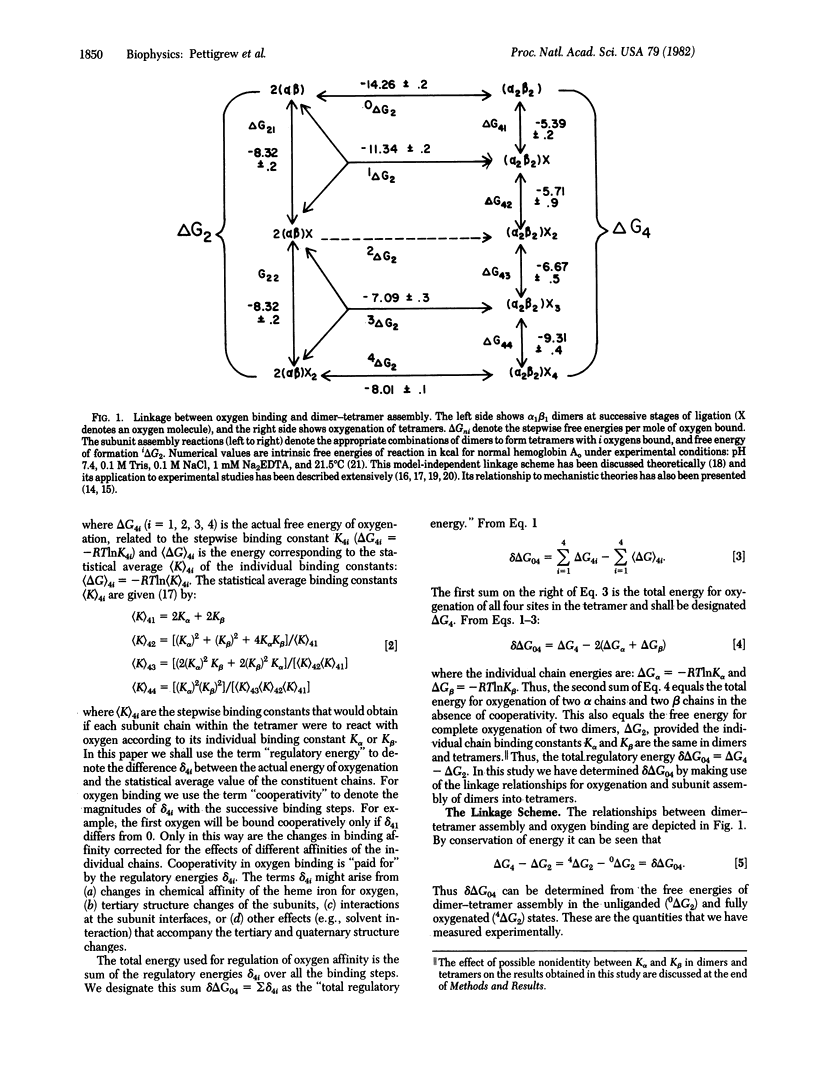
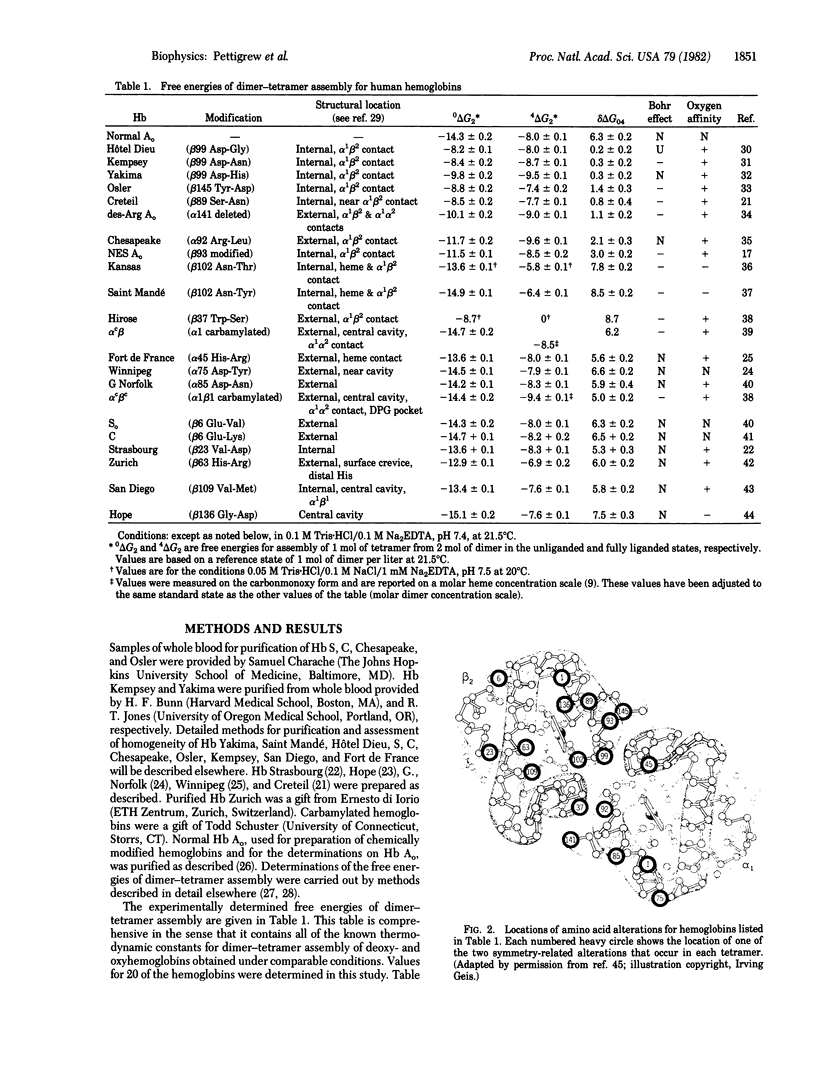
Abstract
The sites of energy transduction within the human hemoglobin molecule for the regulation of oxygen affinity have been determined by an extensive study of the molecule's energetic response to structural alteration at individual amino acid residues. For 22 mutant and chemically modified hemoglobins we have determined the total free energy used by the tetrameric molecule for alteration of oxygen affinity at the four binding steps. The results imply that the regulation of oxygen binding affinity is due to energy changes which are mostly localized at the alpha 1 beta 2 interface. They also indicate a high degree of "internal cooperativity" within this contact region--i.e., the structural perturbations at individual residue sites are energetically coupled. Cooperativity in ligand binding is thus a reflection of cooperativity at a deeper level--that of the protein-protein interactions within the alpha 1 beta 2 interfacial domain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K. Energetics of subunit assembly and ligand binding in human hemoglobin. Biophys J. 1980 Oct;32(1):331–346. doi: 10.1016/S0006-3495(80)84960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackers G. K., Halvorson H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4312–4316. doi: 10.1073/pnas.71.11.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone A., Thillet J., Rosa J. The structure of hemoglobin Creteil (beta 89 Ser replaced by Asn) is similar to that of abnormal human hemoglobins having sequence changes at Tyr 145 beta. J Biol Chem. 1981 Aug 25;256(16):8545–8552. [PubMed] [Google Scholar]
- Arous N., Braconnier F., Thillet J., Blouquit Y., Galacteros F., Chevrier M., Bordahandy C., Rosa J. Hemoglobin Saint Mandé beta 102 (G4) asn replaced by tyr: a new low oxygen affinity variant. FEBS Lett. 1981 Apr 6;126(1):114–116. doi: 10.1016/0014-5793(81)81046-0. [DOI] [PubMed] [Google Scholar]
- Atha D. H., Johnson M. L., Riggs A. F. The linkage between oxygenation and subunit association in human hemoglobin Kansas. Concentration dependence of the oxygen binding equilibria. J Biol Chem. 1979 Dec 25;254(24):12390–12398. [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Blouquit Y., Braconnier F., Galacteros F., Arous N., Soria J., Zittoun R., Rosa J. Hemoglobin Hotel-Dieu beta 99 Asp replaced by Gly (g1). A new abnormal hemoglobin with high oxygen affinity. Hemoglobin. 1981;5(1):19–31. doi: 10.3109/03630268108996908. [DOI] [PubMed] [Google Scholar]
- Braconnier F., Gacon G., Thillet J., Wajcman H., Soria J., Maigret P., Labie D., Rosa J. Hemoglobin Fort de France (alpha2(45)(CD3) His replaced by Arg beta2). A new variant with increased oxygen affinity. Biochim Biophys Acta. 1977 Jul 22;493(1):228–233. doi: 10.1016/0005-2795(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Bucci E., Fronticelli C., Nicklas J., Charache S. Conformation in solution of hemoglobin Osler (alpha 2 A beta 2 145 Tyr replaced by Asp). J Biol Chem. 1979 Nov 10;254(21):10811–10819. [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Wohl R. C., Bradley T. B., Cooley M., Gibson Q. H. Functional properties of hemoglobin Kempsey. J Biol Chem. 1974 Dec 10;249(23):7402–7409. [PubMed] [Google Scholar]
- Chu A. H., Ackers G. K. Mutual effects of protons, NaCl, and oxygen on the dimer-tetramer assembly of human hemoglobin. The dimer Bohr effect. J Biol Chem. 1981 Feb 10;256(3):1199–1205. [PubMed] [Google Scholar]
- Cohen-Solal M., Manesse B., Thillet J., Rosa J. Haemoglobin G Norfolk alpha 85 (F6) Asp leads to Asn. Structural characterization by sequenator analysis and functional properties of a new variant with high oxygen affinity. FEBS Lett. 1975 Feb 1;50(2):163–167. doi: 10.1016/0014-5793(75)80480-7. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal M., North M. L., Thillet J., Albrecht-Ellmer K., Garel M. C., Blouquit Y., Rosa J., Valentin C. Haemoglobin Strasbourg alpha2beta2 23 (B5) Val replaced by Asp: revised structure anf functional properties. FEBS Lett. 1978 Jun 15;90(2):286–288. doi: 10.1016/0014-5793(78)80387-1. [DOI] [PubMed] [Google Scholar]
- Dunn B. M., Chaiken I. M. Relationship between alpha-helical propensity and formation of the ribonuclease-S complex. J Mol Biol. 1975 Jul 15;95(4):497–511. doi: 10.1016/0022-2836(75)90313-7. [DOI] [PubMed] [Google Scholar]
- Flanagan M. A., Ackers G. K., Matthew J. B., Hanania G. I., Gurd F. R. Electrostatic contributions to the energetics of dimer-tetramer assembly in human hemoglobin: pH dependence and effect of specifically bound chloride ions. Biochemistry. 1981 Dec 22;20(26):7439–7449. doi: 10.1021/bi00529a018. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Petsko G. A., Tsernoglou D. Temperature-dependent X-ray diffraction as a probe of protein structural dynamics. Nature. 1979 Aug 16;280(5723):558–563. doi: 10.1038/280558a0. [DOI] [PubMed] [Google Scholar]
- Gelin B. R., Karplus M. Mechanism of tertiary structural change in hemoglobin. Proc Natl Acad Sci U S A. 1977 Mar;74(3):801–805. doi: 10.1073/pnas.74.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. J., Benedict R. C., Fall L., Spokane R., Wyman J. Oxygen binding to sickle cell hemoglobin. J Mol Biol. 1979 May 15;130(2):175–189. doi: 10.1016/0022-2836(79)90425-x. [DOI] [PubMed] [Google Scholar]
- Grütter M. G., Hawkes R. B., Matthews B. W. Molecular basis of thermostability in the lysozyme from bacteriophage T4. Nature. 1979 Feb 22;277(5698):667–669. doi: 10.1038/277667a0. [DOI] [PubMed] [Google Scholar]
- Ho C., Lindstrom T. R., Baldassare J. J., Breen J. J. Magnetic resonance studies of human hemoglobins and their implications to the structure-function relationships in human normal and abnormal hemoglobins. Ann N Y Acad Sci. 1973 Dec 31;222:21–39. doi: 10.1111/j.1749-6632.1973.tb15250.x. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Relation between structure, co-operativity and spectra in a model of hemoglobin action. J Mol Biol. 1973 Jun 25;77(2):207–222. doi: 10.1016/0022-2836(73)90332-x. [DOI] [PubMed] [Google Scholar]
- Imai K. Hemoglobin Chesapeake (92 alpha, arginine--leucine). Precise measurements and analyses of oxygen equilibrium. J Biol Chem. 1974 Dec 10;249(23):7607–7612. [PubMed] [Google Scholar]
- Johnson M. L., Ackers G. K. Thermodynamic analysis of human hemoglobins in terms of the Perutz mechanism: extensions of the Szabo--Karplus model to include subunit assembly. Biochemistry. 1982 Jan 19;21(2):201–211. doi: 10.1021/bi00531a001. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Hewitt J. A., Wootton J. F. Alteration of functional properties associated with the change in quaternary structure in unliganded haemoglobin. J Mol Biol. 1975 Apr 5;93(2):203–218. doi: 10.1016/0022-2836(75)90128-x. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Weber G. Quenching of protein fluorescence by oxygen. Detection of structural fluctuations in proteins on the nanosecond time scale. Biochemistry. 1973 Oct 9;12(21):4171–4179. doi: 10.1021/bi00745a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin E. L., Englander S. W. The slowest allosterically responsive hydrogens in hemoglobin. Completion of the hydrogen exchange survey. J Biol Chem. 1980 Nov 25;255(22):10695–10701. [PubMed] [Google Scholar]
- Matthew J. B., Hanania G. I., Gurd F. R. Electrostatic effects in hemoglobin: Bohr effect and ionic strength dependence of individual groups. Biochemistry. 1979 May 15;18(10):1928–1936. doi: 10.1021/bi00577a012. [DOI] [PubMed] [Google Scholar]
- Mills F. C., Ackers G. K. Quaternary enhancement in binding of oxygen by human hemoglobin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):273–277. doi: 10.1073/pnas.76.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F. C., Johnson M. L., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: experimental studies on the concentration dependence of oxygenation curves. Biochemistry. 1976 Nov 30;15(24):5350–5362. doi: 10.1021/bi00669a023. [DOI] [PubMed] [Google Scholar]
- Nagai M., Nishibu M., Sugita Y., Yoneyama Y. The effects of inositol hexaphosphate on the allosteric properties of two beta-99-substituted abnormal hemoglobins, hemoglobin Yakima and hemoglobin Kempsey. J Biol Chem. 1975 Apr 25;250(8):3169–3173. [PubMed] [Google Scholar]
- Nute P. E., Stamatoyannopoulos G., Hermodson M. A., Roth D. Hemoglobinopathic erythrocytosis due to a new electrophoretically silent variant, hemoglobin San Diego (beta109 (G11)val--met). J Clin Invest. 1974 Jan;53(1):320–328. doi: 10.1172/JCI107553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Electrostatic effects in proteins. Science. 1978 Sep 29;201(4362):1187–1191. doi: 10.1126/science.694508. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Sack J. S., Andrews L. C., Magnus K. A., Hanson J. C., Rubin J., Love W. E. Location of amino acid residues in human deoxy hemoglobin. Hemoglobin. 1978;2(2):153–169. doi: 10.3109/03630267809074782. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Imamura T., Yanase T. Hemoglobin Hirose, a human hemoglobin variant with a substitution at the alpha1beta2 interface. Subunit dissociation and the equilibria and kinetics of ligand binding. J Biol Chem. 1978 Jan 10;253(1):87–94. [PubMed] [Google Scholar]
- The Bohr effect and combination with organic phosphates. Nature. 1970 Nov 21;228(5273):734–739. doi: 10.1038/228734a0. [DOI] [PubMed] [Google Scholar]
- Thillet J., Caburi J., Brun B., Cohen-Solal M., Garel M. C., Minh M. N., Rosa J. Abnormal functional properties of Hb Hope alpha C2A beta2 (H14) Gly-Asp: a low oxygen affinity hemoglobin with decreased DPG effect. FEBS Lett. 1974 Oct 1;47(1):47–52. doi: 10.1016/0014-5793(74)80423-0. [DOI] [PubMed] [Google Scholar]
- Thillet J., Caburi J., Brun B., Cohen-Solal M., Garel M. C., Minh M. N., Rosa J. Abnormal functional properties of Hb Hope alpha C2A beta2 (H14) Gly-Asp: a low oxygen affinity hemoglobin with decreased DPG effect. FEBS Lett. 1974 Oct 1;47(1):47–52. doi: 10.1016/0014-5793(74)80423-0. [DOI] [PubMed] [Google Scholar]
- Turner B. W., Pettigrew D. W., Ackers G. K. Measurement and analysis of ligand-linked subunit dissociation equilibria in human hemoglobins. Methods Enzymol. 1981;76:596–628. doi: 10.1016/0076-6879(81)76147-0. [DOI] [PubMed] [Google Scholar]
- Valdes R., Jr, Ackers G. K. Study of protein subunit association equilibria by elution gel chromatography. Methods Enzymol. 1979;61:125–142. doi: 10.1016/0076-6879(79)61011-x. [DOI] [PubMed] [Google Scholar]
- Vella F., Wiltshire B., Lehmann H., Galbraith P. Hemoglobin Winnipeg: alpha2 75 Asp leads to Tyr beta2. Clin Biochem. 1973 Jun;6(2):66–70. doi: 10.1016/s0009-9120(73)80014-1. [DOI] [PubMed] [Google Scholar]
- Wang C., Yang Y. R., Hu C. Y., Schachman H. K. Communication between subunits in aspartate transcarbamoylase. Effect of active site ligands on the tertiary structure of regulatory chains. J Biol Chem. 1981 Jul 10;256(13):7028–7034. [PubMed] [Google Scholar]
- Welply J. K., Fowler A. V., Zabin I. beta-Galactosidase alpha-complementation. Effect of single amino acid substitutions. J Biol Chem. 1981 Jul 10;256(13):6811–6816. [PubMed] [Google Scholar]
- Williams R. C., Jr, Kim H. Carbamoylated hemoglobins A and S: physical properties. Biochemistry. 1976 May 18;15(10):2207–2211. doi: 10.1021/bi00655a028. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Tsay K. Y. A convenient chromatographic method for the preparation of human hemoglobin. Anal Biochem. 1973 Jul;54(1):137–145. doi: 10.1016/0003-2697(73)90256-x. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Anderson N. M., Amiconi G., Antonini E., Brunori M. Functional properties of hemoglobin Zürich. Eur J Biochem. 1969 Dec;11(3):435–440. doi: 10.1111/j.1432-1033.1969.tb00793.x. [DOI] [PubMed] [Google Scholar]



