Abstract
Rationale
Dependence can develop during chronic opioid use, and the emergence of withdrawal might promote drug taking.
Objective
This study examined how chronic morphine administration or withdrawal modified self administration of heroin or cocaine.
Methods
Four monkeys responded under a fixed ratio 10 schedule to receive i.v. infusions of heroin (0.56–560 µg/kg/infusion) or cocaine (1–100 µg/kg/infusion). Monkeys received morphine twice daily; the final dose was 10 mg/kg/12 h. Dose–effect curves for heroin or cocaine were determined in 150-min sessions throughout morphine administration and during temporary suspension when withdrawal signs were also monitored. Heroin dose–effect curves and withdrawal signs were determined daily following termination of morphine administration.
Results
Before monkeys received morphine, heroin, and cocaine maintained responding with unit doses of 1.78 µg/kg of heroin and 10 µg/kg/injection of cocaine resulting in, on average, 13.4 and 20.8 infusions, respectively. When monkeys received morphine daily, self administration of heroin and cocaine decreased to, on average, 3.1 and 11.3 infusions, respectively. Responding for heroin or cocaine recovered following temporary (17–53 h) suspension of morphine administration. The number of heroin infusions and total withdrawal signs increased when morphine administration was terminated. Withdrawal signs peaked 3–4 days after morphine; however, the number of infusions remained elevated for 8 weeks.
Conclusions
Changes in self administration responding did not precisely covary with signs of withdrawal and responding for small doses of heroin persisted long after discontinuation of morphine, suggesting that non-pharmacologic (e.g., conditioned reinforcing) effects might contribute to the maintenance of lever pressing under these conditions.
Keywords: Morphine, Heroin, Cocaine, Self administration, Withdrawal, Rhesus monkeys
Opioid abuse continues to be a significant public health problem, and the factors that contribute to the initiation and maintenance of opioid abuse are not well established. One hypothesis has been that heroin abuse increases during withdrawal as dependent users try to reduce or avoid withdrawal signs (Koob et al. 1989; Solomon and Corbit 1974), and this hypothesis is supported by studies in nonhumans. For example, in heroin-dependent rhesus monkeys responding under a choice procedure for either food or heroin, discontinuation of daily heroin administration concurrently increased withdrawal scores and choice of heroin over food (Negus 2006; Negus and Rice (2009). In morphine-dependent rats, both withdrawal signs and responding for the μ-opioid receptor agonist remifentanil increased when morphine administration was discontinued for 24 h (Cooper et al. 2008). In another study, heroin intake increased following removal of morphine pellets in rats; however, withdrawal signs were not observed under these conditions, suggesting that this dosing procedure did not induce robust dependence or possibly that even very subtle withdrawal might contribute to changes in responding (Walker et al. 2003). Thus, opioid self administration appears to increase during withdrawal; however, comparisons between the time course of withdrawal signs and heroin self administration have not been examined fully.
In addition to increased heroin abuse during withdrawal, abuse of drugs other than opioids, particularly cocaine, might also increase during opioid withdrawal. Cocaine abuse occurs in opioid dependence, with up to 44% of patients who are participating in methadone-maintenance programs abusing cocaine (Longshore et al. 2005; Peles et al. 2006; Strain et al. 1996). Moreover, patients who used cocaine before or after entering treatment for heroin abuse were significantly more likely to relapse, as compared to patients who did not use cocaine (Gossop et al. 2002). Opioid abusers also report using cocaine to postpone the emergence of withdrawal (Rosen et al. 1992) or to reduce the severity of withdrawal (Kosten et al. 1989).
In the current study, morphine was given in increasing doses to produce physiological dependence. Morphine administration was temporarily suspended for periods of 17 to 53 h. The effects of both administration and short-term withdrawal of morphine were observed on heroin and cocaine self-administration. Finally, morphine administration terminated; withdrawal signs were monitored before and after heroin self-administration sessions to determine whether responding for heroin changed during withdrawal and whether heroin received during sessions reversed withdrawal signs. The purpose of the study was to examine the impact of morphine administration and withdrawal on the reinforcing effects of heroin and cocaine and to determine whether any long-lasting changes in heroin self administration were observed over a 10-week period following termination of morphine administration.
Methods
Subjects
Four adult rhesus monkeys were housed individually with unlimited access to water. The three male monkeys (subjects OS, IG, GR) weighed between 8.3 and 10.4 kg and the female monkey (subject MA) weighed 4.5 kg at the beginning of the experiment; their weights were maintained at 90% of their free-feeding weight by post-session feeding with monkey chow (Harlan Teklad High Protein Monkey Diet, Madison, WI), fresh fruit and peanuts. Lights in the colony room were illuminated daily for 14 h beginning at 0600 h. Prior to these studies, each monkey received other drugs under a variety of conditions (e.g., Gerak et al. 2008; Gerak and France 1999). Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and guidelines of the Committee on Care and Use of Laboratory Animal Resources, National Research Council [Department of Health, Education and Welfare, publication No. (NIH) 85-23, revised 1996].
Surgery
Following induction of anesthesia with 10 mg/kg of ketamine (i.m., Fort Dodge Laboratories, Fort Dodge, IA), catheters were implanted under halothane anesthesia with ventilation sustained by the delivery of oxygen at a flow rate of 2 l/min. A polyurethane catheter (SIMS Deltec Inc., St. Paul, MN) was implanted in the jugular or femoral vein and exteriorized through the skin (OS) or connected to s.c. vascular access ports (Access Technologies, Skokie, IL; IG, MA, GR) according to methods described elsewhere (Wojnicki et al. 1994). OS wore a jacket (Lomir Biomedical Inc., Malone, NY) in which the exteriorized catheter was stored.
Apparatus
During experimental sessions, monkeys were seated in chairs (Primate Products, Redwood City, CA) and placed in sound attenuating, ventilated chambers. Response panels located in each chamber contained response levers, pellet dispensers, food cups, and stimulus lights that could be illuminated red or green. Catheters were connected to 60-cm extension sets (Abbot Laboratories, Stone Mountain, GA) with 18-g needles (exteriorized catheter) or 20-g Huber-point needles (vascular ports) and the opposing end of the extension set was connected to a 30-ml syringe that was mounted in a syringe driver (Razel Scientific Instruments, Inc., Stamford, CT) located outside the chambers. An interface (Med Associates, Inc., East Fairfield, VT) connected response panels to a computer which controlled experimental events and recorded data.
Procedure
During experimental sessions, monkeys responded under a fixed-ratio 10 schedule in order to receive an infusion of cocaine, heroin, or saline. Sessions were divided into five discrete 30-min cycles. Each cycle began with a 5-min timeout period during which lights were not illuminated and responding had no programmed consequence. The end of timeout periods and the beginning of response periods were signaled by delivery of a priming infusion of the solution available for self administration. Immediately thereafter, the green stimulus light located above the right lever was illuminated; in the presence of green light, ten responses on the right lever simultaneously extinguished the green light, illuminated the red light located above the right lever, and began the infusion. The red light remained illuminated for 2 s. Responses on the left lever reset the fixed-ratio requirement on the right lever. The infusion was followed by a 30-s timeout period. Response periods ended after 25 min.
The solution that was available for self administration varied across sessions. When either heroin or cocaine was available, dose–effect curves were determined within a single session by increasing the dose available during each cycle; this type of procedure has proven to be an efficient method for determining dose–effect curves (Winger et al. 1989). The unit dose was increased across cycles in 1/2 log unit increments by increasing the duration of the infusion available for each cycle, which ranged from 2 s for the smallest unit dose to 300 s for the largest unit dose. Before chronic morphine administration, the doses of heroin were 0.056–5.6 µg/kg/infusion for OS and 0.56–56 µg/kg/infusion for IG, MA, and GR; the doses of cocaine were 1–100 µg/kg/infusion for all monkeys. These doses of heroin and cocaine have been shown to maintain responding under similar conditions in monkeys (Gerak et al. 2008; Winger and Woods 2001). Doses were always available in ascending order. Generally, sessions during which the drug was available were separated by sessions during which only saline was available for each of the five cycles.
Monkeys received morphine chronically, beginning with injections of 3.2 mg/kg/12 h. One injection was administered 5 h before sessions. Although these initial dosing conditions were not expected to result in the emergence of robust withdrawal when morphine administration was interrupted, these conditions were selected because they were not expected to have adverse consequences and because they generate modest dependence in monkeys (McMahon et al. 2004). Thus, morphine could be safely administered under these conditions with more robust dependence established by increasing the dose slowly. After monkeys received 3.2 mg/kg/12 h of morphine for at least 7 days, the dose administered 5 h before sessions was increased to 5.6 mg/kg with the second daily morphine dose remaining at 3.2 mg/kg. After at least seven additional days under these dosing conditions, the second daily dose was also increased to 5.6 mg/kg; monkeys received 5.6 mg/kg/12 h of morphine for at least 77 days. Sessions during which the drug was available were separated by sessions during which saline was available with the drug that could be self administered alternating between heroin and cocaine; thus, saline was available every other day whereas heroin and cocaine were available every fourth day. Thereafter, discontinuation studies were conducted (Fig. 1). In monkeys receiving 5.6 mg/kg/12 h of morphine, 17-h morphine withdrawal periods were scheduled to examine changes in heroin and cocaine self administration during withdrawal. The effects of longer withdrawal periods were evaluated following chronic administration of a larger dose of morphine (see below). Saline replaced the normal dose of morphine 5 h before sessions and the interval between the last injection of morphine and sessions was 17 h. For three consecutive sessions, including sessions immediately before and after these discontinuation studies, the same solution was available for self administration; the effects of temporary suspension of daily morphine on self administration of heroin and cocaine were determined on separate occasions and the range of unit doses available for self administration was identical to those available 5 h after morphine. Seven days of uninterrupted dosing with 5.6 mg/kg/12 h of morphine occurred between each discontinuation period. The order of these 17-h discontinuation studies varied across monkeys; for example, heroin was studied first in two monkeys, cocaine was studied first in one monkey and saline was studied first in one monkey. Monkeys received this dose of morphine for 10 days after the last discontinuation study.
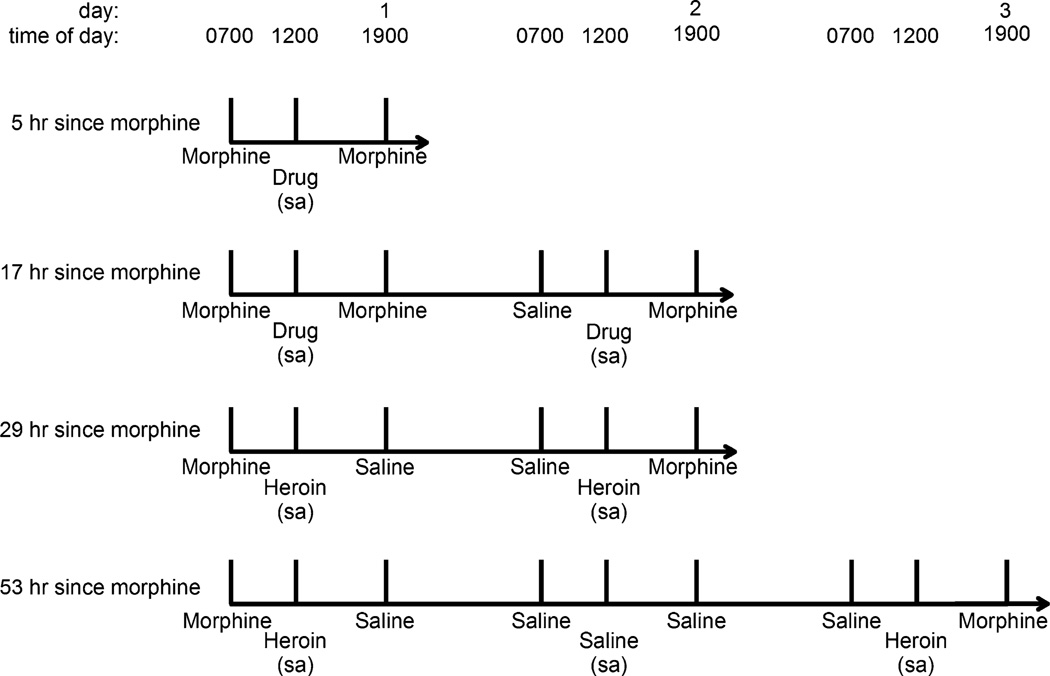
Fig. 1.
Time line for the three different withdrawal periods. When morphine was administered 5 or 17 h before sessions, one of three solutions (saline, heroin, or cocaine) was available for self administration during sessions; thus, these withdrawal periods were studied on three separate occasions. When morphine was last administered 29 or 53 h before sessions, only heroin was available for self administration, and these withdrawal periods were studied once
Subsequently, the daily dose of morphine was increased to 10 mg/kg/12 h for 73 days, and the first discontinuation study during administration of this larger dose of morphine was conducted 8 days after the change in dosing conditions. Three different withdrawal periods were evaluated separately: 17, 29, and 53 h since the last dose of morphine (Fig. 1). Studies investigating self administration of heroin or cocaine 17 h after morphine were similar to those conducted when monkeys received the smaller morphine dose; however, in addition to self administration, directly observable withdrawal signs were also determined. Monkeys were observed in the home cage beginning 30 min before sessions. One person conducted all observations on a single day. Over a 15-min period, monkeys were observed every 5 min for 1 min for a total of three observations per monkey. The following signs were scored as present or absent during each 1-min observation period: retching, yawning, vocalization, grimacing, wet-dog shakes, lying on side, and holding abdomen (Seevers 1936; Katz 1986). Thus, the maximum score for one sign was 3; because seven withdrawal signs were monitored, the maximum possible total withdrawal score was 21 for each monkey.
Discontinuation studies were also conducted when morphine administration was temporarily suspended for 29 or 53 h (Fig. 1), although only heroin self administration was examined following these longer withdrawal periods. To study effects 29 h after the last dose of morphine, saline replaced the normal dose of morphine 5 h and 17 h before sessions; otherwise these studies were identical to those used to study effects 17 h after the last dose of morphine with the same unit doses of the same solution available for three consecutive days and withdrawal signs monitored before each session. To study effects 53 h after the last dose of morphine, saline was administered instead of morphine 7 h after sessions on day 1, for both scheduled injections on day 2 and 5 h before sessions on day 3. On day 1, heroin dose–effect curves were determined 5 h after morphine. During sessions on day 2, saline was available, and during sessions on day 3, which occurred 53 h after the last injection of morphine, heroin was available; withdrawal signs were measured before sessions on all 3 days.
Upon completion of those studies (6 months), morphine administration was terminated and heroin dose–effect curves were determined daily for 10 weeks after the last dose of morphine. Large increases in responding were observed following termination of chronic morphine administration, thereby necessitating decreases in the dose range of heroin available (see Fig. 4). Withdrawal signs were monitored before and after sessions daily.
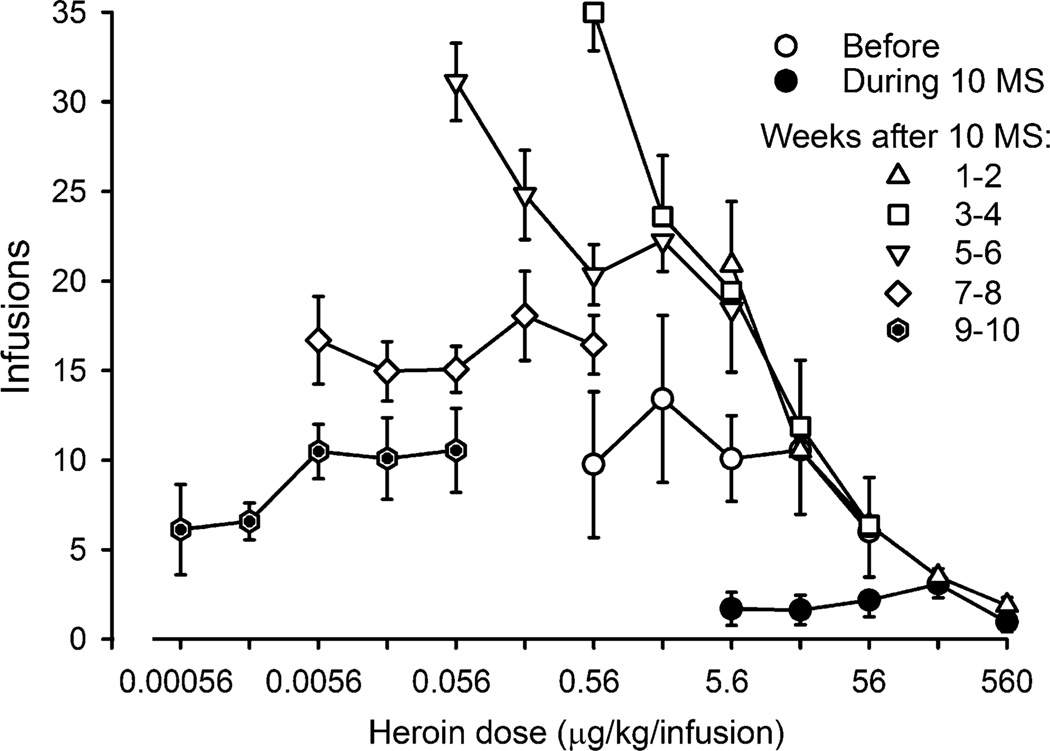
Fig. 4.
Self administration of heroin following termination of morphine administration. Heroin was available for self administration every day and data were averaged across days for 2 weeks to generate the dose–effect curves shown. The number of infusions received in each of the five cycles with different unit doses of heroin available during each cycle (ordinate) is plotted as a function of unit dose of drug (abscissae) expressed in micrograms per kilogram per injection
Data analyses
The number of infusions received per session was plotted as a function of unit dose of drug. Total drug intake (expressed as mg/kg/session) and total withdrawal signs were plotted as a function of time since the last injection of morphine. Dose–effect curves determined prior to chronic morphine administration were obtained by averaging the three heroin dose–effect curves or three cocaine dose–effect curves obtained for each monkey immediately before chronic morphine administration began. On the day preceding determination of each dose–effect curve, saline was available in each of the 5 cycles; consequently, each session during which increasing doses of drug were available was paired with a session during which saline was available. Area under the curve with cycles as the independent variable and number of infusions as the dependent variable was determined for each pair of data. These areas were averaged to obtain a mean value for each drug and the corresponding saline data; drugs were compared to saline using a paired t-test. The mean values for each subject were then combined to obtain a mean (±1 SEM) for the group. In addition to the values determined before chronic treatment, areas under the curve were also determined for dose–effect curves obtained 5, 17, 29, and 53 h after the last dose of morphine as well as after morphine administration was terminated; one-way analyses of variance were performed on these areas under the curve (p < 0.05). When significant differences were found, Tukey’s multiple comparison test was used (p < 0.05). When morphine was administered daily, responding decreased and variability was small; consequently, dose–effect curves for heroin or cocaine were the average of seven curves determined during the first 2 months of administration of 5.6 or 10 mg/kg/12 h of morphine. For discontinuation studies, data represent single determinations in each of four monkeys.
Withdrawal signs were scored as present or absent during three distinct 1-min observation periods. The maximum possible score for each monkey was 21 (seven signs × three observations). Intake and signs during withdrawal were analyzed using one-way repeated measures analysis of variance with time since the last injection of morphine as the factor followed by Tukey’s Multiple Comparison Test when significant differences were detected (p < 0.05).
Drugs
The compounds used in these studies were morphine sulfate, cocaine hydrochloride and heroin hydrochloride (National Institute on Drug Abuse, Research Technology Branch, Rockville, MD). Cocaine and heroin were dissolved in saline and administered i.v. The concentration available for self-administration remained constant throughout a session; the unit dose of drug was increased across cycles by increasing infusion duration and consequently the volume. Morphine was dissolved in sterile water and administered s.c. in a volume of 0.4–1.7 ml.
Results
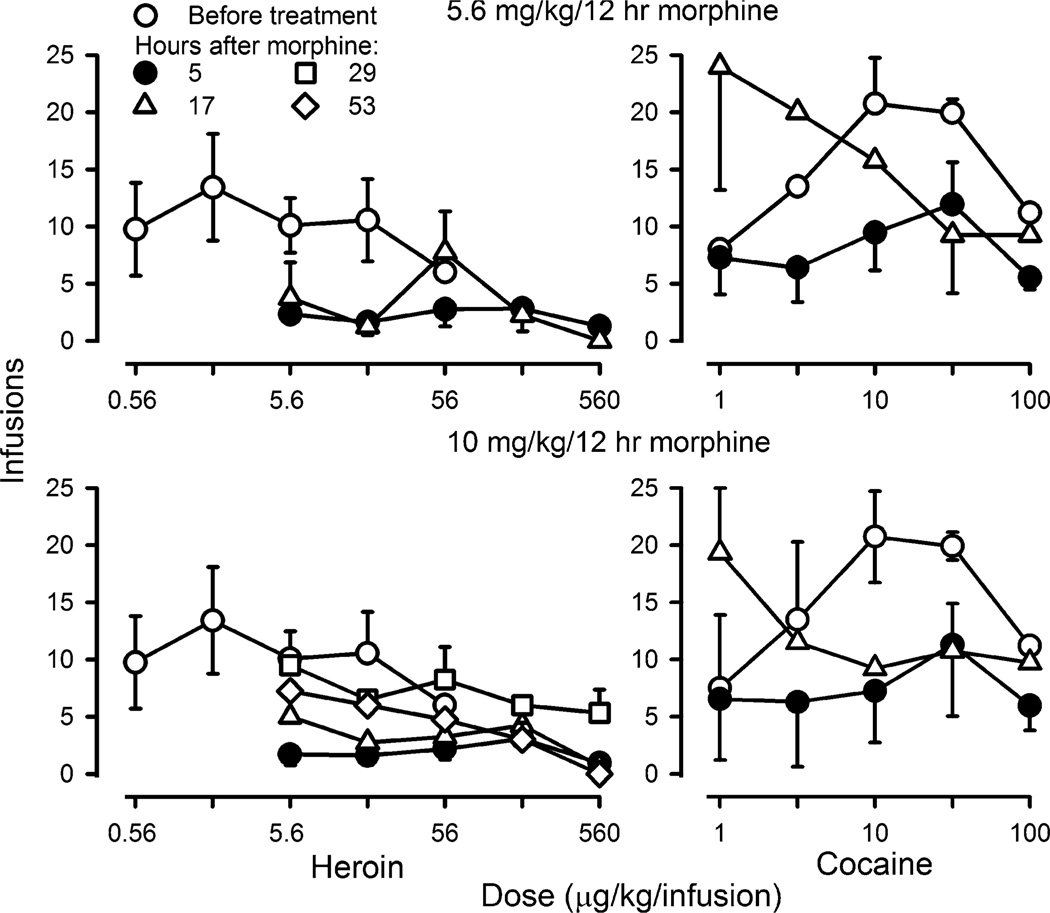
Before chronic morphine administration, responding for saline ranged from 1.4 to 3.3 infusions per cycle. Heroin and cocaine maintained more self-administration responding, as compared to saline (open circles, Fig. 2). Dose–effect curves for heroin and cocaine were inverted U-shaped curves with intermediate unit doses of 1.78 µg/kg of heroin or 10 µg/kg of cocaine, on average, resulting in the largest number of infusions (13.4 ± 4.7 or 20.8 ± 5.0 infusions, respectively). Areas under the curve obtained for heroin and for cocaine were significantly different from the area under the curve for saline for each individual monkey (p < 0.05 in each case). Five hours following administration of either 5.6 mg/kg/12 h (upper panels, Fig. 2) or 10 mg/kg/12 h of morphine (lower panels, Fig. 2), self administration of heroin or cocaine was decreased. Under these conditions, the largest number of infusions self administered for any dose of heroin was 3.1 infusions at a dose of 178 µg/kg in monkeys receiving 10 mg/kg/12 h (closed circles, left panels, Fig. 2). Although the dose-effect curve for cocaine was also shifted downward 5 h after morphine administration, cocaine continued to maintain more self administration responding than heroin.
Fig. 2.
Self administration of heroin (left panels) and cocaine (right panels) in four monkeys before and during chronic administration of 5.6 mg/kg/12 h (upper panels) or 10 mg/kg/12 h (lower panels) of morphine. The number of infusions received in each of five cycles with different unit doses of heroin or cocaine available during each cycle (ordinate) is plotted as a function of unit dose of drug (abscissae) expressed in micrograms per kilogram per injection
Heroin and cocaine dose–effect curves were also determined at various times following temporary suspension of morphine administration; the unit doses available for self administration were the same as those available 5 h after morphine. When monkeys received morphine most recently 17, 29, or 53 h earlier, self administration of heroin and cocaine increased, as compared to self administration that occurred in monkeys receiving morphine daily. The largest number of infusions of heroin received when saline was temporarily substituted for morphine was similar to the largest number of infusions of heroin received before chronic morphine administration, although this effect occurred at different unit doses of heroin. For example, before chronic morphine administration, unit doses of 0.56, 1.78, 5.6, and 17.8 µg/kg resulted in self administration of 9.8 to 13.4 infusions of heroin; temporary suspension of 5.6 mg/kg/12 h of morphine for 17 h resulted in monkeys receiving an average of eight infusions of 56 µg/kg of heroin (upper left panel, Fig. 2). Differences in area under the curve for saline and heroin determined before daily morphine administration and for heroin determined at various times after the last dose of morphine were statistically significant (F(3, 9) = 12.76; p < 0.05), although only the area under the curve for heroin obtained before daily morphine administration was significantly different from saline. When 10 mg/kg/12 h was temporarily discontinued, responding maintained by heroin increased as a function of time since the last morphine injection with the largest increase (average of 9.5 infusions of 5.6 µg/kg/infusion) occurring 29 h after morphine (lower left panel, Fig. 2). Although differences in the areas under the curve were statistically significant (F(3, 15) = 7.79; p < 0.05), only the area under the curve for heroin obtained before daily morphine administration was significantly different from saline. Similarly, responding for cocaine increased 17 h after the last administration of either 5.6 or 10 mg/kg/12 h of morphine. These conditions resulted in the largest number of infusions that were observed during morphine administration and this dramatic increase in responding occurred at the smallest unit dose of cocaine (right panels, Fig. 2). Despite the change in the shape of the cocaine dose–effect curves, there was no significant difference among the areas under the curve for cocaine [5.6 mg/kg/12 h of morphine: F(3, 9) = 2.71; p = 0.11; 10 mg/kg/12 h of morphine: F(3, 9) = 2.89; p = 0.09].
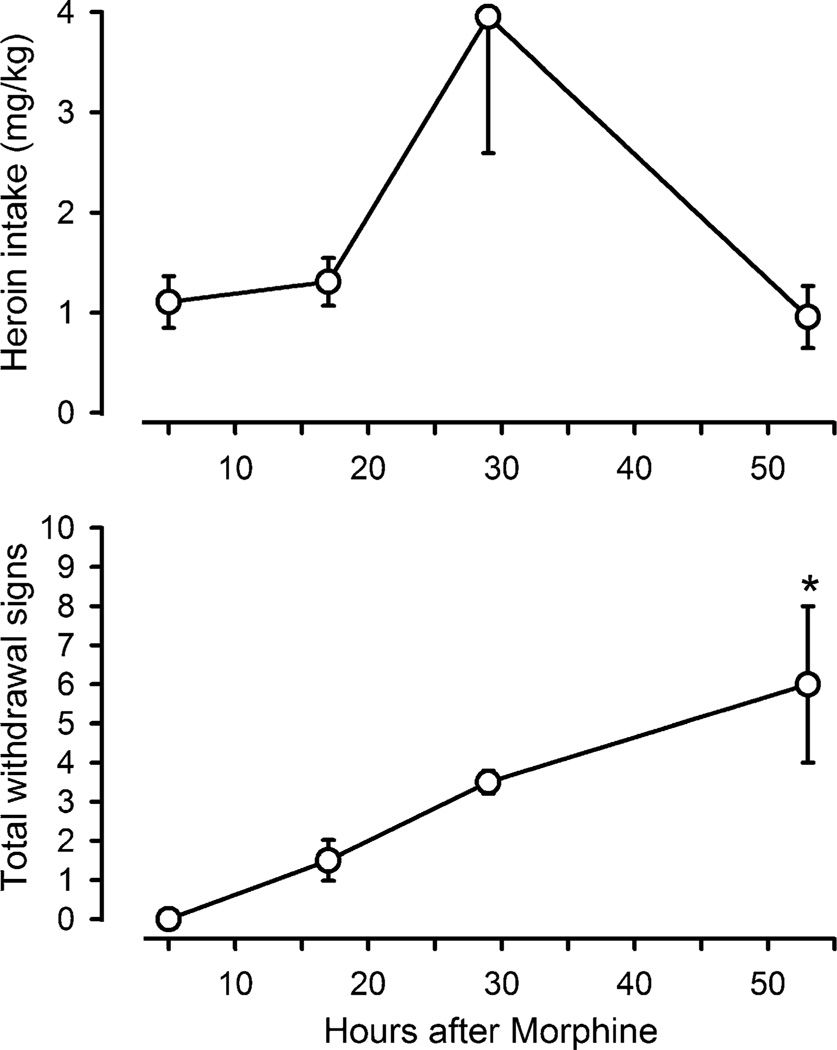
To compare the time course of changes in heroin self administration and the emergence of withdrawal signs, heroin intake for the entire session and total withdrawal signs were plotted as a function of time since the last morphine injection (Fig. 3). Increased heroin intake approached significance (F(3, 15) = 3.612; p = 0.059) and withdrawal signs were significantly elevated after temporary discontinuation of morphine administration (F(3,15) = 6.908; p < 0.05). On average, monkeys received a total of 1.1 mg/kg of heroin during sessions that were conducted 5 h after morphine, and the average number of withdrawal signs (95% CI) that were observed immediately before those sessions was 0.19 (0, 0.42). Heroin intake was similar (1.3 mg/kg) 17 h after morphine, although withdrawal signs were slightly increased at this time point. When studied 29 h after the last injection of morphine, total withdrawal signs increased further and heroin intake was increased to 4 mg/kg. The number of withdrawal signs increased significantly 53 h after morphine, although heroin intake was similar to the intake observed 5 h after morphine.
Fig. 3.
Heroin intake (mg/kg; upper panel) and total withdrawal signs (lower panel) during chronic administration of 10 mg/kg/12 h of morphine are plotted as a function of time (h) since the last morphine injection. Scheduled morphine injections were replaced with saline. Asterisks indicate that data points are significantly different from values obtained 5 h after morphine administration
The effects of discontinuing daily morphine administration (10 mg/kg/12 h) on heroin self administration were evaluated with the dose range of heroin available for self administration decreased every 2 weeks. After termination of morphine administration, responding maintained by heroin increased dramatically (Fig. 4). A unit dose of 5.6 µg/kg of heroin, which resulted in the delivery of 9.4 infusions before chronic morphine administration and 2.2 infusions when monkeys received 10 mg/kg/12 h, resulted in the delivery of, on average, 20.9 infusions during the first 2 weeks after termination of morphine administration. When smaller unit doses of heroin were studied 3–4 weeks after discontinuation of morphine administration, the number of infusions received increased further with a unit dose of 0.56 mg/kg resulting in the delivery of 35 infusions. Heroin self administration decreased over sessions (weeks) and in weeks 9–10, monkeys were receiving similar numbers of infusions, albeit with smaller unit doses, as compared to the number of infusions received before chronic morphine administration. There was a significant difference in areas under the curve for heroin following termination of treatment (F(5, 15) = 27.04; p < 0.05) with the curves determined 3–4 weeks, 5–6 weeks, and 7–8 weeks significantly different from saline.
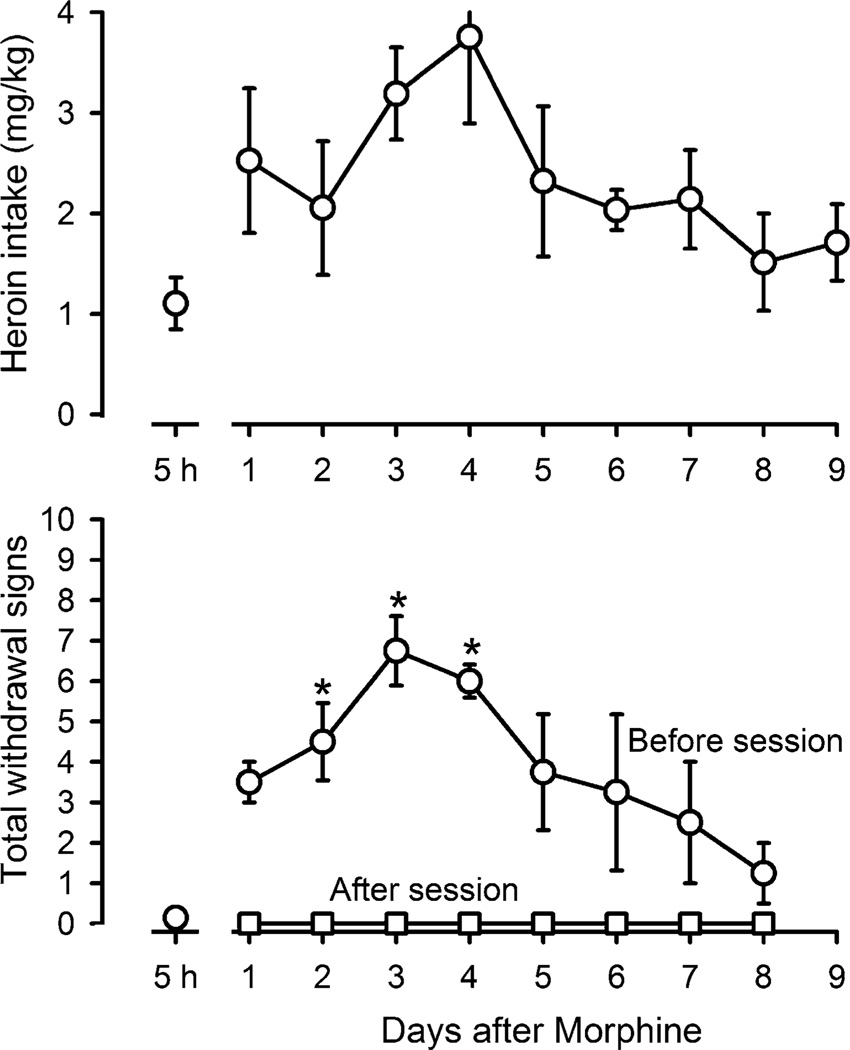
Following termination of morphine administration, withdrawal signs were monitored before and after sessions. Total withdrawal signs observed before sessions increased 1 day after the last administration of morphine and there was an increase in total heroin intake during sessions (lower and upper panels, respectively, Fig. 5). Heroin received during sessions completely reversed withdrawal signs that were evident before sessions (squares, lower panel, Fig. 5). As the time between morphine injections and sessions increased, total withdrawal signs increased significantly (F(8, 35) = 5.416; p < 0.05); withdrawal signs peaked 3 to 4 days after the last administration ofmorphine. Heroin intake increased but failed to reach significance (F(6,27) = 2.246; p = 0.086) as total withdrawal signs increased with the largest intake occurring on days when withdrawal signs were most apparent (3–4 days after discontinuation of morphine). From 4 to 8 days after termination of morphine administration, there was a decrease in total withdrawal signs and heroin intake decreased concurrently.
Fig. 5.
Heroin intake (mg/kg; upper panel) and total withdrawal signs (lower panel) for the first 8 days after termination of chronic administration of 10 mg/kg/12 h of morphine are plotted as a function of days since the last morphine injection. Circles withdrawal signs evident before self administration sessions, squares withdrawal signs determined after monkeys self administered heroin. Points above 5 h indicate the effects obtained during chronic morphine administration, and asterisks indicate that data points are significantly different from values obtained 5 h after morphine administration
Discussion
Dependence developed in monkeys receiving 10 mg/kg/12 h of morphine chronically, as evidenced by the emergence of withdrawal when morphine administration was discontinued. Self administration of heroin decreased during periods of chronic morphine administration and increased following discontinuation of morphine administration. Withdrawal signs were evident before but not after heroin self administration sessions, indicating that self-administered heroin reversed withdrawal signs. One possibility is that under these conditions, responding for heroin was related to the prevention or reduction in withdrawal signs, and this possibility is consistent with the hypothesis that one reason for continued opioid abuse is to avoid or alleviate withdrawal (Koob et al. 1989; Solomon and Corbit 1974).
Despite this view of some that individuals continue to use some drugs so as to avoid withdrawal, there are instances where the emergence of withdrawal does not appear to be related to continued drug taking (Lyvers 1998). On the one hand, it is clear that self administration of heroin suppressed withdrawal signs in the current study; on the other hand, there is other evidence to suggest that responding for heroin was not causally linked to withdrawal. For example, although heroin self administration increased following temporary discontinuation of morphine administration, the largest increase in responding for heroin (29 h) did not occur at the time (53 h) when the largest number of withdrawal signs was observed (Fig. 3). In fact, 53 h after the last morphine injection, heroin intake was similar to intake observed 5 h after morphine, when no withdrawal signs were evident. Self administration of other drugs is not always increased when withdrawal signs peak, although responding can increase as withdrawal signs wane (e.g., ethanol; (Winger 1988). In the current study, further evidence that heroin self administration was not due specifically to the emergence of withdrawal was observed weeks after morphine administration was terminated. Under these conditions, when withdrawal signs were no longer evident, responding was markedly increased, even as the range of unit doses decreased over weeks. This persistent increase in responding might be unrelated to reinforcing effects of heroin available in those sessions because the doses that appeared to be maintaining responding were much smaller than doses that maintain responding under other conditions in rhesus monkeys. A similar study in which saline is substituted for heroin might provide insight with regard to the possible role of non-pharmacologic factors in maintaining responding after morphine administration is discontinued.
In addition to changes in heroin self administration during withdrawal, differences in responding for cocaine were also observed. The potency of cocaine increased during withdrawal, as evidenced by responding for a small unit dose of cocaine 17 h after the last morphine administration. Increased sensitivity to cocaine during withdrawal, observed twice in the current study (i.e., when monkeys received either 5.6 or 10 mg/kg/infusion of cocaine), has also been reported by others (He and Grasing 2004). It is not clear whether increased responding for cocaine (or heroin) is due to positive reinforcing effects per se or to conditioned reinforcing effects associated with drug administration, as has been suggested for the persistent responding for small doses of heroin that is observed for up to 4 weeks following withdrawal from heroin in rats (Zhou et al. 2004).
The apparent dissociation between withdrawal and increased responding for drug in the current study is not due to a failure to generate significant dependence and withdrawal. Although the frequency of administration varies among studies, total daily doses of morphine smaller than those used in the current study have been shown to produce dependence in rhesus monkeys (Bergman and Schuster 1985; Holtzman and Villarreal 1969; Holtzman and Villarreal 1971). Moreover, withdrawal signs observed in the current study were similar to those observed in other studies, including vocalization and lying on side (Becker et al. 2008; Katz 1986; Seevers 1936). Finally, and despite different dosing conditions, the time course of morphine withdrawal is very consistent among studies. Overall, signs emerge by 17 h after morphine administration and peak after 3 days; this time course for withdrawal is similar to that reported in other studies with morphine in rhesus monkeys (Katz 1986) with maximal withdrawal occurring 2–3 days after the last dose of morphine (Seevers 1936). Thus, the dependence and withdrawal observed in the current study are qualitatively and quantitatively similar to results of previous studies.
Taken together, results from the current study and several published studies indicate that drug taking after discontinuation of chronic drug administration cannot be predicted simply by the emergence of withdrawal. For example, directly observable signs that are commonly used to monitor the appearance and disappearance of withdrawal typically are no longer evident 1 week after termination of opioid administration, although increased responding can persist for many weeks after termination of opioid administration (Zhou et al. 2004). However, it is clear that some indices of withdrawal can persist for much longer than 1 week. For example, long-term physiological changes have been reported following discontinuation of chronic opioid administration in humans (Martin and Jasinski 1969; Beswick et al. 2003), and recently it was shown that in rhesus monkeys, some withdrawal signs are evident for weeks after termination of morphine administration (Becker et al. 2008). Thus, discontinuation of chronic morphine administration can produce lingering and sometimes subtle (changes in night-time activity) withdrawal that, in turn, might contribute to persistent increases in responding for heroin.
In summary, responding for heroin increases following temporary discontinuation or termination of opioid administration, although this increase in responding does not precisely covary with signs of withdrawal. Others have reported changes in heroin self administration during withdrawal, although the conditions and procedures used in those studies were different from those used in the current study. For example, some studies evaluated antagonist–precipitated withdrawal (Carrera et al. 1999; Goldberg et al. 1969; 1971) and others used procedures in which monkeys made a choice between responding for food and responding for heroin (Negus 2006; Negus and Rice (2009). In the latter studies, it is possible that increased choice for heroin, relative to food, was influenced by the fact that opioid withdrawal can suppress responding for and consumption of food (Holtzman and Villarreal 1973; Gellert and Sparber 1977). In contrast to other studies, the current study also used longer periods of morphine administration and it monitored monkeys for an extended period following termination of morphine administration. Overall, results from this study suggest that while opioid withdrawal might contribute to drug-taking behavior, it also appears as though conditioned reinforcing effects play a role in drug self-administration during withdrawal; it remains to be seen whether longer lasting and more subtle indices of withdrawal, about which very little is known, influence drug self administration.
Acknowledgments
The authors thank B. Engelhardt and C. Cruz for technical assistance.
This work was supported by United States Public Health Service Grant DA05018 and Senior Scientist Award K05 DA17918 (CPF).
Contributor Information
Lisa R. Gerak, Email: gerak@uthscsa.edu, Department of Pharmacology, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229-3900, USA.
Ruggero Galici, Email: rgalici@prdus.jnj.com, Department of Pharmacology, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229-3900, USA.
Charles P. France, Email: france@uthscsa.edu, Department of Pharmacology, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229-3900, USA; Department Psychiatry, The University of Texas Health Science Center at San Antonio, 7703 Floyd Curl Dr., San Antonio, TX 78229-3900, USA.
References
- Becker GL, Gerak LR, Koek W, France CP. Antagonist-precipitated and discontinuation-induced withdrawal in morphine-dependent rhesus monkeys. Psychopharmacology. 2008;201:373–382. doi: 10.1007/s00213-008-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J, Schuster CR. Behavioral effects of naloxone and nalorphine preceding and following morphine maintenance in the rhesus monkey. Psychopharmacology. 1985;86:324–327. doi: 10.1007/BF00432222. [DOI] [PubMed] [Google Scholar]
- Beswick T, Best D, Rees S, Bearn J, Gossop M, Strang J. Major disruptions of sleep during treatment of the opiate withdrawal syndrome: differences between methadone and lofexidine detoxification treatments. Addict Biol. 2003;8:49–57. doi: 10.1080/1355621031000069882. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Schulteis G, Koob GF. Heroin self-administration in dependent Wistar rats: increased sensitivity to naloxone. Psychopharmacology. 1999;144:111–120. doi: 10.1007/s002130050983. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Truong YN, Shi YG, Woods JH. Morphine deprivation increases self-administration of the fast- and short-acting mu-opioid receptor agonist remifentanil in the rat. J Pharmacol Exp Ther. 2008;326:920–929. doi: 10.1124/jpet.108.139196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharmacol Exp Ther. 1977;201:44–54. [PubMed] [Google Scholar]
- Gerak LR, France CP. Discriminative stimulus effects of flumazenil in untreated and in diazepam-treated rhesus monkeys. Psychopharmacology. 1999;146:252–261. doi: 10.1007/s002130051114. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Galici R, France CP. Self administration of cocaine in monkeys receiving LAAM acutely or chronically. Physiol Behav. 2008;93:20–26. doi: 10.1016/j.physbeh.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–1307. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Nalorphine-induced changes in morphine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1971;176:464–471. [PubMed] [Google Scholar]
- Gossop M, Stewart D, Browne N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97:1259–1267. doi: 10.1046/j.1360-0443.2002.00227.x. [DOI] [PubMed] [Google Scholar]
- He S, Grasing K. Chronic opiate treatment enhances both cocaine-reinforced and cocaine-seeking behaviors following opiate withdrawal. Drug Alcohol Depend. 2004;75:215–221. doi: 10.1016/j.drugalcdep.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Morphine dependence and body temperature in rhesus monkeys. J Pharmacol Exp Ther. 1969;166:125–133. [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Pharmacologic analysis of the hypothermic responses of the morphine-dependent rhesus monkey. J Pharmacol Exp Ther. 1971;177:317–325. [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Operant behavior in the morphine-dependent rhesus monkey. J Pharmacol Exp Ther. 1973;184:528–541. [PubMed] [Google Scholar]
- Katz JL. Effects of clonidine and morphine on opioid withdrawal in rhesus monkeys. Psychopharmacology. 1986;88:392–397. doi: 10.1007/BF00180844. [DOI] [PubMed] [Google Scholar]
- Koob GF, Stinus L, Le Moal M, Bloom FE. Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev. 1989;13:135–140. doi: 10.1016/s0149-7634(89)80022-3. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Jacobsen LK, Kosten TR. Severity of precipitated opiate withdrawal predicts drug dependence by DSM-III-R criteria. Am J Drug Alcohol Abuse. 1989;15:237–250. doi: 10.3109/00952998908993405. [DOI] [PubMed] [Google Scholar]
- Longshore D, Annon J, Anglin MD, Rawson RA. Levo-alpha-acetylmethadol (LAAM) versus methadone: treatment retention and opiate use. Addiction. 2005;100:1131–1139. doi: 10.1111/j.1360-0443.2005.01122.x. [DOI] [PubMed] [Google Scholar]
- Lyvers M. Drug addiction as a physical disease: the role of physical dependence and other chronic drug-induced neurophysiological changes in compulsive drug self-administration. Exp Clin Psychopharmacol. 1998;6:107–125. doi: 10.1037//1064-1297.6.1.107. [DOI] [PubMed] [Google Scholar]
- Martin WR, Jasinski DR. Physiological parameters of morphine dependence in man-tolerance, early abstinence, protracted abstinence. J Psychiatr Res. 1969;7:9–17. doi: 10.1016/0022-3956(69)90007-7. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Sell SL, France CP. Cocaine and other indirect-acting monoamine agonists differentially attenuate a naltrexone discriminative stimulus in morphine-treated rhesus monkeys. J Pharmacol Exp Ther. 2004;308:111–119. doi: 10.1124/jpet.103.058917. [DOI] [PubMed] [Google Scholar]
- Negus SS. Choice between heroin and food in nondependent and heroin-dependent rhesus monkeys: effects of naloxone, buprenorphine, and methadone. J Pharmacol Exp Ther. 2006;317:711–723. doi: 10.1124/jpet.105.095380. [DOI] [PubMed] [Google Scholar]
- Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2008.127. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E, Schreiber S, Adelson M. Factors predicting retention in treatment: 10-year experience of a methadone maintenance treatment (MMT) clinic in Israel. Drug Alcohol Depend. 2006;82:211–217. doi: 10.1016/j.drugalcdep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Rosen MI, Wallace EA, Sullivan MC, Stine S, Kosten TR. Use of cocaine to prevent opiate withdrawal. Am J Psychiatry. 1992;149:1609. doi: 10.1176/ajp.149.11.1609b. [DOI] [PubMed] [Google Scholar]
- Seevers MH. Opiate addiction in the monkey. I. Methods of study. J Pharmacol Exp Ther. 1936;56:147–156. [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Liebson IA, Bigelow GE. Buprenorphine versus methadone in the treatment of opioid dependence: self-reports, urinalysis, and addiction severity index. J Clin Psychopharmacol. 1996;16:58–67. doi: 10.1097/00004714-199602000-00010. [DOI] [PubMed] [Google Scholar]
- Walker JR, Chen SA, Moffitt H, Inturrisi CE, Koob GF. Chronic opioid exposure produces increased heroin self-administration in rats. Pharmacol Biochem Behav. 2003;75:349–354. doi: 10.1016/s0091-3057(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Winger G. Effects of ethanol withdrawal on ethanol-reinforced responding in rhesus monkeys. Drug Alcohol Depend. 1988;22:235–240. doi: 10.1016/0376-8716(88)90023-3. [DOI] [PubMed] [Google Scholar]
- Winger G, Woods JH. The effects of chronic morphine on behavior reinforced by several opioids or by cocaine in rhesus monkey. Drug Alcohol Depend. 2001;62:181–189. doi: 10.1016/s0376-8716(00)00166-6. [DOI] [PubMed] [Google Scholar]
- Winger G, Palmer RK, Woods JH. Drug-reinforced responding: rapid determination of dose–response functions. Drug Alcohol Depend. 1989;24:135–142. doi: 10.1016/0376-8716(89)90076-8. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44:491–494. [PubMed] [Google Scholar]
- Zhou W, Zhang F, Tang S, Liu H, Lai M, Yang G. Low dose of heroin inhibits drug-seeking elicited by cues after prolonged withdrawal from heroin self-administration in rats. Neuroreport. 2004;15:727–730. doi: 10.1097/00001756-200403220-00031. [DOI] [PubMed] [Google Scholar]