 hours, demonstrated tenofovir concentrations ranging from 2.1 × 102 – 1.4 × 106 ng/ml with a median Cmax of 2.2 × 105 ng/ml [45]. In a crossover study, the tissue concentrations of the active tenofovir diphosphate were found to be 2 log10 higher following vaginal dosing than oral dosing [46].
Although, vaginal fluid and tissue concentration ranges have not been established for protection against HIV infection, data from the CAPRISA 004 study indicate that HIV incidence was considerably lower in women with tenofovir cervicovaginal concentrations of 1000ng/ml or more when compared to placebo drug or concentrations <1000ng/ml [47]. In summary, tenofovir concentrations with oral TDF are approximately 100-fold higher in rectal than vaginal tissues and 2.5 fold higher in the cervicovaginal fluid than blood plasma. TFV gel demonstrates 1000-fold higher concentration in vaginal tissues compared to oral TDF.
5. Safety and efficacy of oral and topical formulations of tenofovir in preventing HIV-1 infection in humans (Phase 1-3)
Following the promising anti-HIV results from animal studies the tenofovir research agenda advanced rapidly to comprehensively testing both oral and topical tenofovir in humans. Studies of topical TFV gel and oral TDF span a decade of research that includes clinical assessment of safety, acceptability, tolerance, pharmacokinetics (PK) and pharmacodynamics (PD) in differing populations and with various patterns of use.
Several ongoing phase 1 studies are assessing safety in pregnancy and during lactation, adherence and PK comparing the safety of oral TDF to TFV gel. In addition, these studies are assessing safety, acceptability and PK with rectal use by women and men (including a separate study in young men); altered gel formulation characteristics to aid rectal use and resistance screening. Two phase 3 studies; one testing oral TDF as PrEP among injection drug users, and the other assessing effectiveness of coitally linked topical tenofovir gel are also ongoing.
5.1 Phase 1 studies
Topical TFV gel
Two concentrations (0.3% and 1%) of topical tenofovir have been shown to be safe and well tolerated in a phase 1 trial among 84 sexually abstinent HIV-uninfected women. The HPTN 050 study [48], showed that a two week course of 1% TFV gel used twice daily was as well tolerated as 0.3% used once daily by all 84 women and 24 male sexual partners. The highest practical dose and frequency (HPDF) was determined to be 1% twice daily. Adverse events (AEs) were mainly those that occurred in the genital tract, with 92% of women reporting at least one AE and 87% of the AEs being mild and short lived. Serum tenofovir levels were low but detectable in 14 of the 25 women who had PK evaluations completed. No new HIV resistance mutations were detected after 2 weeks of twice daily TFV gel use in the HIV infected women. Quantitative results indicate that tenofovir vaginal gel was acceptable to almost all users (94%), while qualitative findings indicate that acceptability is complex - lubrication, leakage, sexual pleasure, and the possibilities for covert use being important considerations to women [49]. A male tolerability study of TFV gel has shown that multiple topical gel exposures on the penis were well tolerated by both circumcised and uncircumcised men [50] .
A number of pharmacology studies investigating local and systemic absorption, genital tract drug concentrations, and the impact of TFV gel on mucosal immune mediators have also been completed. One study among 45 HIV-negative, sexually abstinent women (A04-095) [51] has shown that high genital tract and cervicovaginal fluid levels, and low blood plasma concentrations were detectable up to 24 hours following a single or multiple doses of 1% TFV gel. The active tenofovir diphosphate concentration was high in endocervical cells and detectable in about 40% of vaginal tissue biopsy samples at exposures similar to or higher than what is seen in PMBCs after oral exposure[52]. Candidate biomarkers of microbicide pharmacodynamics and safety were evaluated in a double blind, placebo-controlled trial (TFV 010) with 30 women randomized to apply single daily dose of TFV or placebo gel for 14 consecutive days [53]. A significant increase in anti-HIV activity was detected in cervicovaginal lavage (CVL) from women who applied TFV gel compared to those using the placebo and this activity persisted in thpresence of virus infected semen. The gels were well tolerated and AEs were similar in both arms. Repeated vaginal application of TFV gel was not associated with reduction in endogenous antimicrobial activity, loss of protective mediators or pro-inflammatory response.
An important sub-population for gel use is pregnant and lactating women. The Microbicide Trials Network (MTN 002) study was the first phase 1 trial to evaluate the safety and PK of a single dose of 1% TFV gel administered approximately two hours before planned caesarean to 16 pregnant women [54]. There were no serious AEs among mothers or neonates related to TFV gel exposure. Single application of the gel in term pregnancy produces low overall serum levels consistent with levels reported in non-pregnant women. While TFV does appear to cross the placenta, foetal exposure after vaginal dosing was low, with only trace amounts being passed to the foetus [55].
Collectively these phase 1 studies indicate that the 1% TFV gel is safe, well tolerated and acceptable to users. Additionally, there is compelling evidence for anti-HIV activity and availability of high drug concentrations at the site of HIV exposure.
Phase 1 safety studies of TFV gel when used rectally are ongoing. Results of the first phase 1 trial assessing the systemic safety of 1% TFV gel when applied rectally in 18 sexually-abstinent HIV-uninfected men and women in the US is currently being analysed and another trial of rectal safety and acceptability of a new TFV gel formulation (low glycerine concentration) MTN 007 [56] is currently underway in 60 men and women.
A number of new phase 1 studies are also planned. These include a phase 1 study that will expand on the positive results from MTN 002 to provide important PK information of the use of TFV gel daily for seven days in pregnant and lactating women[57] and a phase 1 study comparing the PK/PD in 100 sexually active, US women using either a daily 1% TFV gel dose, a peri-coital (dosed either 1 hr before or 1 hr after sex) dose or BAT 24 dosing [58].
Oral TDF
The review of phase 1 studies on the use of oral TDF for HIV prevention are restricted to ongoing trials only as the safety and tolerability of oral TDF for daily use has been well established from extensive experience with this formulation of the drug for HIV treatment.
The ongoing phase 1 studies of oral TDF for prevention focus on drug PK in special populations and participant acceptability with PK assessment of the oral formulation compared to the gel formulation. MTN 006 is a safety, acceptability and PK assessment comparing rectally applied TFV gel with oral TDF in 18 HIV negative US men and women [59] and will provide valuable data to support the vaginal microbicide application, should vaginal efficacy be demonstrated in other trials; and additional safety data on rectal microbicides for use by men and women. Another ongoing phase 1 trial in Malawi and Brazil, HPTN057, is assessing the safety and PK of oral TDF in 110 HIV-infected women and their infants and will provide data to inform the optimal regimen for a subsequent MTCT efficacy trial, if indicated.
5.2 Phase 2 studies
Table 2 provides an overview of oral TDF and topical TFV gel phase 2 and 3 studies, completed and in progress for tenofovir safety and effectiveness determination against HIV-1.
Table 2.
Summary of key TFV oral and gel phase 2 and 3 safety and effectiveness studies for HIV prevention
| Phase 2/2b - Safety and Effectiveness Studies | |||||
|
FHI West Africa Study (9780)[60] Safety and Preliminary Effectiveness 2007 |
Ghana, Cameroon, Nigeria | Sexually active HIV- women, (N=936) |
Active: Daily TDF 300mg tablet Control: Daily placebo tablet |
Complete | No clinical or laboratory safety concerns Effectiveness not evaluable due to a small number of HIV infections observed. |
|
HPTN 059[64] Safety and acceptability 2008 |
US, India | Sexually active HIV- women, (N = 200) |
Active: Daily TFV 1% gel (N=50) Coital use: TFV 1% gel (N=50) Control: Daily placebo gel (N=50) Coital Use – placebo gel (N=50) |
Complete | No safety or acceptability differences or concerns between the arms |
|
CAPRISA 004[15] Expanded safety and effectiveness study 2010 |
South Africa | Sexually active HIV- women, (N = 889) |
Active: TFV 1% Gel (N=445) Control: Placebo Gel (N= 444) *BAT 24 |
Complete | 39% (95%CI: 6-60%) effective against HIV 51% (95%CI: 22-70%) effective against HSV-2[100] Higher gel adherence 54% effective in preventing HIV |
|
CDC 4323 [61, 62] Extended behavioural safety 2010 |
US | Sexually active HIV- MSM, (N=400) |
Active: Daily TDF 300mg tablet Control: Daily placebo tablet Group 1: Started at enrolment Group 2: Started 9 months after enrolment |
Complete | Preliminary analysis in July 2010 No serious safety concerns No increased risk in men taking PrEP compared to those not taking it. |
|
MTN 001[46, 66] Adherence and PK study 2011 |
US, South Africa, Uganda | Sexually active, HIV- women, (N=144) | Daily TFV 1% gel (N = 144) Daily TDF 300 mg tablet (N=144) Both (N=144) |
Complete | No safety or acceptability differences Tablet preferred in US women Vaginal tissue concentrations of TFV-DP-2log10 higher after vaginal dosing than oral dosing. |
|
MTN 003 (VOICE)[93] Safety and effectiveness study Initiated September 2009 |
South Africa, Zimbabwe, Uganda | Sexually active, HIV- women, (N=5000) |
Active: Daily TFV 1% gel (N=1000) Daily TDF 300mg tablet (N=1000) Daily TDF/FTC 300/200 mg tablet (N = 1000) Control: Placebo gel (N = 1000) Oral placebo tablet (N = 1000) |
Ongoing | Oral TDF arm stopped for futility September 2011 1% TFV gel arm stopped for fultility November 2011 No serious safety concerns Results expected in 2013 |
| Phase 3 - Effectiveness Studies | |||||
|
Partners PrEP study [12] Safety and effectiveness study Initiated May 2008 |
Kenya, Uganda | Sero-discordant heterosexual couples (N=4758) |
Active: Daily oral TDF tablet or Daily oral TDF/FTC tablet Control: Placebo tablet |
Ongoing | Preliminary analysis: safety concerns - SAEs similar in all arms. TDF effectiveness: 62% (95%CI: 34-78%) TDF/FTC effectiveness 73% (95%CI: 49-85%) |
|
CDC 4370 Bangkok Tenofovir Study Safety and effectiveness study Initiated June 2005 |
Bangkok | Injection drug users (N=2400) |
Active: Daily oral TDF tablet Control: Placebo tablet |
Ongoing | Results expected in 2012 |
|
FACTS 001 [76] Safety and effectiveness study including HSV-2 infection as a primary endpoint Initiated October 2011 |
South Africa | HIV-uninfected sexually active women (N=2200) |
Active: BAT 24* - TDF 1% Gel Control: HEC Placebo Gel |
Ongoing | Results expected in 2014 |
BAT24 Regimen - within 12 hours BEFORE coitus, as soon as possible within 12 hours AFTER coitus and up to TWO doses in a 24 hour period
Oral TDF
The first study to assess oral PrEP in women, under the direction of FHI 360, evaluated the safety and preliminary effectiveness of daily oral TDF vs placebo in preventing HIV in 936 African women [60]. Although this West African study showed that oral TDF was safe, it was unable to assess effectiveness due to the small number of HIV infections observed [60]. The use of oral TDF in preventing HIV acquisition was also evaluated by the US CDC in 400 HIV-negative men who have sex with men (MSM) (CDC 4323) [61]. The preliminary analysis (July 2010) suggested no serious safety concerns and no increased risk compensation in men taking a study pill compared to those not taking prophylactic pills [62].
Topical TFV gel
The evaluation of expanded safety and effectiveness of topical tenofovir includes two completed studies and one ongoing study. The first trial, HPTN 059, which compared 1% TFV gel coitally-dependent use (up to twice daily) to daily vaginal use versus a placebo gel over 24 weeks in HIV-1 uninfected women, showed no difference in safety, acceptability and adherence. The gel was widely acceptable and 90% of the women reported that they would use the gel if reduced risk of HIV infection [63, 64] .
The CAPRISA 004 study, a phase 2b study compared 1% TFV gel vs placebo (applied intra-vaginally by women, up to 12 hours before sex and as soon as possible within 12 hours after sex but not more than twice in 24 hours-termed BAT 24) in 889 South African women aged 18 – 40 years. It was the first microbicide effectiveness trial to demonstrate proof- of-concept that a gel containing an antiretroviral agent can protect women from acquiring HIV. The trial showed that TFV gel use reduced HIV infection in women by 39% overall [15]. The reduction in HIV risk reached 54% in women who used the gel consistently (> 80% of sex acts were covered by gel). No tenofovir related resistance mutations were detected and AE rates were similar in the two study arms. The trial also showed that TFV gel reduced the risk of HSV-2 infection by 51% [65].
Combination of oral TDF tablets and topical TFV gel
An adherence and PK study has also been completed. The MTN 001 study randomized 144 sexually active HIV uninfected women at four US and three African sites to a sequence of oral TDF, topical TFV gel, and both formulations daily for 6 weeks, with a one week, no drug, washout period between sequences. All 3 regimens were well tolerated and acceptable, with high self-reported adherence. Of note, daily use of the vaginal gel achieved a more than 100 fold higher concentration of active drug in vaginal tissue compared to the oral tablet but compared to the gel, the tablet used daily was associated with a 20 fold higher active drug concentration in blood. Women in the US preferred the tablets over the gel, while African women showed a preference for the gel [66, 67].
Currently in the field, the MTN 003 (VOICE) trial is examining the safety and effectiveness of two oral antiretroviral agents (TDF and FTC-TDF) taken daily and 1% TFV gel also administered daily to reduce the risk of HIV acquisition in women. The study enrolled 5,029 African women (approximately 1,000 in each study group) [68]. On 16 September 2011, the TDF tablet component of the VOICE study was discontinued after interim results showed that it was no better than placebo in preventing HIV in the study women. Two months later, on 17 November 2011, a scheduled review of the VOICE study's data by the independent Data Safety and Monitoring Board (DSMB) revealed that the incidence rate of HIV infection in the women assigned to daily TFV gel was 6.0% compared to 6.1% in women assigned to placebo gel. The FTC-TDF tablet arm is continuing to study completion. The DSMB found no safety concerns with any of the products used in the study [69-71]. Final results are expected in late 2012.
5.3 Phase 3 studies
Three phase three trials, two examining TDF tablets and one evaluating TFV gel are currently underway. The Partners PrEP trial, which was initiated in serodiscordant couples in May 2008, is investigating whether once daily PrEP (TDF or FTC-TDF) taken by an HIV uninfected person can reduce their risk of acquiring HIV from their infected partner [72]. In July 2011, an interim analysis indicated a strong HIV prevention effect and the placebo arm was discontinued. The preliminary data showed that HIV was reduced by 62% in the TDF arm and by 73% in the FTC/TDF arm when compared to placebo [12, 73]. The trial is continuing and will provide additional safety and effectiveness data in 2012 or 2013. Another ongoing trial, the Bangkok tenofovir study (CDC 4370), is assessing the safety and efficacy of daily oral TDF to prevent parenteral HIV infection among injection drug users in Thailand [74, 75], with results expected in 2012.
The FACTS 001 (Follow on consortium on tenofovir studies) study is evaluating the safety and effectiveness of 1% TFV gel in preventing HIV and HSV-2 infection in young South African women [76]. The study enrolled their first participant on 21 October 2011 and results are expected in 2014. This important study will contribute additional data to the CAPRISA 004 results and is critical for potential licensure of 1% TFV gel for HIV prevention in women.
5.4 Observational / Exploratory Studies
Several small observational and exploratory studies are underway to provide additional information on tenofovir PrEP. MTN 003B is a sub-study of the MTN 003 trial monitoring changes in bone mineral density after one year among MTN 003 participants receiving oral TDF and FTC-TDF compared with oral placebo [77]. MTN 003C will explore how community and household factors affect adherence in VOICE participants [78]. MTN 015 is comparing the plasma HIV-1 RNA level twelve months after HIV-1 seroconversion among ART naive participants assigned to an active study product compared to control participants [79]. MTN 016 is evaluating the prevalence of spontaneous loss in mothers and congenital abnormalities in infants of mothers exposed to an active study agent during pregnancy as compared to that in mothers not exposed to an active study agent during pregnancy [80].
CAPRISA 009, an open label RCT, will assess the impact of prophylactic exposure to TFV gel on the therapeutic effect of subsequent TFV-containing ART on viral suppression [81].
Together these studies will provide important information to supplement primary outcome date required for product licensure.
5.5 HIV prevention trials investigating the FTC-TDF combination tablet
Although the scope of this review is limited to the tenofovir compound we provide here a brief summary of trial outcomes when oral TDF was tested in combination with FTC.
In November 2010, the Pre-exposure Prophylaxis Initiative (iPrEX) trial provided the first evidence that oral antiretroviral drugs can effectively prevent HIV acquisition in men who have sex with men (MSM). The trial was conducted Brazil, Peru, Ecuador, Thailand and South Africa among 2,499 men or transgender women who have sex with men, showed that the daily oral combined FTC-TDF, reduced HIV incidence by 44% (95% CI 15;63) (4). During 3,324 person-years of follow-up, there were 100 HIV infections; 36 in the FTC-TDF group and 64 in the placebo group[13]. Further, evidence for the effectiveness of daily oral pre-exposure prophylaxis (PrEP) in heterosexual men (and women) comes from results of two studies presented at the 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention in Rome in July 2011.
The PartnersPrEP trial [82], involving 4,758 HIV discordant couples from Kenya and Uganda, found that daily oral TDF and FTC-TDF reduced HIV incidence by 62% (95% CI 34;78) and 73% (95% CI 49;85) respectively. The Bostwana TDF2 trial [83], conducted in 1,200 heterosexual men and women from the general population, found that daily oral FTC-TDF reduced HIV incidence by 63% (95% CI 21;48).
In contrast, the FEM-PREP study, having enrolled 1952 women to assess the effectiveness of FTC-TDF tablets in preventing HIV infection was terminated early by its DSMB for futility. A total of 56 new HIV infections had occurred, with an equal number of infections in those participants assigned to FTC-TDF and those assigned to a placebo pill [84].
6. Regulatory pathway for licensure
At present no antiretrovirals are licensed for prevention of sexual transmission of HIV as an indication. In order for candidate agents to be considered for registration, two independent well conducted trials showing efficacy are generally needed. At a WHO consultation in 2010, the US Food and Drug Administration (FDA) has agreed to review the TFV gel licensing submission under a “fast track” designation, which also allows for a rolling submission of relevant data that can be reviewed by the regulatory authority as the data becomes available [85]. The FDA had also agreed to consider a combination of CAPRISA 004 and the VOICE trials for licensure of tenofovir gel, despite their differences in dosing. This plan is no longer applicable as the VOICE trial's daily dosing of tenfovir gel did not demonstrated protection against HIV. For both the oral TDF and TFV gel, positive results from confirmatory studies are necessary in order to establish expanded safety and efficacy data that is required for licensure. In addition, safety data in generally excluded populations such as adolescents, pregnant women and Hepatitis B positive individuals would be required by regulators to effectively complete the drug dossier.
7. Conclusion
Tenofovir exhibits distinct biological and pharmacologic properties that make this agent an ideal candidate for HIV prevention. Extensive and comprehensive testing of tenofovir in animal models has demonstrated efficacy in this model for advancement in humans for HIV prevention. Both the topical and systemic forms of tenofovir have been shown in phase 2, 2b and 3 studies to be protective against HIV infection by sexual transmission, with a tolerable side effect profile and with adequate monitoring, demonstrated low risk for the development of tenofovir resistance. However research, particularly in women, has demonstrated apparently conflicting results in two important studies which may potentially hamper progress in finding a women controlled prevention. Once positive confirmatory results from similarly conducted, comparable effectiveness trials become available, tenofovir in its gel and/or oral form should be advanced for licensure for HIV prevention.
8. Expert opinion
In the last 18 months, there has been a sea-change in HIV prevention. A series of studies since July 2010, have generated a confluence of new evidence that has brought new hope that antiretrovirals can potentially change the course of the HIV epidemic, when used as early treatment for prevention [86], as topical [15] or oral pre-exposure prophylaxis[72]. Tenofovir has been central to this achievement; every HIV prophylaxis trial tested tenofovir, either alone or in combination with FTC. Tenofovir is the first choice antiretroviral for HIV prophylaxis because of its safety profile, efficacy in suppressing viral replication, long half-life, rapid absorption and evidence of efficacy in animal challenge studies, Tenofovir has reinvigorated the HIV prevention field and created new found optimism that it may be possible to control the HIV epidemic.
For women who are unable to convince their male partners to be faithful or use condoms, tenofovir represents a completely new approach to HIV prevention. Tenofovir, either as tablets or gel, is the first HIV prevention technology that women can genuinely control themselves and enables women to take charge of their HIV risk. In South Africa alone, this new prevention technology could avert an estimated 1.3 million new HIV infections and 800,000 AIDS deaths over the next 20 years[87]. Implemented on a broader scale, Tenofovir, used as prophylaxis, could save millions of lives.
In addition to its effect on HIV acquisition, the gel formulation of tenofovir also reduced genital herpes infections by 51%, an effect not found with oral TDF, since the oral drug does not achieve the high drug concentrations observed at the site of HIV exposure in the genital tract observed with topical administration [47, 88]. A recent study confirmed the mechanism of action of high doses of tenofovir, against HSV-2 and provided further evidence from tissue culture and animal models for this effect [88]. Genital herpes infections are the most important global cause of genital ulcer disease with up to a quarter of sexually active adults estimated to be infected with HSV-2. Women who have genital herpes are also significantly more likely to acquire HIV than those who do not. Besides general safe sex practices, there is no known prevention or cure for genital herpes infection, thereby making TFV gel an important breakthrough against HSV-2 infection.
However, this new hope can only be realised when there is adequate robust evidence of efficacy of oral and topical tenofovir to achieve licensure by a medicines regulator, such as the FDA. As at the end of 2011, the available evidence that tenofovir is efficacious is strong. Data from cell culture, tissue explants, mice and monkeys demonstrate consistent results that tenofovir is efficacious in preventing HIV or SIV. Human efficacy trials confirm these pre-clinical observations; tenofovir gel and oral tenofovir, either singly or in combination with FTC have demonstrated effectiveness in humans. However, two trials conducted among women have produced apparently conflicting results. It is unclear at this time as to whether adherence or some other reason is responsible for the differences in the outcomes from these studies. A detailed analysis of the study data from these two trials, anticipated in late 2012, will be critical to understanding the reasons for these perplexing results.
Based on the CAPRISA 004 results on coital dosing of tenofovir gel, there was high hope that the VOICE study (MTN003) of daily TFV gel would show similar or better results. Instead, the VOICE trial did not demonstrate that tenfovoir gel and TDF tablets were effective in preventing HIV. As highlighted in previous microbicide studies, to be able to demonstrate effectiveness of a microbicide gel, women have to consistently use the right amount of the right drug to get the right drug levels in the right cells at the right time. At present, it is unclear whether the VOICE study's unexpected outcome could be due to inadequate or non-use of the products by women in the study, to insufficient drug levels in the genital tract of the women at the time of HIV exposure during sex, or to some other reason. Indeed there have been opinions expressed prior to the results, that non-coitally dependent, daily use of a product may be tiresome, lead to poor adherence and possibly product failure [89]. The FACTS 001 study, which is currently underway, is designed to confirm the CAPRISA 004 trial's coital use of tenofovir gel in preventing HIV and HSV-2 in order to generate the data needed for licensure.
One of the most crucial challenges in HIV prevention in Africa is reducing the high infection rates among young women. Young women in Africa bear the brunt of the HIV epidemic, with HIV rates up to 8 higher than the rates in their male counterparts. For women unable to negotiate mutual faithfulness and/or consistent condom use with their male partners, microbicides are a critically important technology. The need for a woman-controlled HIV prevention technology remains urgent.
The PartnersPrEP and TDF2 trials, which have not been published yet, produced the first evidence that antiretroviral tablets can also prevent HIV in serodiscordant couples and the general heterosexual population. In women, however, additional recent data on oral PrEP have not demonstrated consistent results. Two trials – FEMPrEP [84] and VOICE [90] – found no protection against HIV in women using daily oral FTC-TDF and TDF respectively. These trials are currently being analysed to help determine the reasons the results. Until these data are available, it is unclear whether the mixed results from oral PrEP studies in women is due to differing adherence (behavior) or due to differing efficacy (biology).
In several countries throughout the world, MSM constitute a major sub-group where new HIV infections are taking place, especially in concentrated epidemics. Traditional safe sex messages have only had limited impact on the HIV epidemic in this group. The iPrEX trial produced the first evidence that antiretroviral tablets can prevent HIV in MSM. This introduces a new approach which could be used in combination with condom promotion and educational safe sex programs to prevent HIV in this MSM. Since FTC-TDF, known by its trade name - Truvada®, is already a licensed antiretroviral drug for AIDS treatment, it is already being prescribed off-label for HIV prevention in MSM. Based on the results of the iPrEX study, the Centers for Disease Control and Prevention and other US Public Health Service agencies have issued guidance on the use of PrEP among MSM in the United States as part of a comprehensive set of HIV prevention services [91]. However, concerns about adherence, drug resistance[92] and behavioural disinhibition have hampered the widespread roll out of FTC-TDF for HIV prevention among MSM. Within the iPrEX trial, tolerability to FTC-TDF was not compromised, side effects were minimal and generally well tolerated, no behavioural disinhibition was observed, TDF resistance was not reported and FTC resistance was only reported in patients who were already infected with HIV at entry into the trial.
The new findings on the effectiveness of tenofovir in preventing HIV, despite some conflicting data from the FEM-PrEP and VOICE trials, have reinvigorated the HIV prevention field and created new found optimism that it may be possible to impact the spread of HIV in the general heterosexual population through implementation in high risk sub-groups in generalized epidemics. Large-scale implementation, post-licensure, will require vigilance and surveillance to monitor behaviour changes, adverse events, adherence levels, drug resistance patterns, and the effect of drug resistance on later AIDS treatment.
9. Drug summary box.
Acknowledgments
The authors wish to acknowledge Lise Werner for graphical assistance with Figure 2. CAPRISA is supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grant no. AI51794). The authors were investigators in the CAPRISA 004 tenofovir gel trial, which was supported by the United States Agency for International Development (USAID), Family Health International (FHI) [co operative agreement # GPO-A-00-05-00022-00, contract # 132119], and LIFElab, a biotechnology centre of the South African Department of Science & Technology. Professor Salim S Abdool Karim is the Principal Investigator of the TRAPS (Tenofovir gel Research for AIDS Prevention Science) Program, which is funded by CONRAD, Eastern Virginia Medical School [cooperative grant #GP00-08-00005-00, subproject agreement # PPA-09-046] with the United States Agency for International Development (USAID); an Executive Committee Member of the NIH-Funded Microbicide Trials Network, which is undertaking the VOICE trial of oral and topical PrEP; and is a co-inventor of two pending patents (61/354.050 and 61/357,892) of tenofovir gel against HSV-1 and HSV-2 with scientists from Gilead Sciences. The Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP grant # D43TW00231) has supported Ayesha BM Kharsany and Tanuja N Gengiah's professional development. The views expressed by the authors do not necessarily reflect the views of NIH, USAID, Eastern Virginia Medical School, CONRAD or Gilead Sciences.
References
(*of interest, **of considerable interest)
- 1.Buchbinder SP. HIV epidemiology and breakthroughs in prevention 30 years into the AIDS epidemic. Top Antivir Med. 2011 May-Jun;19(2):38–46. [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS . UNAIDS World AIDS Day Report 2011. Joint United Nations Programme on HIV/AIDS.; Geneva: 2011. [24 November 2011]. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf. [Google Scholar]
- 3.Leclerc-Madlala S. Age-disparate and intergenerational sex in southern Africa: the dynamics of hypervulnerability. AIDS. 2008 Dec;22(Suppl 4):S17–25. doi: 10.1097/01.aids.0000341774.86500.53. [DOI] [PubMed] [Google Scholar]
- 4.Karim QA, Kharsany AB, Frohlich JA, et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials. 2011;12:67. doi: 10.1186/1745-6215-12-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5**.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999 Sep 4;354(9181):795–802. doi: 10.1016/S0140-6736(99)80008-7. (This seminal study demonstrated the potential for affordable single dose nevirapine to prevent mother to child transmission of HIV developing countries)
- 6**.de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011 Mar;11(3):171–80. doi: 10.1016/S1473-3099(10)70288-7. (This study indicated that triple antiretroviral prophylaxis during pregnancy and breastfeeding is safe and reduces the risk of HIV transmission to infants)
- 7.Young TN, Arens FJ, Kennedy GE, et al. Antiretroviral post-exposure prophylaxis (PEP) for occupational HIV exposure. Cochrane Database Syst Rev. 2007;(1):CD002835. doi: 10.1002/14651858.CD002835.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padian NS, McCoy SI, Karim SS, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011 Jul 16;378(9787):269–78. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly CG, Shattock RJ. Specific Microbicides in the Prevention of HIV Infection. J Intern Med. 2011 Sep 14;270(6):509–19. doi: 10.1111/j.1365-2796.2011.02454.x. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq E, Sakuma T, Baba M, et al. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987 Dec;8(5-6):261–72. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 11.Bronson JJ, Kim CU, Ghazzouli I, Hitchcock MJM, Kern ER, Martin JC. Synthesis and antiviral activity of phosphonylmethoxyethyl derivatives of purine and pyrimidine bases. In: Martin JC, editor. ACS Symposium Series 401: Nucleosite Analogues as Antiviral Agents. American Chemical Society; Washington, DC: 1989. pp. 72–87. [Google Scholar]
- 12.Partners PrEP Study [2011 15 November];Press Release: Pivotal Study finds that HIV Medications are Highly Effective as Prophylaxis Against HIV Infection in Men and Women in Africa. 2011 Available from: http://depts.washington.edu/astda/resources/PrEP_PressRelease-UW_13Jul2011.pdf.
- 13**.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010 Dec 30;363(27):2587–99. doi: 10.1056/NEJMoa1011205. (This important rabdomised controlled trial demonstrated that daily dosing with oral FTC-TDF provided protection against HIV infection in men who have sex with men)
- 14**.Thigpen MKP, Smith D, Segolodi T, Soud F, Chillag K, Chirwa L, Kasonde M, Mutanhaurwa R, Henderson F, Pathak S, Gvetadze R, Rose C, Paxton L. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study. IAS; Rome: 2011. 2011. (This randomised controlled trial demonstrated that that FTC-TDF was safe and effective in preventing HIV infection in a heterosexual population)
- 15**.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–74. doi: 10.1126/science.1193748. (This study provided the first clinical trial evidence that the antiretroviral agent, tenofovir, formulated as a topical microbicide and used before and after sex can prevent sexual transmission of HIV in women)
- 16**.De Clercq E, Descamps J, De Somer P, Holy A. (S)-9-(2,3-Dihydroxypropyl)adenine: an aliphatic nucleoside analog with broad-spectrum antiviral activity. Science. 1978 May 5;200(4341):563–65. (This study provided the first description of DHPA demonstrating antiviral activity)
- 17.De Clercq E, Holy A, Rosenberg I, et al. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986 Oct 2-8;323(6087):464–7. doi: 10.1038/323464a0. (This study provided the first description of DHPA as an antiretroviral agent.)
- 18.De Clercq E. The acyclic nucleoside phosphonates from inception to clinical use: historical perspective. Antiviral research. 2007 Jul;75(1):1–13. doi: 10.1016/j.antiviral.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. J Med Virol. 1993;(Suppl 1):2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- 20.Elion GB, Furman PA, Fyfe JA, et al. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–20. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Votruba I, Holy A, De Clercq E. Metabolism of the broad-spectrum antiviral agent, 9-S)-(2,3-dihydroxypropyl) adenine, in different cell lines. Acta Virol. 1983 May;27(3):273–6. [PubMed] [Google Scholar]
- 22.De Clercq E. Acyclic nucleoside phosphonates: past, present and future. Bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochemical pharmacology. 2007 Apr 1;73(7):911–22. doi: 10.1016/j.bcp.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 23*.De Clercq E, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nature reviews. 2005 Nov;4(11):928–40. doi: 10.1038/nrd1877. (This paper reviews the development, mechanism of action, spectrum of activity and future potential of acyclic nucleosides phosphonates)
- 24.Balzarini J, Holy A, Jindrich J, et al. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrobial agents and chemotherapy. 1993 Feb;37(2):332–8. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balzarini J, Aquaro S, Perno CF, et al. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem Biophys Res Commun. 1996 Feb 15;219(2):337–41. doi: 10.1006/bbrc.1996.0234. [DOI] [PubMed] [Google Scholar]
- 26.Maudgal PC, De Clercq E, Huyghe P. Efficacy of (S)-HPMPA against thymidine kinase-deficient herpes simplex virus-keratitis. Investigative ophthalmology & visual science. 1987 Feb;28(2):243–8. [PubMed] [Google Scholar]
- 27**.Tsai CC, Follis KE, Sabo A, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995 Nov 17;270(5239):1197–9. doi: 10.1126/science.270.5239.1197. (First study to demonstrate the potential of tenofovir to prevent SIV infection)
- 28**.Van Rompay KK, Berardi CJ, Aguirre NL, et al. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS. 1998 Jun 18;12(9):F79–83. doi: 10.1097/00002030-199809000-00001. (This study provided evidence that 2 doses of tenofovir; one used before and used one after, can prevent SIV)
- 29.Van Rompay KK, Miller MD, Marthas ML, et al. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J Virol. 2000 Feb;74(4):1767–74. doi: 10.1128/jvi.74.4.1767-1774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30**.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998 May;72(5):4265–73. doi: 10.1128/jvi.72.5.4265-4273.1998. (This study demonstrated that the timing and duration of tenofovir treatment is important in the prevention of SIV infection)
- 31.Otten RA, Smith DK, Adams DR, et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol. 2000 Oct;74(20):9771–5. doi: 10.1128/jvi.74.20.9771-9775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CONRAD . Investigators Brochure:Tenofovir Gel (GS-1278) CONRAD; Arlington, Virgina: Nov 12, 2010. 2010. [Google Scholar]
- 33**.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal SHIV exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol. 2009;83(20):10358–65. doi: 10.1128/JVI.01073-09. (This study shows that a single dose of tenofovir gel, 30 minutes prior to viral exposure, was protective)
- 34.Miller C, Rosenberg Z, Bischofberger N. Use of topical PMPA to prevent vaginal transmission of SIV.. 9th International Conference on Antiviral Research; Japan. 1996.1996. [Google Scholar]
- 35**.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal SHIV infection in macaques by tenofovir gel and its relationship to tissue drug levels. J Virol. 2011 Nov 9; doi: 10.1128/JVI.05842-11. (This study in macaques demonstrates that tenofovir gel provides durable protection from HIV challenge up to 3 days after application and that TFV-DP in vaginal lymphocytes is a good predictor of efficacy)
- 36.Shattock M. Protection of macaques against rectal SIV challenge by mucosally-applied PMPA.. Abstract OA15. Microbicides 2006.; Cape Town, South Africa. 2006. [Google Scholar]
- 37.Van Rompay KK, Schmidt KA, Lawson JR, et al. Topical administration of low-dose tenofovir disoproxil fumarate to protect infant macaques against multiple oral exposures of low doses of simian immunodeficiency virus. J Infect Dis. 2002 Nov 15;186(10):1508–13. doi: 10.1086/344360. [DOI] [PubMed] [Google Scholar]
- 38.Van Rompay KK, Kearney BP, Sexton JJ, et al. Evaluation of oral tenofovir disoproxil fumarate and topical tenofovir GS-7340 to protect infant macaques against repeated oral challenges with virulent simian immunodeficiency virus. J Acquir Immune Defic Syndr. 2006 Sep;43(1):6–14. doi: 10.1097/01.qai.0000224972.60339.7c. [DOI] [PubMed] [Google Scholar]
- 39.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006 Oct 1;194(7):904–11. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 40**.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS medicine. 2008 Feb;5(2):e28. doi: 10.1371/journal.pmed.0050028. (This study shows that short but potent intermittent PrEP can provide protection comparable to that of daily PrEP).
- 41.Gilead Sciences Inc. Full prescribing information: Viread®. 2011 Sep; [cited; Available from: http://rsc.tech-res.com/safetyandpharmacovigilance/PIList.aspx.
- 42.Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet. 2004;43(9):595–612. doi: 10.2165/00003088-200443090-00003. [DOI] [PubMed] [Google Scholar]
- 43**.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007 Sep 12;21(14):1899–907. doi: 10.1097/QAD.0b013e328270385a. (This was the first study to evaluate antiretroviral drug exposure in the female genital tract after oral dosing)
- 44**.Patterson KBPH, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, Cohen MS, Kashuba ADM. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112re4) doi: 10.1126/scitranslmed.3003174. (This study describes vaginal and rectal tissue penetration of FTC and TDF following oral dosing of the drug combination)
- 45.Schwartz JL, Rountree W, Kashuba AD, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6(10):e25974. doi: 10.1371/journal.pone.0025974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46*.Hendrix CMA, Guddera V, Riddler S, Salata R, Nakabiito C, Hoesley C, Justman J, Soto-Torres L, Richardson B. MTN-001: A Phase 2 Cross-over Study of Daily Oral and Vaginal TFV in Healthy, Sexually Active Women Results in Significantly Different Product Acceptability and Vaginal Tissue Drug Concentrations.. Abstract#: 35LB. 18th Conference on Opportunistic Infections and Retroviruses; Boston, MA, USA. 2011 Feb 27-March 22, 2011; 2011. (This pharmacokinetic study showed that vaginal tissue concentrations are significantly higher after vaginal dosing compared to oral dosing)
- 47**.Abdool Karim SS, Kashuba A, Werner L, Abdool Karim Q. Drug concentrations following topical and oral antiretroviral pre-exposure prophylaxis: Implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. (This study provides insights into the potential threshold tenofovir concentrations needed to prevent HIV infection in women)
- 48**.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006 Feb 28;20(4):543–51. doi: 10.1097/01.aids.0000210608.70762.c3. (This study was the first phase 1 safety and tolerability study of tenofovir gel)
- 49.Rosen RK, Morrow KM, Carballo-Dieguez A, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008 Apr;17(3):383–92. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz JL, Poindexter A, Wheeless A, et al. Safety evaluation of 1% tenofovir gel in healthy men. Int J STD AIDS. 2009 Jun;20(6):384–6. doi: 10.1258/ijsa.2008.008309. [DOI] [PubMed] [Google Scholar]
- 51.ClinicalTrials.gov [22 September 2011];A04-095: Pharmacokinetic Study of the Vaginal Microbicide Agent 1% Tenofovir Gel. 2007 Available from: http://clinicaltrials.gov/ct2/show/NCT00561496.
- 52**.Schwartz JL, Rountree W, Kashuba AD, et al. A multi-compartment, single and multiple dose pharmacokinetic study of the vaginal candidate microbicide 1% tenofovir gel. PLoS One. 2011;6(10):e25974. doi: 10.1371/journal.pone.0025974. (This PK study assessed TFV exposure in the blood plasma and genital tract following single and mutliple doses of the gel - high genital tract concentrations and minimal systemic concentrations were observed.)
- 53.Keller MJ, Madan RP, Torres NM, et al. A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One. 2011;6(1):e16475. doi: 10.1371/journal.pone.0016475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MicrobicideTrialsNetwork [14 November 2011];MTN 002: Phase I Study of the Maternal Single-Dose Pharmacokinetics and Placental Transfer of Tenofovir 1% Vaginal Gel among Healthy Term Gravidas. 2011 Available from: http://www.mtnstopshiv.org/news/studies/mtn002.
- 55.Microbicide Trials Network Press Release: Researchers report results of first microbicide trial in pregnant women. [15 November 2011];Study indicates small amount of drug passes to fetus. Available from: http://www.mtnstopshiv.org/node/1846.
- 56.MicrobicideTrialsNetwork [11 November 2011];MTN 007: A Phase 1 Randomized, Double-Blinded, Placebo-Controlled Rectal Safety and Acceptability Study of Tenofovir 1% Gel. 2011 Available from: http://www.mtnstopshiv.org/node/912.
- 57.MicrobicideTrialsNetwork [10 November 2011];MTN 008: Expanded Safety of Tenofovir 1% Gel in Pregnancy and Lactation. 2011 Available from: http://www.mtnstopshiv.org/node/1637.
- 58.ClinicalTrials.gov [22 September 2011];A10-113: Pharmacokinetic and Pharmacodynamic Study of Tenofovir 1% Gel. 2011 Available from: http://clinicaltrials.gov/ct2/show/NCT01369303?term=A10-113&rank=1.
- 59.Microbicide Trials Network [14 November 2011];MTN 006: Phase 1 rectal microbicide safety and acceptability trial of topically applied tenofovir compared with oral tablet. Available from: http://www.mtnstopshiv.org/node/911.
- 60*.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. (This is the first study to assess safety of oral PrEP in women)
- 61.ClinicalTrials.gov [15 November 2011];CDC 4323: Extended Safety Study of Tenofovir Disoproxil Fumarate (TDF) Among HIV-1 Negative Men. Available from: http://clinicaltrials.gov/ct2/show/NCT00131677?term=CDC+4323&rank=1.
- 62.Centers for Disease Control and Prevention [15 November 2011];Preliminary Results from First Safety Study of Daily Tenofovir for HIV Prevention Among MSM Find No Significant Concerns. 2010 Available from: http://www.cdc.gov/hiv/prep/resources/factsheets/extended_PrEB-safety-trial.htm.
- 63.Ramjee G, Doncel GF, Mehendale S, et al. Microbicides 2008 conference: from discovery to advocacy. AIDS Res Ther. 2008;5:19. doi: 10.1186/1742-6405-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hillier S,L. Safety and acceptability of coitally dependent use of 1% tenofovir over six months of use.. Abstract No. 655 Microbicides 2008; New Delhi, India. 2008. [Google Scholar]
- 65.Cates W., Jr After CAPRISA 004: time to re-evaluate the HIV lexicon. Lancet. 2010 Aug 14;376(9740):495–6. doi: 10.1016/S0140-6736(10)61200-7. [DOI] [PubMed] [Google Scholar]
- 66.Microbicide Trials Network [15 November 2011];MTN 001: Phase 2 Adherence and Pharmacokinetics Study of Oral and Vaginal Preparations of Tenofovir. Available from: http://www.mtnstopshiv.org/node/71.
- 67*.Hendrix CMA, Guddera V, Riddler S, Salata R, Nakabiito C, Hoesley C, Justman J, Soto-Torres L, Richardson B. MTN-001: A Phase 2 Cross-over Study of Daily Oral and Vaginal TFV in Healthy, Sexually Active Women Results in Significantly Different Product Acceptability and Vaginal Tissue Drug Concentrations. CROI; Boston: 2011. 2011. [Google Scholar]
- 68.MicrobicideTrialsNetwork [15 November 2011];MTN 003: Phase 2B Safety and Effectiveness Study of Tenofovir 1% Gel, Tenofovir Disoproxil Fumarate Tablet and Emtricitabine/Tenofovir Disoproxil Fumarate Tablet for the Prevention of HIV Infection in Women. 2011 Available from: http://www.mtnstopshiv.org/node/70.
- 69.Microbicide Trials Network [15 November 2011];Questions and Answers on Decision to Modify VOICE. Available from: http://www.mtnstopshiv.org/node/3622.
- 70.Microbicide Trials Network [25 November 2011];MTN Statement on Decision to Discontinue Use of Tenofovir Gel in VOICE, a Major HIV Prevention Study in Women. Available from: http://www.mtnstopshiv.org/node/3909.
- 71.National institute of allergy and infectious diseases [25 November 2011];NIH Discontinues Tenofovir Vaginal Gel in ‘VOICE’ HIV Prevention Study. 2011 Available from: http://www.niaid.nih.gov/news/newsreleases/2011/Pages/VOICEdiscontinued.aspx.
- 72.ClinicalTrials.gov [22 September 2011];PartnersPrEP: Pre-Exposure Prophylaxis to Prevent HIV-1 Acquisition Within HIV-1 Discordant Couples. Available from: http://clinicaltrials.gov/ct2/show/NCT00557245?term=PartnersPrEP&rank=1.
- 73.Buchbinder SP, Liu A. Pre-exposure prophylaxis and the promise of combination prevention approaches. AIDS Behav. 2011 Apr;15(Suppl 1):S72–9. doi: 10.1007/s10461-011-9894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov [2011 22 September];CDC 4370: Bangkok Tenofovir Study. Available from: http://clinicaltrials.gov/ct2/show/NCT00119106?term=cdc+4370&rank=1.
- 75.Martin M, Vanichseni S, Suntharasamai P, et al. Enrollment characteristics and risk behaviors of injection drug users participating in the Bangkok Tenofovir Study, Thailand. PLoS One. 2011;6(9):e25127. doi: 10.1371/journal.pone.0025127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.ClinicalTrials.gov [09 November 2011];FACTS 001: Safety and Effectiveness of Tenofovir Gel in the Prevention of Human Immunodeficiency Virus (HIV-1) Infection in Young Women and the Effects of Tenofovir Gel on the Incidence of Herpes Simplex Virus (HSV-2) Infection. Available from: http://clinicaltrials.gov/ct2/show/NCT01386294?term=facts+001&rank=1.
- 77.ClinicalTrials.gov [15 November 2011];MTN 003B: Bone Mineral Density Substudy - An Ancillary Study to MTN-003. Available from: http://clinicaltrials.gov/ct2/show/NCT00729573?term=mtn003b&rank=1.
- 78.Microbicide Trials Network [15 November 2011];MTN 003C: Household and Community Level Factors Associated with Study Product Adherence in VOICE: A Substudy of MTN-003. Available from: http://www.mtnstopshiv.org/node/1087.
- 79.ClinicalTrials.gov [15 November 2011];MTN 015: Observational Study of HIV Infected Women Previously Enrolled in Other Microbicide Trials. Available from: http://clinicaltrials.gov/ct2/show/NCT00514098?term=mtn015&rank=1.
- 80.ClinicalTrials.gov [15 November 2011];MTN 016: Evaluation of Maternal and Baby Outcome Registry After Chemoprophylactic Exposure (EMBRACE) Available from: http://clinicaltrials.gov/ct2/show/NCT01209754?term=mtn016&rank=1.
- 81.ClinicalTrials.gov [24 November 2011];Trial to Assess the Impact of PrEP to Tenofovir Gel on the Efficacy of Tenofovir-containing ART on Viral Suppression (TOAST) Available from: http://clinicaltrials.gov/ct2/show/NCT01387022?term=CAPRISA+009&rank=1.
- 82**.Partners PrEP study press release [13 July 2011];Pivotal study finds that HIV medications are highly effective as prophyalxis against HIV infection in men and women in Africa. 2011 Available from: http://depts.washington.edu/uwicrc/research/studies/files/PrEP_PressRelease-UW_13Jul2011.pdf. (This is the first randomised controlled trial to confirm the success of oral PrEP in serodiscordant couples)
- 83.Centers for Diseases Control and Prevention [13 July 2011];CDC Trial and another major study find PrEP can reduce risk of HIV infection among heterosexuals. CDC Press release. 2011 Available from: http://www.cdc.gov/nchhstp/newsroom/PrEPHeterosexuals.html.
- 84.FHI [18 April 2011];FHI Statement on the FEM-PrEP HIV Prevention Study. 2011 Available from: http://minilicious.wordpress.com/2011/04/18/fhi-statement-on-thefem-prep-hiv-prevention-study/
- 85.WHO/UNAIDS Next Steps with 1% Tenofovir Gel - Meeting Report 25-26 August 2010 Johannesburg; South Africa. 25 November 2010; 2010. [Google Scholar]
- 86**.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. (This landmark study indicated that early ART initiation dramatically reduced rates of sexual transmission of HIV)
- 87*.Williams BG, Abdool Karim SS, Gouws E, Abdool Karim Q. Potential impact of tenofovir gel on the HIV epidemic in South Africa. J Acquir Immune Defic Syndr. 2011;58:207–10. doi: 10.1097/QAI.0b013e3182253c19. (This paper estimates the potential impact that tenofovir gel could have on the HIV epidemic in South Africa by modeling data from the CAPRISA 004 trial)
- 88**.Andrei G, Lisco A, Vanpouille C, et al. Topical Tenofovir, a Microbicide Effective against HIV, Inhibits Herpes Simplex Virus-2 Replication. Cell Host & Microbe. 2011;10(4):379–89. doi: 10.1016/j.chom.2011.08.015. (This laboratory-based study confirmed the mechanism of action of high doses of tenofovir, which is achieved with tenofovir gel but not oral TDF, against HSV-2 and provided further evidence from tissue culture and animal models for this effect)
- 89*.Vermund SH, Van Damme L. HIV prevention in women: next steps. Science. 2011 Jan 21;331(6015):284. doi: 10.1126/science.331.6015.284-a. (This commentary provides interesting explanations for why daily dosing of tenofovir gel may result in poor adherence and may hamper advancement of this product)
- 90.Microbicide Trials Network [1 November 2011];Microbicide Trials Network Statement on Decision to Discontinue Use of Oral Tenofovir Tablets in VOICE, a Major HIV Prevention Study in Women. Available from: http://www.mtnstopshiv.org/node/3619.
- 91.Centers for Disease Control and Prevention Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011 Jan 28;60(3):65–8. [PubMed] [Google Scholar]
- 92.Hurt CB, Eron JJ, Jr., Cohen MS. Pre-Exposure Prophylaxis and Antiretroviral Resistance: HIV Prevention at a Cost? Clin Infect Dis. 2011 Dec;53(12):1265–70. doi: 10.1093/cid/cir684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Microbicide Trials Network [15 November 2011];MTN 003: Phase 2B Safety and Effectiveness Study of Tenofovir 1% Gel, Tenofovir Disoproxil Fumarate Tablet and Emtricitabine/Tenofovir Disoproxil Fumarate Tablet for the Prevention of HIV Infection in Women. Available from: http://www.mtnstopshiv.org/node/70.
- 94.Van Rompay KK, Marthas ML, Lifson JD, et al. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS research and human retroviruses. 1998 Jun 10;14(9):761–73. doi: 10.1089/aid.1998.14.761. [DOI] [PubMed] [Google Scholar]
- 95**.Van Rompay KK, McChesney MB, Aguirre NL, et al. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J Infect Dis. 2001 Aug 15;184(4):429–38. doi: 10.1086/322781. (This study showed that tenofovir (PMPA) can potentially protect human newborns against intrapartum HIV infection)
- 96.Miller C, Rosenberg Z, Bischofberger N. Use of topical PMPA to prevent vaginal transmission of SIV.. 9th International Conference on Antiviral Research.; Japan. 1996. [Google Scholar]
- 97**.Cranage M, Sharpe S, Herrera C, et al. Prevention of SIV Rectal Transmission and Priming of T Cell Responses in Macaques after Local Pre-exposure Application of Tenofovir Gel. PLoS Med. 2008 Aug 5;5(8):e157. doi: 10.1371/journal.pmed.0050157. (This is the first study to demonstrate that tenofovir gel used rectally can prevent SIV infections in monkeys)
- 98**.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008 Feb;5(2):e28. doi: 10.1371/journal.pmed.0050028. (This study shows that short but potent intermittent PrEP can provide protection comparable to that of daily PrEP)
- 99.Van Rompay KK, Singh RP, Heneine W, et al. Structured treatment interruptions with tenofovir monotherapy for simian immunodeficiency virus-infected newborn macaques. J Virol. 2006 Jul;80(13):6399–410. doi: 10.1128/JVI.02308-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdool Karim Q AKSobotCTG CAPRISA 004: effectiveness and safety of vaginal microbicide 1% tenofovir gel for prevention of HIV infection in women. Session TUSS05.. Program and abstracts of the 18th International AIDS Conference; Vienna, Austria. 2010 July 18-23; 2010. [Google Scholar]