Abstract
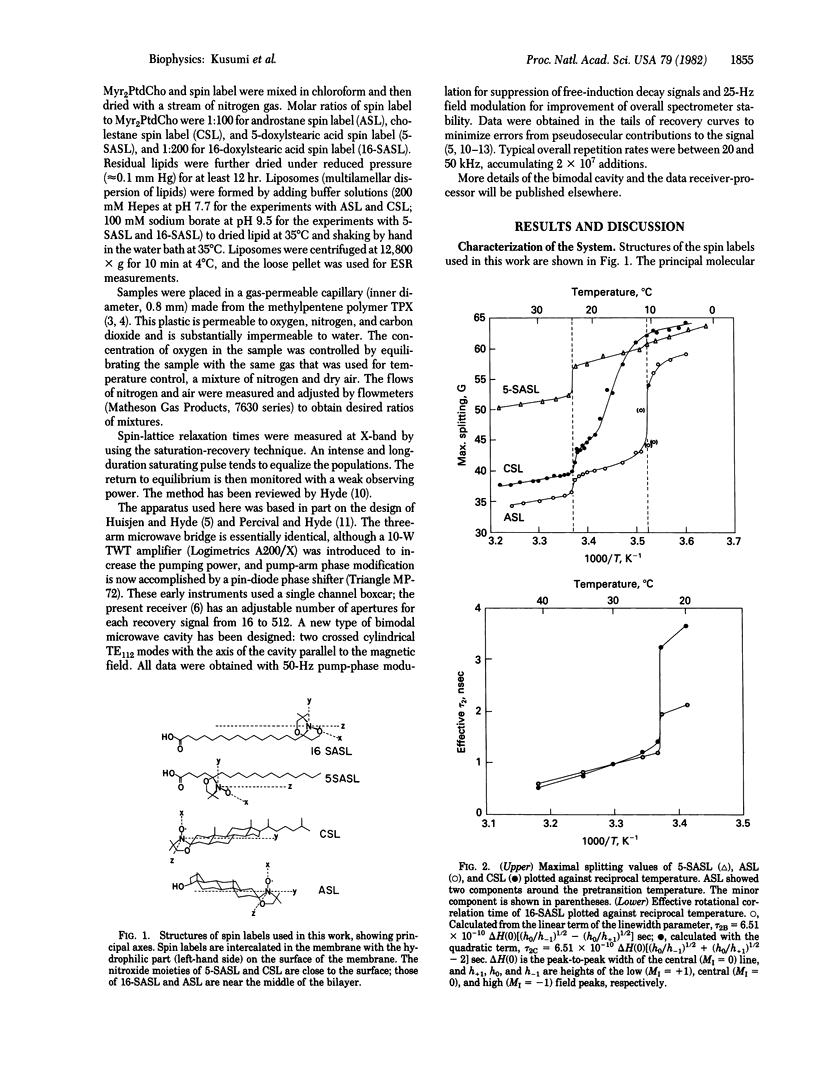
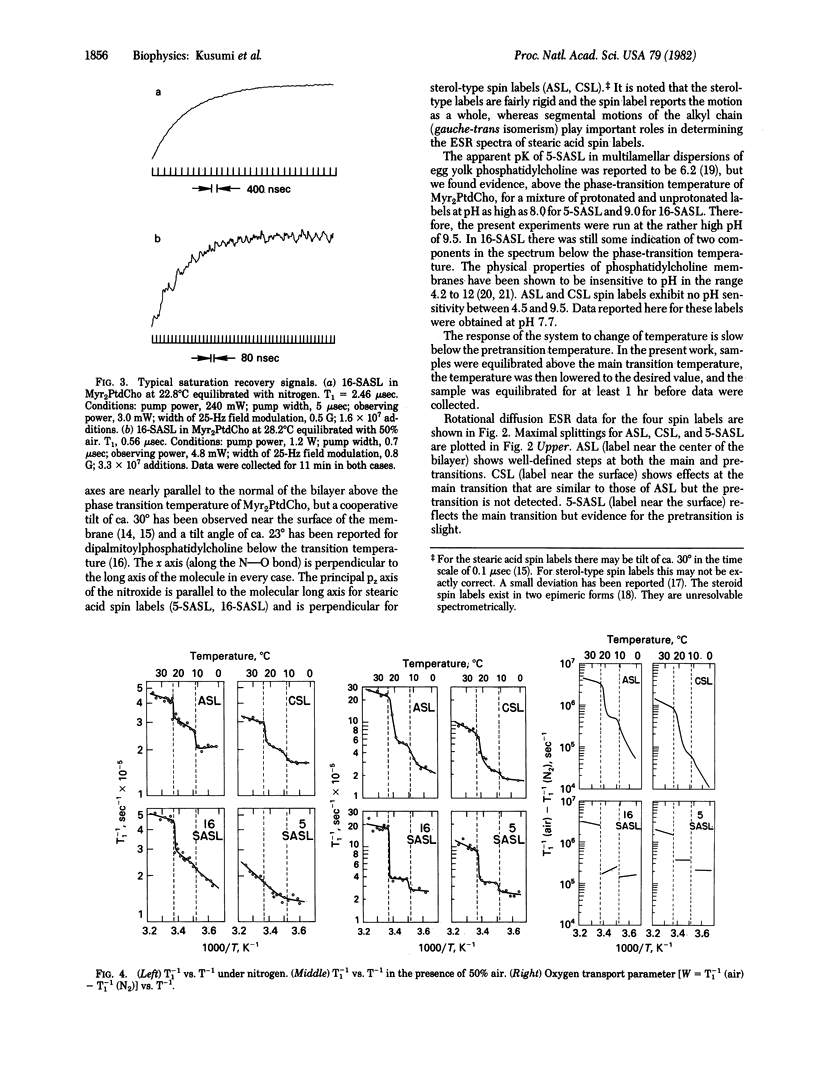
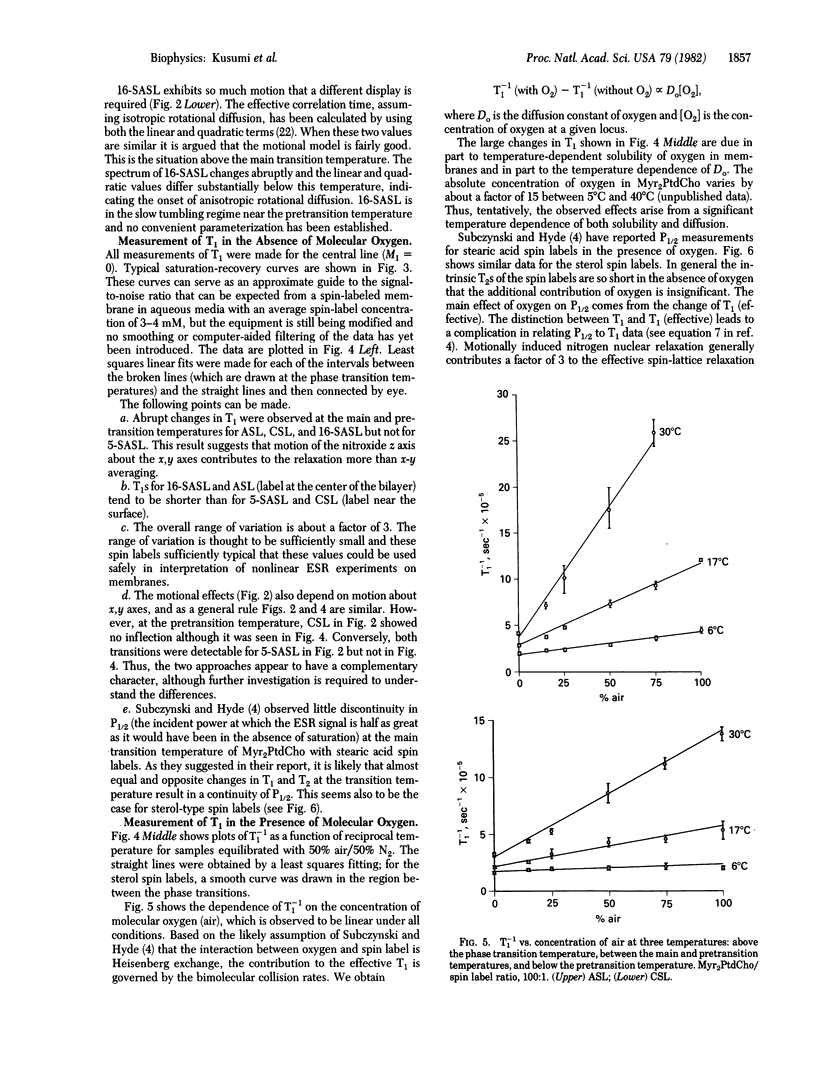
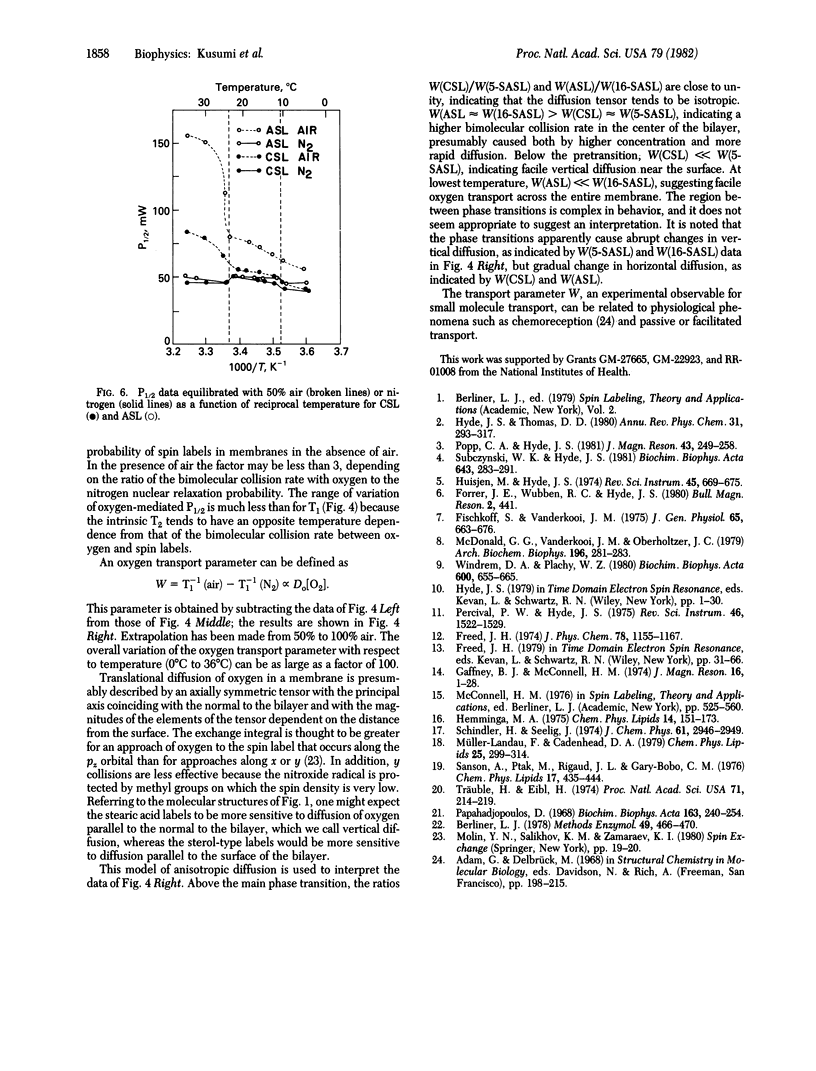
Spin-lattice relaxation time (T1) measurements of nitroxide radical spin labels in membranes have been made by using the saturation-recovery technique. Stearic acid and sterol-type labels were used as probes of dimyristoylphosphatidylcholine liposomes from 0 degrees C to 36 degrees C. In the absence of oxygen, the range of variation of T1 over all samples and conditions is about a factor of 3. Heisenberg exchange between oxygen and spin labels is an effective T1 mechanism for the spin labels. The full range of variation of T1 in the presence of air is about a factor of 100. It is suggested that the oxygen transport parameter W = T1(-1) (air) - T1(-1) (N2) is a useful new monitor of membrane fluidity that reports on translational diffusion of small molecules. The values of W change at the prephase and main phase transitions and vary in complex ways. Arguments are advanced that the data are indicative of anisotropic translational diffusion of oxygen.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fischkoff S., Vanderkooi J. M. Oxygen diffusion in biological and artificial membranes determined by the fluorochrome pyrene. J Gen Physiol. 1975 May;65(5):663–676. doi: 10.1085/jgp.65.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminga M. A. An ESR Spin label study of structural and dynamical properties of oriented lecithin-cholesterol multibilayers. Chem Phys Lipids. 1975 Apr;14(2):151–173. doi: 10.1016/0009-3084(75)90057-2. [DOI] [PubMed] [Google Scholar]
- McDonald G. G., Vanderkooi J. M., Oberholtzer J. C. Oxygen diffusion in phospholipid artificial membranes studied by Fourier transform nuclear magnetic resonance. Arch Biochem Biophys. 1979 Aug;196(1):281–283. doi: 10.1016/0003-9861(79)90577-0. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Sanson A., Ptak M., Rigaud J. L., Gary-Bobo C. M. An ESR study of the anchoring of spin-labeled stearic acid in lecithin multilayers. Chem Phys Lipids. 1976 Nov;17(4):435–444. doi: 10.1016/0009-3084(76)90045-1. [DOI] [PubMed] [Google Scholar]
- Subczynski W. K., Hyde J. S. The diffusion-concentration product of oxygen in lipid bilayers using the spin-label T1 method. Biochim Biophys Acta. 1981 May 6;643(2):283–291. [PubMed] [Google Scholar]
- Träuble H., Eibl H. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc Natl Acad Sci U S A. 1974 Jan;71(1):214–219. doi: 10.1073/pnas.71.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem D. A., Plachy W. Z. The diffusion-solubility of oxygen in lipid bilayers. Biochim Biophys Acta. 1980 Aug 14;600(3):655–665. doi: 10.1016/0005-2736(80)90469-1. [DOI] [PubMed] [Google Scholar]



