Abstract
Background
The purpose of this study was to investigate the relationship between retrobulbar circulation and visual field change in eyes with primary open-angle glaucoma following unilateral trabeculectomy.
Methods
Twenty-one patients with primary open-angle glaucoma were prospectively enrolled. Retrobulbar circulation was evaluated using color Doppler imaging. The peak systolic velocity, end-diastolic velocity, and resistive index were evaluated in the central retinal artery, temporal site of the short posterior ciliary artery (t-SPCA), and nasal site of the short posterior ciliary artery (n-SPCA). Visual field examinations were performed using a Humphrey visual field analyzer before surgery and trimonthly for 12 months postoperatively.
Results
In the operative eyes, the end-diastolic velocity was significantly increased in the central retinal artery (P = 0.005, analysis of variance), t-SPCA (P = 0.005), and n-SPCA (P = 0.027). The resistive index was significantly decreased in the central retinal artery (P = 0.003), t-SPCA (P = 0.000), and n-SPCA (P = 0.010) postoperatively compared with preoperatively. The nonoperative contralateral eyes did not show a significant change in end-diastolic velocity or resistive index for either the SPCA or central retinal artery. The mean deviation slope in the operative eyes (−0.26 ± 0.64 dB/year) was significantly slower than that in the nonoperative eyes (−0.65 ± 0.70 dB/year; P = 0.047, Mann-Whitney U test).
Conclusion
These results suggest that trabeculectomy improves the retrobulbar circulation and prevents the progression of visual field changes in patients with primary open-angle glaucoma.
Keywords: trabeculectomy, retrobulbar circulation, visual field, color Doppler imaging
Introduction
Intraocular pressure is the most important known risk factor for the onset and progression of primary open-angle glaucoma, and its reduction remains the only evidence-based intervention found to slow or halt disease progression. Although medical therapy delays the progression of visual field defects, greater intraocular pressure reduction can often be achieved with filtration surgery. Surgical intervention may produce greater intraocular pressure reduction and fewer intraocular pressure fluctuations than medical treatment, thus preventing future visual field loss better than medical therapy.1–5
In addition to the reduction in intraocular pressure, the effects of treatment for glaucoma on ocular blood flow also have important clinical implications. Trabeculectomy is commonly performed in patients with glaucoma when medical therapy has failed to control intraocular pressure. Because of the large intraocular pressure reduction, the patient can expect a significant increase in the ocular perfusion pressure after trabeculectomy. An increase in ocular blood flow in patients undergoing trabeculectomy has been shown in several reports,6–9 but not in all studies.10,11 The reasons for the discrepancies remain to be elucidated, but may be related to the use of different techniques, heterogeneous patient groups, small sample sizes, and the confounding effects of ocular hypotensive agents. To the best of our knowledge, no published clinical report has evaluated the relationship between ocular hemodynamics and visual field progression after surgical intraocular pressure reduction at each postoperative visit.
The purpose of this study was to investigate the effect of trabeculectomy on retrobulbar blood flow in patients with primary open-angle glaucoma using color Doppler imaging and to evaluate the effect of intraocular pressure reduction in preventing the progression of visual field defects.
Materials and methods
Patients
This study protocol was approved by the ethics review committee of the Nihon University School of Medicine, Tokyo, Japan. Subjects for whom trabeculectomy with adjunctive mitomycin C was planned were studied between January 2003 and February 2004 at the Glaucoma Service, Nihon University Hospital, Tokyo, Japan. Subjects meeting the inclusion criteria were enrolled in this study after written informed consent for participation in the study was obtained. The inclusion criteria were: primary open-angle glaucoma with intraocular pressure that was considered unsatisfactory despite maximum tolerable medication; no history of operative procedures including filtering, cataract, and refractive surgery, but excluding laser trabeculoplasty, in either eye before the study; the absence of other coexisting ocular or systemic disorders, including cataract that may affect visual field examination; preoperative best corrected visual acuity at least 40/50; and two or more reproducible visual field examination results using the Swedish interactive threshold algorithm standard 24-2 program of the Humphrey visual field analyzer (Carl Zeiss Meditec, Dublin, CA) before trabeculectomy.
Finally, 21 patients with primary open-angle glaucoma (mean age 64.7 ± 11.2 years; 15 males and six females) were enrolled in the study. Four patients received topical antiglaucoma monotherapy, 10 patients received two types of topical antiglaucoma medication, and the remaining seven patients received three types of topical antiglaucoma medication at the time of preoperative assessment. Seventeen patients received 0.005% latanoprost, 17 patients received 0.5% timolol, and eight patients received 1% dorzolamide.
Trabeculectomy
The surgery was performed by the same surgeon (YY), and the surgical technique was the same in every subject. Trabeculectomy was performed with a limbus-based conjunctival flap. After a 4 × 4 mm scleral flap was prepared, 0.04% mitomycin C in a cut surgical sponge was applied to the bared sclera for 3 minutes. The operative field was washed with 200 mL of balanced salt solution. Following resection of a 1 × 3.5 mm sclerocorneal block and peripheral iridectomy, the flap was closed with 10–0 nylon sutures, which were adjusted so that slight leakage around the margin of the flap was observed without decreasing the depth of the anterior chamber. Postoperative medications comprised a topical corticosteroid and antibiotic, each given four times daily, and 1% atropine once daily.12 Corticosteroids were used to inhibit inflammation and fibrosis for 8–10 weeks. Broad-spectrum antibiotics were used as theoretical prophylaxis against bleb infection and endophthalmitis for 4 weeks. The 1% atropine was continued for 4–6 weeks to deepen the anterior chamber and reduce the risk of postoperative malignant glaucoma. Topical antiglaucoma medications were discontinued in the operated eye. If necessary, laser suture lysis or needle bleb revision was performed. Topical antiglaucoma medications for the nonoperative eyes and systemic medications remained unchanged before and after surgery.
Clinical follow-up
Prior to surgery, all patients underwent a comprehensive examination. Baseline data were obtained within one month before the trabeculectomy. Measurement of equivalent spherical diopter power (refraction) and best corrected visual acuity, slit-lamp examination, Goldmann applanation tonometry, gonioscopy, visual field examination with the 24-2 Swedish interactive threshold algorithm standard programs of the Humphrey visual field analyzer, and dilated funduscopy were performed. Baseline visual field examination had reliability indices of less than 25% fixation losses, false-positive responses, or false-negative responses. Visual fields with unreliable test results or those with results that were incompatible with the previous results were retested. Brachial arterial pressure was also measured with a standard sphygmomanometer. The ocular perfusion pressure was calculated by the following formula:
where mBP was the mean brachial arterial pressure.
Hemodynamics in the short posterior ciliary arteries (SPCAs) and central retinal artery were evaluated using a color Doppler imaging scanner (SSA-550A, Toshiba, Japan) with a 7.0 MHz sector-phased transducer (PSM-70AT; Toshiba) to acquire the Doppler spectral analysis information. The estimated in situ spatial real-time average intensity determined by spectral analysis was 32 mW/cm2, which is within Food and Drug Administration safety guidelines. Color Doppler imaging examinations were carried out with the patient in a sitting position and eyes closed. Each eye was initially examined with a B-scan in both the transverse and sagittal planes. Using the color flow as a map, the central retinal artery was identified and analyzed in the center of the optic nerve shadow, as far forward as possible but always well behind the area of the lamina cribrosa. SPCAs are multiple and varied in number,13 so it is impossible to know how many of the vessels are being imaged simultaneously. However, the SPCAs could be depicted in the nasal and temporal positions from the optic nerve shadow with the probe in a transverse plane. The SPCAs were identified and analyzed in the nasal (n-SPCA) and temporal sites (t-SPCA) adjacent to the optic nerve and posterior to the sclera with the probe in the transverse plane. The vessels were identified by a consistent color flow pattern in the site before specific spectral measurements of the vessel were made. If more than one SPCA was visible, the velocity was measured in the largest one. From these data, three values were obtained, ie, the peak systolic velocity, the end-diastolic velocity, and the resistive index. The resistive index was calculated according to the following formula:
Postoperative assessments were performed every 3 months until 12 months after surgery. All procedures at the preoperative examination (described above) were repeated for each of these evaluations. The blood flow measurements were performed with color Doppler imaging in the same site as the baseline measurement with reference to the preoperative B-scan image. The visual field examinations with the 24-2 Swedish interactive threshold algorithm standard programs of the Humphrey visual field analyzer were also performed with the same schedule as used for the color Doppler imaging examinations. When the visual field examinations could not fulfill the reliable indices, a retest was performed within one week. The progression of visual field changes was qualitatively assessed by modified linear regression analysis of the mean deviation of the visual field obtained during the preoperative and postoperative period using a statistical analysis package (STAPAC 2; Carl Zeiss Meditec, Dublin, CA), and the mean deviation slope in decibels (dB) per year was calculated.
Statistical analysis
Analysis of variance (ANOVA) for repeated measures was used to evaluate the postoperative changes in intraocular pressure, ocular perfusion pressure, color Doppler imaging parameters, and mean deviation. Mann-Whitney U tests were used to investigate the mean deviation slope. A P value of < 0.05 was considered to be statistically significant.
Results
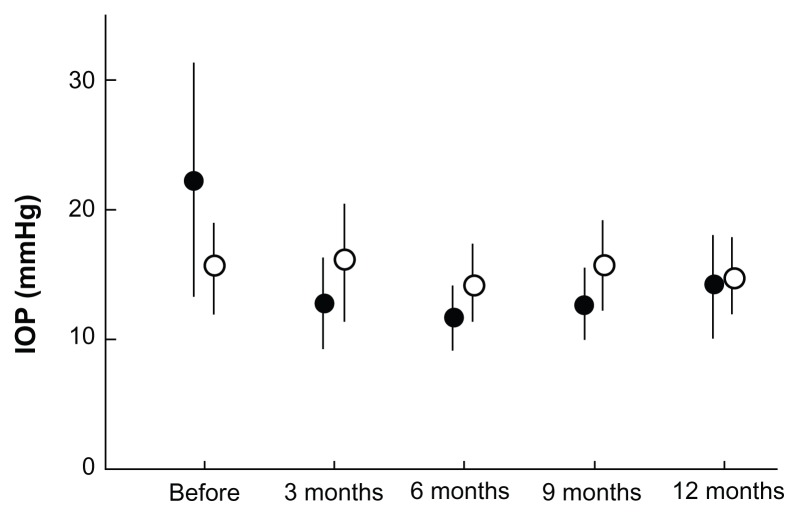
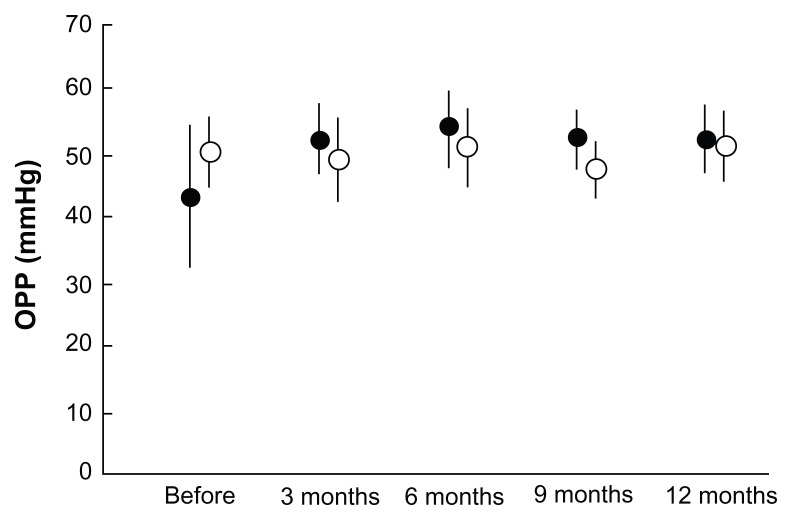
All 21 patients with primary open-angle glaucoma who met the inclusion criteria completed the follow-up plan of 12 months. The intraocular pressure, mBP, and ocular perfusion pressure during the preoperative and postoperative follow-up periods are shown in Figures 1–3. In the operative eyes, the intraocular pressure was significantly decreased (P = 0.001, ANOVA) and the ocular perfusion pressure was significantly increased (P = 0.004, ANOVA) postoperatively. However, in the nonoperative contralateral eyes, the intraocular pressure and ocular perfusion pressures did not change significantly at any of the postoperative time points. There was no significant change in mBP from baseline at any time point.
Figure 1.
Comparison of IOP at each postoperative interval between operative (closed circles) and nonoperative (open circles) eyes.
Notes: Error bars indicate the standard deviation of the mean. There was a significant decrease in IOP in operative eyes (P = 0.001).
Abbreviation: IOP, intraocular pressure.
Figure 3.
Comparison of OPP at each postoperative interval between operative (closed circles) and nonoperative (open circles) eyes.
Notes: Error bars indicate the standard deviation of the mean. There was a significant increase in OPP in operative eyes (P = 0.004).
Abbreviation: OPP, ocular perfusion pressure.
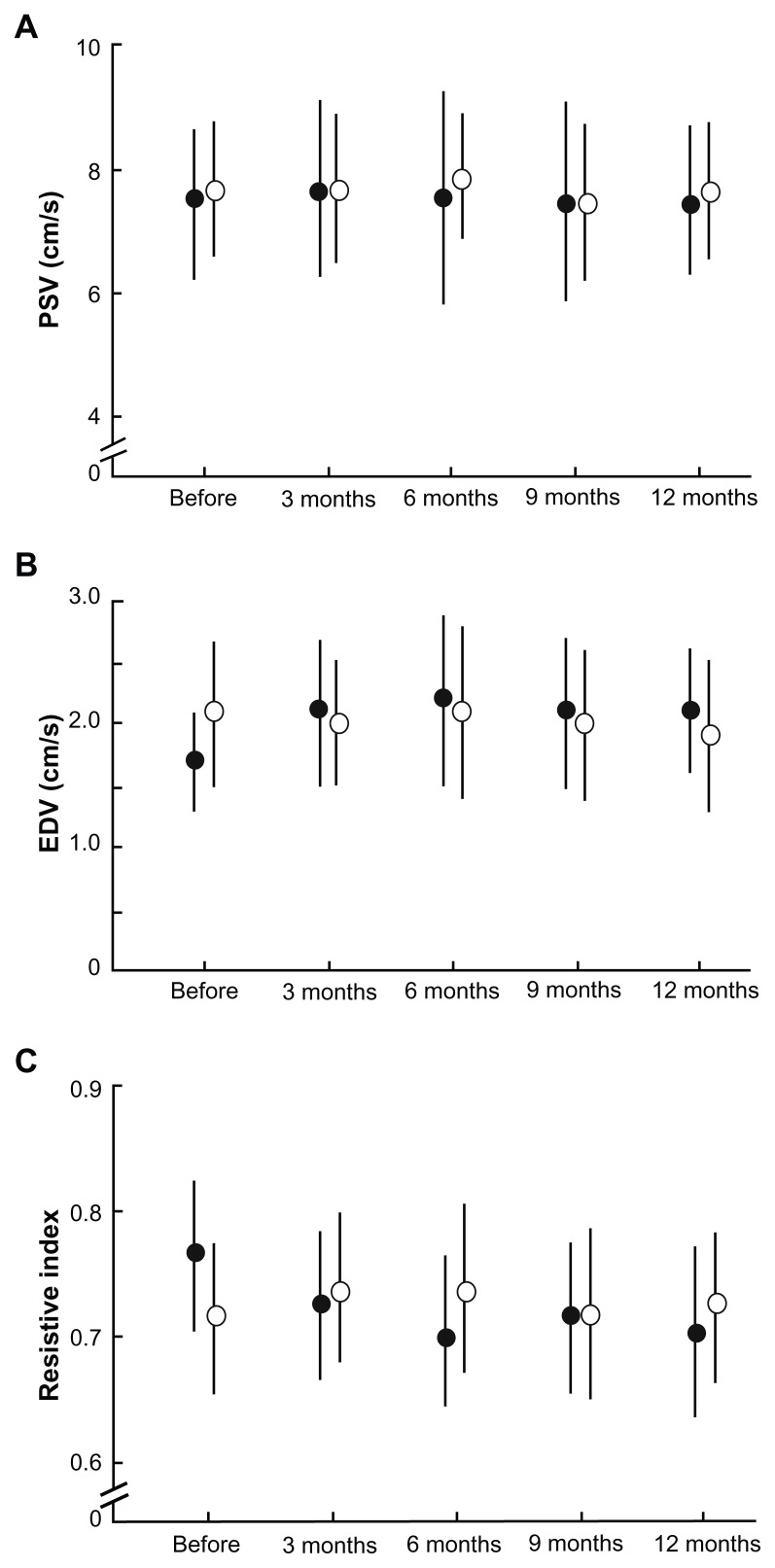
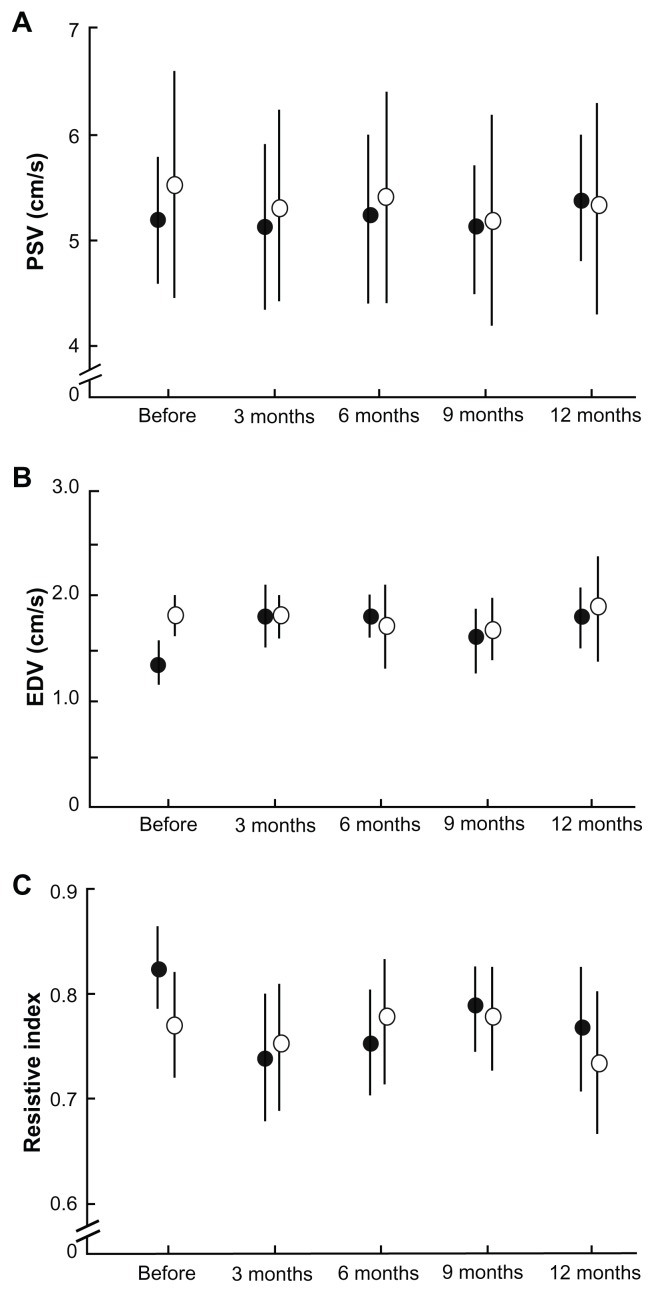
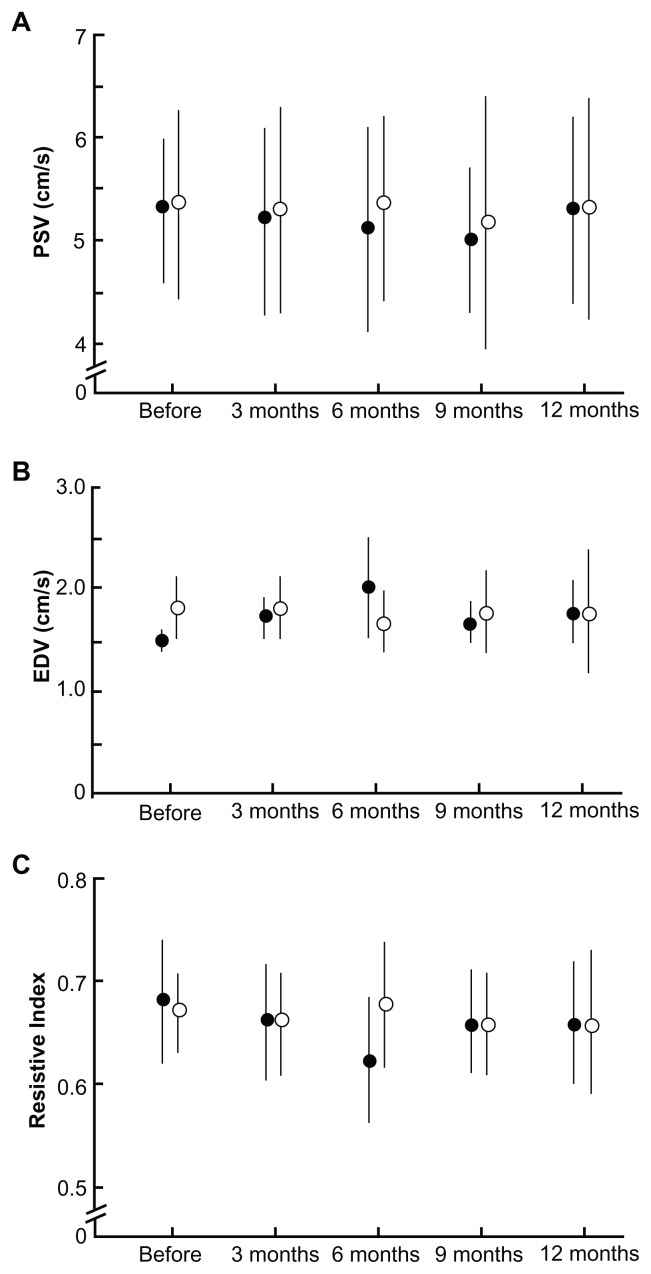
The operative eyes showed improvement in retrobulbar hemodynamics after trabeculectomy. Figure 4 shows the changes in blood flow velocities and resistive index for the central retinal artery. The operative eyes showed significantly increased end-diastolic velocity from baseline after surgery (P = 0.005, ANOVA). Vascular resistance, as measured by the resistive index, was significantly reduced from baseline after surgery (P = 0.003, ANOVA), whereas the peak systolic velocity did not show a significant change at any of the time points. The SPCA demonstrated a statistically significant postoperative change (Figures 5 and 6). The t-SPCA showed a significantly increased end-diastolic velocity (P = 0.005, ANOVA) and a significantly decreased resistive index (P = 0.000, ANOVA) postoperatively. The n-SPCA also had a significantly increased end-diastolic velocity (P = 0.027, ANOVA) and significantly decreased resistive index (P = 0.010, ANOVA) postoperatively. The nonoperative contralateral eye did not show a significant change in end-diastolic velocity or resistive index for either the SPCA or central retinal artery.
Figure 4.
Comparisons of blood flow velocity in the central retinal artery at each postoperative interval between operative (closed circles) and nonoperative (open circles) eyes. (A) PSV. (B) There was a significant increase in the EDV of the central retinal artery in operative eyes (P = 0.005). (C) Significant decreases in the resistive index of the central retinal artery were seen in operative eyes (P = 0.003).
Note: Error bars indicate the standard deviation of the mean.
Abbreviations: EDV, end-diastolic velocity; PSV, peak systolic velocity.
Figure 5.
Comparisons of blood flow velocities in the temporal site of the short posterior ciliary artery at each postoperative interval between operative (closed circles) and nonoperative (open circles) eyes. (A) PSV. (B) EDV. There was a significant increase in the EDV of the temporal site of the short posterior ciliary artery in operative eyes (P = 0.005). (C) Significant decreases in the resistive index of the temporal site of the short posterior ciliary artery were seen in operative eyes (P = 0.000).
Note: Error bars indicate the standard deviation of the mean.
Abbreviations: EDV, end-diastolic velocity; PSV, peak systolic velocity.
Figure 6.
Comparisons of blood flow velocities in the nasal site of the short posterior ciliary artery (SPCA) at each postoperative interval between operative (closed circles) and nonoperative (open circles) eyes. (A) PSV. (B) There was a significant increase in the EDV of the temporal site of the SPCA in operative eyes (P = 0.005). (C) Significant decreases in the resistive index of the temporal site of the SPCA were seen in operative eyes (P = 0.003).
Note: Error bars indicate the standard deviation of the mean.
Abbreviations: EDV, end-diastolic velocity; PSV, peak systolic velocity.
Table 1 shows the postoperative mean deviation change and mean deviation slope. The mean deviation in the operative eyes did not show significant changes postoperatively, whereas the nonoperative eyes showed significant deterioration (P = 0.011, ANOVA). The mean deviation slope was −0.26 ± 0.64 dB/year in the operative eyes and −0.65 ± 0.70 dB/year in the nonoperative eyes. The difference in mean deviation slope between the operative eyes and nonoperative eyes was statistically significant (P = 0.047, Mann-Whitney U test).
Table 1.
Comparison of mean deviation (MD) change and MD slope
| Before | 3 months | 6 months | 9 months | 12 months | P value | MD slope (dB/year) | |
|---|---|---|---|---|---|---|---|
| Operative eyes (dB) | −19.7 ± 5.6 | −19.6 ± 5.7 | −20.3 ± 5.4 | −19.8 ± 5.3 | −20.4 ± 5.1 | 0.194 | −0.26 ± 0.64# |
| Nonoperative eyes (dB) | −8.8 ± 8.0 | −10.2 ± 8.4 | −9.3 ± 8.3 | −9.8 ± 8.8 | −9.5 ± 8.4 | 0.011 | −0.65 ± 0.70# |
Notes: Values are mean ± standard deviation. P values were derived by ANOVA for repeated measures.
P = 0.047 (Mann-Whitney U-test).
Abbreviations: MD, mean deviation; dB, decibel.
Discussion
The present study demonstrates that trabeculectomy associated with a decreased intraocular pressure produces statistically significant and sustained changes in the retrobulbar circulation of patients with primary open-angle glaucoma. The findings of the present study are comparable with those reported by several investigators.6–8,14 In the present study, there was a correlation between the increase in ocular perfusion pressure and the changes in ocular hemodynamic variables measured with color Doppler imaging after trabeculectomy, indicating that the ocular blood flow response was due to the reduction in intraocular pressure.
Among the semiologic methods used to evaluate the vascular component in glaucoma, color Doppler imaging seems to be the most valuable because it is noninvasive and reproducible. It probably provides the most useful data, allowing study of the vessels that are more involved in supplying blood to the optic nerve head. The most indicative parameters of optic nerve head perfusion are end-diastolic velocity and resistive index. End-diastolic velocity is indicative of the average blood flow during the longest phase of the circadian cycle, which represents an instantaneous variation in blood flow. Resistive index, the most reproducible and reliable color Doppler imaging parameter, is a measure of peripheral vascular resistance, with lower values indicating decreased impedance in the territory supplied by the examined vessel.15
Evidence of decreased autoregulatory capacity in patients with glaucoma was provided by a previous report.16 In the present study, there was a correlation between the increase in ocular perfusion pressure and the change in ocular hemodynamic parameters measured with color Doppler imaging, indicating that the ocular blood flow response was due to reduction of intraocular pressure postoperatively.
The present study revealed significant visual field deterioration in nonoperative eyes; however, operative eyes showed postoperative stability of visual field defects. We previously reported that eyes with stable visual field defects in patients with primary open-angle glaucoma showed a significantly higher end-diastolic velocity and a lower resistive index in retrobulbar blood flow compared with eyes with progressive visual field defects.15 It has been proposed that the retrobulbar circulation is correlated with the risk of visual field deterioration in patients with primary open-angle glaucoma.16,17 Our results suggest that changes in retrobulbar circulation, which correlate with intraocular pressure reduction, could delay the progression of visual field defects. However, detection of visual field progression requires understanding of variability, the magnitude of change that is considered clinically significant, and the number of visual field examinations required to detect visual field changes with adequate statistical power.18 In the present study, the visual field examination frequency was low and the postoperative observation period may have been too short to identify the progression of visual field changes. Furthermore, there are several definitions for the progression of glaucomatous visual field defects. Further studies are needed to clarify the relationship between the change in retrobulbar circulation and progression of visual field defects after trabeculectomy.
In contrast with the present study, others have reported that, despite a significant reduction in intraocular pressure, there were no significant differences in any ocular blood flow parameters before and after trabeculectomy.10 However, in these studies, a washout period for antiglaucoma medications before trabeculectomy was not included.10 There is a possibility that the topical antiglaucoma medications may have influenced ocular hemodynamics, and thus may have had a confounding effect on the postoperative evaluation. The effect of antiglaucoma medications on retrobulbar hemodynamics is still controversial.19–26 However, the effect mostly disappeared at least 4 weeks after discontinuation. For ethical reasons, it is clinically impossible to discontinue topical antiglaucoma medications for more than 4 weeks before trabeculectomy. In the present study, there was no change in topical antiglaucoma medications before and after surgery in the nonoperative contralateral eyes. However, postoperative discontinuation of the prescribed medication in the operative eyes might have had some effect on the retrobulbar circulation. The effects of topical therapy on ocular blood flow require additional studies.
The present study demonstrates that surgical reduction of intraocular pressure is effective in delaying visual field progression. However, cataract formation is the most prominent postoperative complication after trabeculectomy, possibly overshadowing the beneficial effect of intraocular pressure reduction on the progression of visual field defects.27 If it were not for the visual effects of cataract, many patients would be more satisfied with their postoperative vision. We must consider adjusting the treatment modality to improve the quality of vision for individual patients; for example, triple procedures for patients with glaucoma who are apparently destined to develop cataract.
In conclusion, the present study demonstrated a significant increase in end-diastolic velocity and decrease in resistive index in the central retinal artery and SPCA in the operated eyes of patients with primary open-angle glaucoma compared with the nonoperative contralateral eyes. The visual field defects were stable in the operative eyes but progressive in the nonoperative contralateral eyes. These findings suggest that trabeculectomy may improve the retrobulbar circulation and prevent visual field defect progression in patients with primary open-angle glaucoma. Further longitudinal studies are needed to investigate the relationship between retrobulbar circulation and visual field defect progression in patients with glaucoma.
Figure 2.
Time course change in mean BP.
Note: Error bars indicate the standard deviation of the mean.
Abbreviation: BP, blood pressure.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gandolfi SA. Improvement of visual field indices after surgical reduction of intraocular pressure. Ophthalmic Surg. 1995;26(2):121–126. [PubMed] [Google Scholar]
- 2.Bhadari A, Crabb DP, Poinoosawmy D, et al. Effect of surgery on visual field progression in normal-tension glaucoma. Ophthalmology. 1997;104(7):1131–1137. doi: 10.1016/s0161-6420(97)30172-9. [DOI] [PubMed] [Google Scholar]
- 3.Hagiwara Y, Yamamoto T, Kitazawa Y. The effect of mitomycin C trabeculectomy on the progression of visual field defect in normal-tension glaucoma. Graefes Arch Clin Exp Ophthalmol. 2000;238(3):232–236. doi: 10.1007/s004170050349. [DOI] [PubMed] [Google Scholar]
- 4.Folgar FA, De Moraes CGV, Prata TS, et al. Glaucoma surgery decreases the rate of localized and global visual field progression. Am J Ophthalmol. 2010;149(2):258–264. doi: 10.1016/j.ajo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression; results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 6.Trible JR, Sergott RC, Spaeth GL, et al. Trabeculectomy is associated with retrobulbar hemodynamic change. Ophthalmology. 1994;101(2):340–351. doi: 10.1016/s0161-6420(13)31332-3. [DOI] [PubMed] [Google Scholar]
- 7.Berisha F, Schmetterer K, Vass C, et al. Effect of trabeculectomy on blood flow. Br J Ophthalmol. 2005;89(2):185–188. doi: 10.1136/bjo.2004.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galassi F, Giambene B, Corvi A, et al. Retrobulbar hemodynamics and corneal surface temperature in glaucoma surgery. Int Ophthalmol. 2008;28(6):399–405. doi: 10.1007/s10792-007-9160-8. [DOI] [PubMed] [Google Scholar]
- 9.James CB. Effect of trabeculectomy on pulsatile ocular blood flow. Br J Ophthalmol. 1994;78(11):818–822. doi: 10.1136/bjo.78.11.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantor LB. The effect of trabeculectomy on ocular hemodynamics. Trans Am Ophthalmol Soc. 2001;99:241–252. [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki Y, Araie M, Hasegawa T, Nagahara M. Optic nerve head circulation after intraocular pressure reduction achieved by trabeculectomy. Ophthalmology. 2001;108(3):627–632. doi: 10.1016/s0161-6420(00)00541-8. [DOI] [PubMed] [Google Scholar]
- 12.Katz LJ, Costa VP, Spaeth GL. Filtration surgery. In: Ritch R, Shield MB, Krupin T, editors. The Glaucomas. 2nd ed. Vol. 3. St Louis, MO: Mosby; 1996. [Google Scholar]
- 13.Hayreh SS. The ophthalmic artery. III. Branches. Br J Ophthalmol. 1962;46(4):212–247. doi: 10.1136/bjo.46.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trible JR, Costa VP, Sergott RC, et al. The influence of primary open-angle glaucoma upon the retrobulbar circulation: baseline, postoperative and reproducibility analysis. Trans Am Ophthalmol Soc. 1993;91:245–281. [PMC free article] [PubMed] [Google Scholar]
- 15.Yamazaki Y, Drance SM. The relationship between progression of visual field defects and retrobulbar circulation in patients with glaucoma. Am J Ophthalmol. 1997;124(3):287–295. doi: 10.1016/s0002-9394(14)70820-7. [DOI] [PubMed] [Google Scholar]
- 16.Galassi F, Sodi A, Ucci F, et al. Ocular hemodynamics and glaucoma prognosis. A color Doppler imaging study. Arch Ophthalmol. 2003;121(12):1711–1715. doi: 10.1001/archopht.121.12.1711. [DOI] [PubMed] [Google Scholar]
- 17.Martinez A, Sanchez M. Predictive value of colour Doppler imaging in prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol Scand. 2005;83(5):716–722. doi: 10.1111/j.1600-0420.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92(4):569–573. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flammer J, Orgul S, Costa VF, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(2):359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 20.Harris A, Spaeth GL, Sergott RC, et al. Retrobulbar arterial hemodynamic effects of betaxolol and timolol in normal-tension glaucoma. Am J Ophthalmol. 1995;120(2):165–175. doi: 10.1016/s0002-9394(14)72604-2. [DOI] [PubMed] [Google Scholar]
- 21.Nicolela MT, Walman BE, Buckley AR, et al. A comparative study of the effects of timolol and latanoprost on blood flow velocity of the retrobulbar vessels. Am J Ophthalmol. 1996;122(6):784–789. doi: 10.1016/s0002-9394(14)70374-5. [DOI] [PubMed] [Google Scholar]
- 22.Bergstrand IC, Heijl A, Harris A. Dorzolamide and ocular blood flow in previous untreated glaucoma patients: a controlled double-masked study. Acta Ophthalmol Scand. 2002;80(2):176–182. doi: 10.1034/j.1600-0420.2002.800211.x. [DOI] [PubMed] [Google Scholar]
- 23.Costa VP, Harris A, Stefānsson E, et al. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res. 2003;22(6):769–805. doi: 10.1016/s1350-9462(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 24.Altan-Yaycioglu R, Turder G, Akdol S, et al. The effects of beta-blockers on ocular blood flow in patients with primary open angle glaucoma: a color Doppler imaging study. Eur J Ophthalmol. 2001;11(1):37–46. doi: 10.1177/112067210101100108. [DOI] [PubMed] [Google Scholar]
- 25.Vetrugno M, Cantatore F, Gigante G, et al. Latanoprost 0.0005% in POAG: effects on IOP and ocular blood flow. Acta Ophthalmol Scand. 1998;227(Suppl):40–41. doi: 10.1111/j.1600-0420.1998.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 26.Martinez A, Gonzalez F, Capeans C, et al. Dorzolamide effect on ocular blood flow. Invest Ophthalmol Vis Sci. 1999;40(6):1270–1275. [PubMed] [Google Scholar]
- 27.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]