Abstract
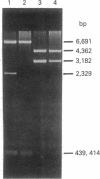
3T3 cells containing hepatitis B virus DNA sequences can be efficiently selected by exposure to methotrexate after cotransfection with cloned viral DNA and DNA coding for a methotrexate-resistant dihydrofolate reductase. More than 75% of methotrexate-resistant cells isolated after cotransfection with a head-to-tail tandem of the hepatitis B virus genome synthesized viral surface antigen. The antigen was released into the culture medium in the form of 22-nm particles with buoyant density of 1.20 g/ml. No other virally coded proteins were detected in the cells or the culture medium. Application of selective pressure by increasing the concentration of methotrexate resulted in an amplification of viral DNA sequences and a concomitant increase in the rate of synthesis and release of hepatitis B surface antigen. The ability to produce large amounts of surface antigen appears to be a stable trait and has been maintained in these cultures through more than 30 passages.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J., Bey E. M., Geddes E. W., Lecatsas G. Establishment of a continuously growing cell line from primary carcinoma of the liver. S Afr Med J. 1976 Dec 18;50(54):2124–2128. [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugaiczyk A., Boyer H. W., Goodman H. M. Ligation of EcoRI endonuclease-generated DNA fragments into linear and circular structures. J Mol Biol. 1975 Jul 25;96(1):171–184. doi: 10.1016/0022-2836(75)90189-8. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Garfinkel E., Hirschman S. Z., Thung S. N., Panagiotatos T. Immune and enzyme histochemical studies of a human hepatocellular carcinoma cell line producing hepatitis B surface antigen. J Immunol. 1981 Mar;126(3):1085–1089. [PubMed] [Google Scholar]
- Gerin J. L., Holland P. V., Purcell R. H. Australia antigen: large-scale purification from human serum and biochemical studies of its proteins. J Virol. 1971 May;7(5):569–576. doi: 10.1128/jvi.7.5.569-576.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Gerber M., Garfinkel E. Purification of naked intranuclear particles from human liver infected by hepatitis B virus. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3345–3349. doi: 10.1073/pnas.71.9.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman S. Z., Price P., Garfinkel E., Christman J., Acs G. Expression of cloned hepatitis B virus DNA in human cell cultures. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5507–5511. doi: 10.1073/pnas.77.9.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Moriarty A. M., Hoyer B. H., Shih J. W., Gerin J. L., Hamer D. H. Expression of the hepatitis B virus surface antigen gene in cell culture by using a simian virus 40 vector. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2606–2610. doi: 10.1073/pnas.78.4.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Gray P., Quiroga M., Zaldivar J., Goodman H. M., Rutter W. J. Nucleotide sequence of the gene coding for the major protein of hepatitis B virus surface antigen. Nature. 1979 Aug 30;280(5725):815–819. doi: 10.1038/280815a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Perucho M., Kurtz D., Dana S., Pellicer A., Axel R., Silverstein S. Transformation of mammalian cells with an amplifiable dominant-acting gene. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3567–3570. doi: 10.1073/pnas.77.6.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]