Abstract
Objective
The goals of this study were to describe the clinical and anatomic features of infants undergoing Kasai portoenterostomy (KPE) for biliary atresia (BA), and to examine associations between these parameters and outcomes.
Methods
Infants enrolled in the prospective Childhood Liver Disease Research and Education Network, who underwent KPE were studied. Patients enrolled in a blinded, interventional trial were excluded from survival analysis. Primary end-points were successful surgical drainage (total bilirubin less than 2 mg/dL within the first three months), transplant-free survival (Kaplan-Meier), and time to transplant/death (Cox regression).
Results
KPE was performed in 244 infants (54% female; mean age 65± 29 days). Transplant-free survival was 53.7% and 46.7% at 1 and 2 years post-KPE. The risk of transplant/death was significantly lower in the 45.6% of patients who achieved successful bile drainage within 3 months post-KPE (HR 0.08, p<0.001). The risk of transplant/death was increased in patients with porta hepatis atresia (Ohi Type II and III vs. Type I; HR 2.03, p=0.030), non-patent common bile duct (Ohi Subtype b, c, and d vs. a; HR 4.31, p=0.022), BA splenic malformation syndrome (HR 1.92, p=0.025), ascites > 20 ml (HR=1.90, p=0.0230), nodular liver appearance compared to firm (HR=1.61, p=0.008), and age at KPE ≥ 75 days (HR 1.73, p<0.002). Outcome was not associated with gestational age, gender, race, ethnicity, or extent of porta hepatis dissection.
Conclusion
Anatomic pattern of BA, BASM, presence of ascites and nodular liver appearance at KPE, and early postoperative jaundice clearance are significant predictors of transplant-free survival.
Keywords: Biliary atresia, Kasai portoenterostomy, jaundice, hepatobiliary, Biliary Atresia Research Consortium
Introduction
Biliary atresia (BA) is an idiopathic neonatal hepatobiliary disease characterized by progressive fibrosing obstruction of the extrahepatic biliary tree. BA is the most common cause of neonatal direct hyperbilirubinemia, occurring in approximately 1 in 8,000 to 18,000 live births.1 In the United States, there are approximately 250 to 400 cases of BA annually. The only effective treatments of BA are surgical drainage of the biliary tree or liver transplantation.1 Without drainage, BA inevitably progresses to cirrhosis, end-stage liver failure and death within three years of life.2 BA is the most common indication for pediatric liver transplantation in the world, accounting for nearly 50% of all transplants transplants in children and 10% of all transplants.1, 3 In the United States, $77 million is spent annually on pediatric liver transplantation-related costs, disproportionately representing 0.2% of total health care expenditures for only 0.0006% of the entire pediatric population.1
The pathogenesis of BA is incompletely understood but appears to be multifactorial. Between 10 and 20 percent of patients with BA have associated congenital malformations, such as abdominal and thoracic heterotaxia, polysplenia, asplenia, intestinal malrotation, and preduodenal portal vein. The association of BA with these anomalies suggests a developmental defect in ductal plate formation.4 BA has also been associated with prenatal exposure to viruses such as cytomegalovirus, reovirus, and rotavirus.5–8 Additionally, environmental toxins and neonatal immune dysregulation have been implicated in the pathogenesis of BA.9, 10 Indeed, BA may represent a final common pathway of bile duct injury in response to a combination of these factors.1, 11
In 1959, Kasai first reported the surgical technique of portoenterostomy for the treatment of BA.12 In the Kasai procedure, the obliterated biliary remnant is excised and the portal plate is drained with a Roux-en-Y hepatojejunostomy. Successful drainage of the biliary tree is essential for transplant-free survival. However, successful drainage does not necessarily predict transplant-free survival as progressive or irreversible liver injury can occur despite adequate drainage. Over the past several decades, numerous clinical, surgical, and pathologic factors predictive of a successful portoenterostomy and/or transplant-free survival have been defined.1, 13–15 Early diagnosis, absence of associated congenital malformations, certain anatomic variants of BA, and freedom from postoperative ascending cholangitis are factors that are predictive of drainage and survival.16 Defining accurate prognostic factors in children with BA has been limited because most studies have been from single institutions, are retrospective, and limited in size. To date, there has been no large-scale prospective analysis of the clinical and surgical factors affecting outcome after portoenterostomy in the United States.
The Biliary Atresia Research Consortium (BARC) was formed in 2002 as a National Institutes of Health (NIH)–sponsored collaborative network of 10 pediatric institutions and a data coordinating center for the purpose of conducting prospective clinical and basic research in BA.17 In 2010, BARC merged with the Cholestatic Liver Disease Consortium (CLiC) to form the NIDDK-supported Childhood Liver Disease Research and Education Network (ChiLDREN). There are currently 16 children’s hospitals and medical centers in North America, which participate in ChiLDREN. Investigators from each site included pediatric hepatologists, pediatric surgeons, pediatric pathologists, and others. Herein, we report on prospectively collected clinical, surgical, and outcome data of 244 contemporaneously treated BA patients who have undergone Kasai portoenterostomy. From this prospective database, we attempt to identify clinical and surgical variables associated with subsequent successful biliary drainage, as defined by achieving a total serum bilirubin concentration of less than 2.0 mg/dL in the first three months post drainage as well as longer term transplant-free survival.
Methods
Study participants
Between June 1, 2004, and March 31, 2010, 530 infants were enrolled in a prospective longitudinal study of cholestasis in infancy (PROBE: Clinicaltrials.gov NCT00061828) performed by ChiLDREN. To be eligible to enroll in PROBE, subjects needed to present to a participating clinical center prior to 180 days of age with cholestasis, defined as a serum direct or conjugated bilirubin greater than or equal to 2 mg/dL and greater than 20% of total bilirubin. Infants who had undergone previous hepatobiliary surgery with dissection or excision of biliary tissue prior to presentation at a ChiLDREN site were not eligible for enrollment. Informed consent was obtained from the study participant’s parents or guardians, and the protocol was carried out under IRB approval.
Of the subjects enrolled in PROBE, 267 were subsequently diagnosed with BA and they represent the baseline cohort in this report. After September 1, 2005, PROBE subjects diagnosed with BA who underwent a drainage procedure were also eligible to be enrolled in a prospective randomized double-blinded, placebo-controlled trial of corticosteroid therapy after hepatoportoenterostomy for BA (START: Clinicaltrials.gov NCT 00294684). While ChiLDREN has successfully reached the target enrollment (n=140) for START, the subjects are in active follow-up and the study remains blinded. As a result, the outcomes analysis reported herein excludes all PROBE subjects who were also enrolled in START in this baseline cohort (n=108), resulting in 159 patients studied.
Data collection
Data were collected by study research coordinators and clinical investigators and were entered into a centralized database at the DCC. Baseline data collected included demographics, medical history, and laboratory studies. Operative details were recorded on a case report form by the attending surgeon. Variables included an assessment of the gross appearance of the liver and biliary tree (Ohi type and subtypes, Figure 1), as well as the presence and amount of ascites. Other data recorded included the presence of specific abdominal anomalies (polysplenia, asplenia, intestinal malrotation, situs inversus, midline liver, and pre-duodenal portal vein); type of drainage procedure performed and the extent of hilar dissection at the portal plate (left-to-right and anterior-to-posterior); intraoperative diagnosis; complications at surgery; and volume of blood transfusions. The presence of splenic malformations (polysplenia or asplenia) documented anytime during the evaluation and treatment phase was used to classify the infant as having BA splenic malformation syndrome (BASM). Subjects not classified as BASM, but who were noted intraoperatively to have any of the other above abdominal anomalies, were classified as having non-BASM abdominal anomalies.
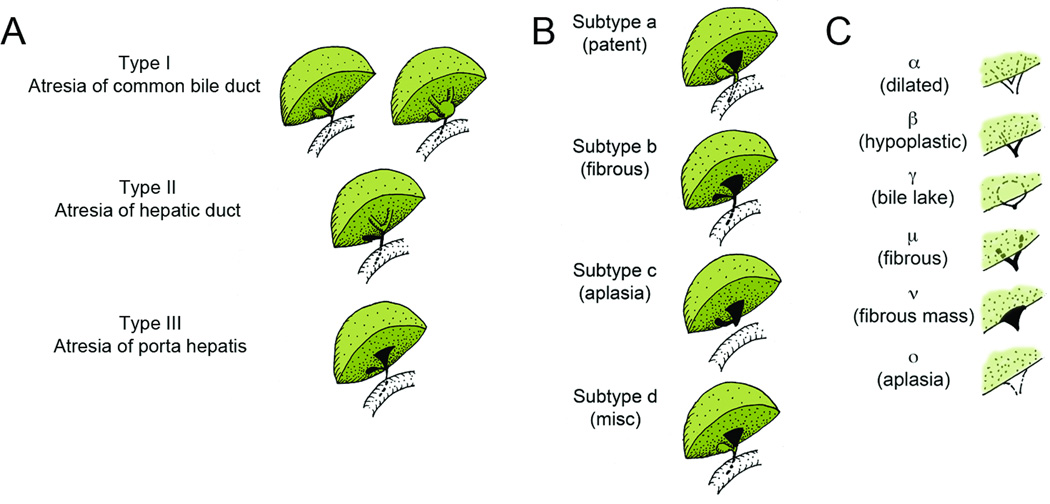
Figure 1.
Schematic of Ohi (A) main types, (B) distal subtypes, and (C) proximal subtypes.
Follow-up visits for data collection occurred at one, two, three, six months, and eighteen months post operatively. Thereafter, annual follow-up was performed on the subject’s birth date. Follow-up continued until liver transplant, death, withdrawal from the study, or January 31, 2011.
Liver biopsy histology obtained during the initial evaluation process or at time of surgery was evaluated centrally by the ChiLDREN Pathology Committee using a validated standardized assessment instrument.18 Fibrosis was quantified using the Ishak scoring system. This Pathology Committee evaluation of liver and bile duct histology is an integral aspect of the PROBE study, and is blinded with respect to diagnosis and outcomes.
Data analysis
Descriptive data were summarized as the mean ± standard deviation (SD) for continuous variables and as percentages for categorical variables. Outcomes were summarized using Kaplan Meier analysis. In addition to the descriptive analysis of baseline and intraoperative variables, we evaluated their impact on outcomes following surgical drainage. The probability of achieving a successful surgical drainage, defined as achieving a total serum bilirubin less than 2.0 mg/dL in the first 3 months post drainage19 was evaluated using univariate conditional (matching on study site) logistic regression. The role of these factors in transplant-free survival was evaluated using Cox models for time to transplant or death with native liver, after ensuring assumptions of proportionality were maintained and clustering for study site. In both outcome analyses, the extent and randomness of missing data was evaluated. Missing baseline data were managed with multiple imputation utilizing the entire baseline cohort. The functional forms of specific individual variables were explored and optimized. Of specific note, age at KPE and liver biopsy fibrosis scores were evaluated as both continuous variables and as categorical variables with thresholds determined by inflection in the risk of transplant or death. In all outcomes analyses, subjects were censored at date of last follow-up. As mentioned previously, all outcome analyses were restricted to the 159 PROBE subjects with BA who were not enrolled in the START trial.
All analyses were performed using SAS/STAT (SAS Institute Inc. 2008. SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc).
Results
Baseline
A total of 267 infants enrolled in PROBE were diagnosed with BA. Of these infants, 17 did not undergo any surgery and 6 underwent surgery but no drainage procedure was performed. The primary reason cited for not proceeding with drainage was severity of liver disease and/or late age at presentation. Of patients without surgical drainage, all but a few progressed to end-stage liver disease and death or transplant within the first year of life (Figure 2). The remaining 244 underwent surgical exploration and drainage of the biliary tree. Nearly all who underwent a drainage procedure had a Roux en-Y Kasai performed (n=237). There were 6 subjects who had a portocholecystostomy (gallbladder Kasai) and 1 subject underwent a choledochojejunostomy. Five of the drainage procedures were performed laparoscopically. Infants who did not undergo surgery or who were explored but did not undergo drainage, were significantly older at time of enrollment (142.8 ± 37.6 and 155.2 ± 69.7 days, respectively) compared to those who underwent a drainage procedure (61.8 ± 25.7 days).
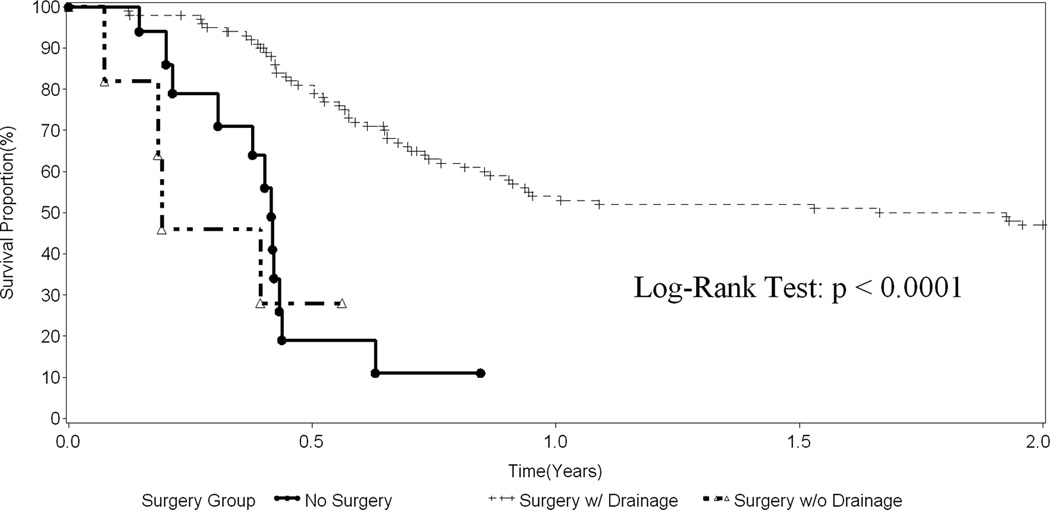
Figure 2.
Transplant-free survival in infants with BA stratified by no surgery (n=17), surgery but no drainage procedure (n=6), and surgery with drainage procedure (n=136).
Baseline demographics of the 244 subjects who underwent surgical drainage are summarized in Table 1. There was a female predominance and a history of prematurity (gestational age < 37 weeks) in 26.6 % of infants. Operative findings are summarized in Table 2. Mean age at surgery was 64.6 ± 25.1 days. Intestinal malrotation was the most frequent abdominal malformation noted (9.4%). Polysplenia or asplenia was noted intraoperatively in 5.7% of infants. BASM, as defined as polysplenia or asplenia determined at any time during evaluation or treatment, was reported in 19 (7.7%) of subjects.
Table 1.
Baseline demographics of 244 BA subjects who underwent surgical drainage
| N (%) or Mean ±SD | |
|---|---|
| Gender: | (N) |
| Male | 113 (46.3%) |
| Female | 131 (53.7%) |
| Race: | |
| White | 146 (59.8%) |
| Black/African American | 38 (15.6%) |
| American Indian/Alaska Native | 2 (0.8%) |
| Asian | 18 (7.4%) |
| Native Hawaiian/Pacific Islander | 3 (1.2%) |
| Other | 29 (11.9%) |
| Unknown | 3 (1.2%) |
| Refused to Answer | 5 (2.0%) |
| Ethnicity: | |
| Hispanic | 55 (22.5%) |
| Non-Hispanic | 188 (77.0%) |
| Unknown | 1 (0.4%) |
| Gestational Age (Weeks) | 38.1 ± 2.32 (233) |
| Gestational Age Categorical: | |
| <= 37 Weeks | 65 (26.6%) |
| > 37 Weeks | 168 (68.9%) |
Table 2.
Operative details for 244 BA subjects who underwent surgical drainage
| N (%) or Mean ±SD | |
|---|---|
| Age at Surgery (Days) | 64.6 ± 25.1 (244) |
| Age at Surgery Adjusted for Gestational Age (Days) | 50.8 ± 27.2 (233) |
| Liver Appearance: | |
| Firm | 170 (69.7%) |
| Nodular | 49 (20.1%) |
| Normal | 24 (9.8%) |
| Ascites | |
| No Ascites or <=20 cc | 205 (81.0%) |
| Ascites > 20 cc | 39 (19.0%) |
| CBD Inflamed: | |
| Yes | 77 (31.6%) |
| No | 143 (58.6%) |
| Abdominal Anatomy Abnormalities: | |
| No abnormality identified | 118 (48.4%) |
| Intestinal Malrotation | 23 (9.4%) |
| Situs Inversus | 7 (2.9%) |
| Midline Liver | 9 (3.7%) |
| Polysplenia | 13 (5.3%) |
| Asplenia | 1 (0.4%) |
| Pre-duodenal Portal Vein | 10 (4.1%) |
| Other | 23 (9.4%) |
| Hilar dissection | |
| Left to Right (LR) Dissection (mm) | 18.0 ± 12.8 (182) |
| Anterior to Posterior (AP) Dissection (mm) | 9.9 ± 8.5 (182) |
| Total Dissection Area (LR × AP; mm2) | 285.9 ± 1026.6 (182) |
At time of exploration, the attending surgeon also evaluated the Ohi classification. The distribution of Ohi types, subtypes, and subgroups is summarized in Table 3. We evaluated the distribution of Ohi type and subtype across quartiles of age and found no evidence suggesting an association of age at surgery and the distribution of Ohi type and subtype (data not shown). Intraoperative blood transfusions were given in 44 cases (18.0%).
Table 3.
Distribution of Ohi Type, Subtype, and Subgroup in 244 subjects who underwent surgical drainage.
| Ohi Type | Ohi Subtype (Patterns of distal ducts) |
Ohi Subgroup (Pattern of radicles at porta hepatis) |
|---|---|---|
| I (CBD atresia): 10.2% | a (Patent CBD): 20.1% | α (Dilated hepatic ducts): 0% |
| II (Hepatic duct atresia): 7.4% | b (Fibrous CBD): 45.9% | β (Hypoplastic hepatic ducts): 2.9% |
| III (Atresia at porta hepatis): 80.7% | c (Aplasia of CBD): 24.6% | γ (Bile lake): 2.5% |
| d (Miscellaneous): 4.1% | μ (Fibrous hepatic ducts): 7.4% | |
| ν (Fibrous mass): 78.7% | ||
| ο (Aplasia of hepatic ducts): 7.0% |
CBD = common bile duct
Outcomes Analysis
Outcomes were evaluated in the infants diagnosed with BA who were not enrolled in the START trial (i.e. PROBE only subjects; n=159). Of these infants, 17 had no surgery performed and 6 underwent abdominal exploration without any drainage procedure. The impact of no surgical drainage on transplant-free survival is demonstrated in Figure 2, comparing these two groups with the 136 subjects who had a drainage procedure. Among the infants who did not undergo surgical drainage, transplant-free survival was poor. Transplant-free survival in the subjects who underwent a drainage procedure was 53.7% and 46.7% at 1 and 2 years post drainage, respectively. The demographic and baseline characteristics for infants who underwent a drainage procedure in the outcomes analysis subgroup were similar to the baseline population (data not shown). In the drainage procedure population, there were 132 Roux-en-Y Kasai’s, 3 gallbladder Kasai’s, and 1 choledochojejunostomy. Nearly all of the procedures were done via an open surgical approach. After exclusion of two patients who were enrolled in START, three patients undergoing a laparoscopic Kasai procedure remained available for analysis. Two of the three patients who underwent a laparoscopic procedure underwent liver transplant within two years. Given the small number and unique characteristics associated with these laparoscopic cases, they were excluded from subsequent univariate analyses.
A primary aim of our study was to evaluate the impact of specific factors on achieving successful surgical drainage, as defined by achieving a total bilirubin less than 2.0 mg/dL anytime within the first 3 months following surgery. Overall, 45.6% (n=62) of the 136 infants who had a drainage procedure achieved successful drainage by this definition. The impact of specific factors was evaluated using logistic regression (Table 4). An odds-ratio greater than 1 indicates the variable is associated with a better outcome, i.e. greater probability of achieving a total bilirubin less than 2 mg/dl within 3 months. Ohi type II or III (atresia at the porta hepatis) was associated with a lower probability of achieving successful drainage compared to Ohi Type I (atresia of the common bile duct or so called correctable atresia) although this difference did not achieve statistical significance (OR 0.29, p=0.086). Ohi subtypes b, c, or d were not as likely to be associated with surgical success as subtype a (patent common bile duct), but this also did not achieve significance (OR 0.43, p=0.080). While the presence of BASM had no effect on the probability of successful drainage, none of the five infants with non-BASM abnormalities achieved successful drainage. Older age at portoenterostomy was associated with lower likelihood of surgical success, but was not statistically significant when age was evaluated as a continuous or categorical variable. In addition to the other non-significant variables reported in Table 4, gender, race, ethnicity, gestational age, age at portoenterostomy corrected for gestational age, gross CBD inflammation, liver appearance, and intraoperative blood transfusion were not associated with outcome. Additionally, the extent of hilar dissection, as defined by left-right dimension, anterior-posterior dimension, or area (left-right dimension × anterior-posterior dimension) had no effect.
Table 4.
Univariate analysis of variables on probability of achieving total bilirubin < 2 by 3 months post drainage and risk of transplant or death with native liver.
| Probability of Achieving Total Bilirubin < 2.0 by 3 Months |
Risk of Transplant or Death with Native Liver |
|||
|---|---|---|---|---|
| Variable | Odds Ratio | P-Value | HR | P-Value |
| Ohi Type II + III (Ref= Type I) | 0.29 | 0.086 | 2.03 | 0.030 |
| Ohi Subtypes b, c, or d (Ref=a) | 0.43 | 0.080 | 4.31 | 0.022 |
| Ascites > 20 (Ref=None or <=20) | 0.41 | 0.065 | 1.90 | 0.023 |
| Liver appearance Normal (Ref = Firm) | 1.16 | 0.785 | 1.21 | 0.473 |
| Liver appearance Nodular (Ref = Firm) | 0.55 | 0.216 | 1.61 | 0.008 |
| BASM (Ref = no anomaly) | 0.33 | 0.108 | 1.92 | 0.025 |
| Isolated non BASM Abdominal anomaly (Ref =no anomaly) | * | * | 2.33 | 0.088 |
| Fibrosis (Ishak): Stages 3–6 (Ref=Stages 0–2) | 0.51 | 0.089 | 1.54 | 0.074 |
| Age at Kasai (days, continuous) | 0.99 | 0.075 | 1.01 | 0.316 |
| Age at Kasai (≥ 75 days) | 0.47 | 0.086 | 1.73 | 0.002 |
| Total Bilirubin < 2 by 3 Months | - | - | 0.08 | <0.0001 |
Odds ratio cannot be determined as none of the 5 infants achieved a total bilirubin less than 2 mg/dL in the first 3 months.
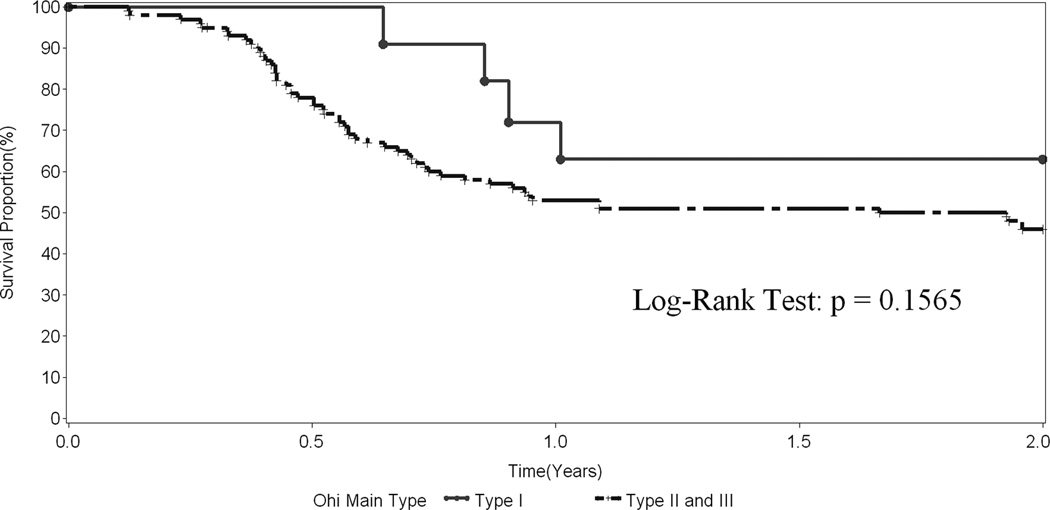
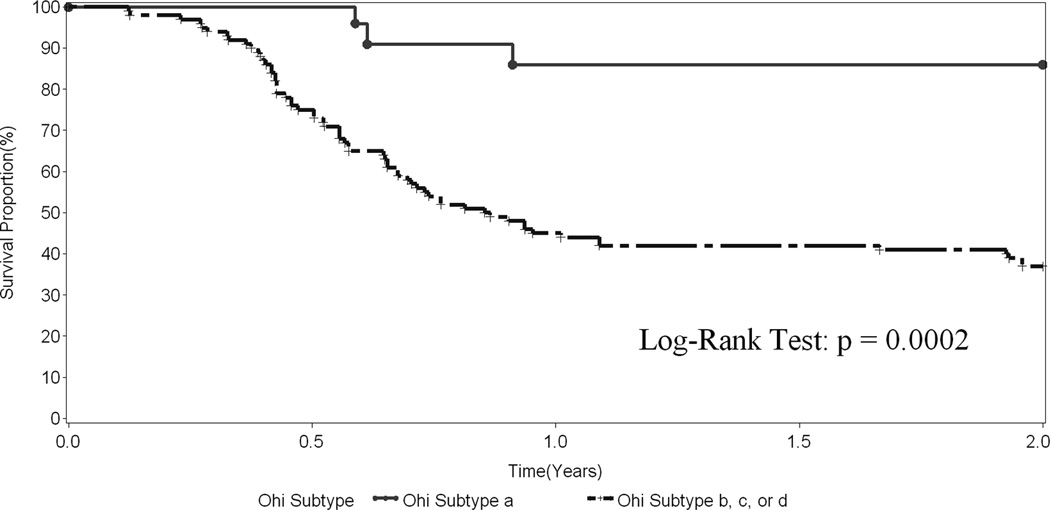
We also evaluated risk factors associated with transplant-free survival over the follow-up period using Cox models (Table 4). A hazard-ratio greater than 1 indicates the variable is associated with a worse outcome, i.e. a greater probability of transplant or death prior to transplant. In contrast to the weaker association of anatomical features with bile drainage, these variables impacted significantly on transplant-free survival. Paralleling the observations related to successful drainage, Ohi classifications were associated with survival. Specifically, Ohi type II or III (atresia at the porta hepatis) was associated with a greater risk of transplant or death compared to Ohi Type I (HR 2.03, p=0.030). The non-patent Ohi subtypes b, c, or d were associated with a greater risk of transplant or death than subtype a (patent common bile duct) (HR 4.31, p=0.022). The effect of Ohi Type and subtype on transplant-free survival is demonstrated in Figures 3 and 4, respectively. The presence of greater than 20 cc of ascites and a nodular appearance of the liver noted at the time of the Kasai procedure were associated with poor outcome. BASM was similarly associated with a worse prognosis (Table 4). Children 75 days of age and older fared less well than those who were younger (HR 1.73, p=0.002). Successful surgical drainage was the best predictor for the risk of transplant or death (HR 0.08, p < 0.0001). Although there was a trend towards lower transplant-free survival, histological severity of liver fibrosis as measured by the 6 grade Ishak score was not significantly associated with worse outcomes (Table 4). In addition to the other non-significant variables reported in Table 4, gender, race, ethnicity, gestational age, gross CBD inflammation at surgery, and intraoperative blood transfusion were not associated with probability of transplant or death. Additionally, the extent of hilar dissection had no effect.
Figure 3.
Transplant-free survival in 136 infants with BA who underwent a surgical drainage procedure stratified by Ohi Type.
Figure 4.
Transplant-free survival in 136 infants with BA who underwent a surgical drainage procedure stratified by Ohi Subtype.
Discussion
BA remains an enigmatic disease, almost 100 years after it was first described and more than a half century since the Kasai portoenterostomy was first reported.12, 20 Since the initial description by Kasai, the operation has undergone a number of modifications aimed at improving chances of establishing and maintaining bile flow.21, 22 However, even when bile flow is re-established and jaundice resolves, progression to biliary cirrhosis and end-stage liver disease develops in a substantial portion of patients.23, 24 As a result, outcomes for infants with BA following portoenterostomy still remain unpredictable, unsatisfying, and relatively disappointing. This study represents the first report from our North American-based, multi-institutional collaboration to prospectively collect clinical data on infants with BA cared for at 16 academic medical centers and children’s hospitals with specialized expertise in hepatobiliary diseases.
Published data regarding the impact of age at time of surgery on outcome are somewhat conflicting. Younger age at diagnosis and surgery was originally cited by Kasai as an important prognostic factor; however, this was not corroborated in the larger subsequent report from the Japanese registry.25, 26 Since then, other authors also reported age at time of surgery as an important determinant of outcome. Notably, a recent analysis of a large cohort of Taiwanese children showed that increasing the fraction of children who underwent surgery before 60 days of age with earlier diagnosis through screening, improved the rate of resolution of jaundice as well as 3 and 5 years survival rates with their native livers.27 Paradoxically, Davenport reported a 5-year 45% transplant-free survival in a limited number of children who had surgery at more than 100 days of age.28
In our study, we observed resolution of jaundice in 46% of infants postoperatively, similar to results reported by the French (40%), Swiss (39.5%), and British (56%) although lower than that reported by the Japanese (60%).8, 29–31 Average age at time of the portoenterostomy procedure was 58 days, also consistent with those reported by the British (58 days), French (60 days), American (61 days) and Japanese (60–70 days).19 Disappointingly, there has been no appreciable improvement in terms of earlier referral and diagnosis of patients with BA in the U.S. between 1997 and 2006 in a survey of both academic and non-academic centers, as reported by Raval et al.32 In our study, there was no improvement in age at portoenterostomy compared to previous reports. In fact, nearly 10% of infants were deemed to be too old or as having such advanced liver disease, that they did not undergo portoenterostomy but were listed for primary liver transplant.
We found that infants younger than age 75 days undergoing Kasai portoenterostomy were no more likely than infants older than 75 days to achieve a total bilirubin less than 2.0 mg/dL within the first 3 months. However, being less than 75 days old at time of surgery was associated with improved transplant-free survival compared to the older infants. Previous studies indicate a difference in the outcome of portoenterostomy between extremes of age with patients younger than 30 days attaining greater transplant-free survival than those older than 120 days.33, 34 In our study, the impact of age on outcomes may have been blunted by the limited number of infants who underwent Kasai portoenterostomy at an early age. Additionally, there were a significant number of older children who did not undergo Kasai portoenterostomy because it was felt that this intervention would be unlikely to impact the need for transplantation. The decision to list these infants for transplant is affected by other indicators of chronic liver disease such as failure to thrive, portal hypertension, and cholangitis, and not solely based on persistence of jaundice. It may be that age is only a gross reflection of the cumulative damage to the liver and that many other factors may alter the tempo of progression to cirrhosis and irreversible damage. Others have suggested that age at surgery may not be independent of other variables such as inflammation, fibrosis, size of ductules in the excised specimen, hepatic stellate cell activation, poor nutritional status, and phenotype of the BA.30, 35–39
Extra-hepatic malformations have historically been associated with BA, particularly splenic anomalies known as BASM, which has been associated with a poorer prognosis.40 We categorized our associated anomalies either as those associated with BASM or those not associated with BASM. In both instances, the presence of BASM or non-BASM associated anomalies did not influence the clearing of jaundice after portoenterostomy but was associated with decreased transplant-free survival over two years.
The incidence of the various types, subtypes, and subgroups in our series did not differ dramatically from those reported by Ohi.41, 42 Consistent with the published literature, patients in the Ohi type III group had significantly worse outcome than those in the other two groups, and patients with subtype “a” had a higher probability of clearing jaundice after surgery and surviving two years without a transplant than patients in the other subgroups. Unfortunately, there were not enough patients to test the hypothesis that patients with subgroup ”a” had better results and survival with a “gallbladder” Kasai than with a conventional portoenterostomy.
This study was not designed to address the issue of predicting outcome based on histological criteria. The gross appearance of the liver at the time of surgery was predictive of survival, but the histologic grade of liver fibrosis did not have an association with either the clearance of the jaundice or the 2-year transplant free survival.
In our series, there were five laparoscopically-performed Kasai portoenterostomies. We opted to include the laparoscopic cases in our outcomes analyses since there were so few cases. Although not statistically significant, these patients tended to do poorer than those who underwent open Kasai procedures. This is consistent with the recently published observations of Ure et al, where infants undergoing laparoscopically-performed Kasai procedures did so poorly compared to conventional open, historical controls that the authors halted the study.43 It is unclear why patients undergoing laparoscopically-performed Kasai procedures do poorly. We speculate that the complexity of the procedure combined with the very limited experience any one surgeon is likely to amass likely contribute to poorer outcomes for patients who undergo laparoscopically-performed procedures. Regardless, currently, the conventional, open Kasai portoenterostomy remains the standard approach for re-establishing biliary drainage in BA patients.
Success of the Kasai procedure in achieving biliary drainage is modest at best. Beyond this however, successful drainage is not a perfect predictor of survival with one’s native liver. As such, efforts to improve transplant-free survival should extend beyond “fine-tuning” the surgical procedure alone. Our analysis partially supports the concept that diagnosis and treatment of BA is associated with better drainage and transplant-free survival. Accordingly, efforts to identify and treat infants with BA at an earlier age may be worthwhile. Given the high rates of liver transplantation in BA patients, efforts to improve drainage or halt the progression of liver fibrosis towards cirrhosis and end-stage liver disease remain critical. We have recently completed enrollment of patients in our prospective, randomized, double-blinded, placebo-controlled trial investigating the efficacy of corticosteroids to improve biliary drainage following portoenterostomy and results of the trial will become available in two years. Continued efforts to better understand the pathogenesis of BA and to identify intervention points within the disease progression remain ongoing.
In conclusion, this study represents the first contemporary North American collaborative effort to prospectively collect demographic and operative data in a group of children with biliary atresia. Our findings largely validate those of past retrospective and registry analyses. The distribution of anatomic variants is essentially unchanged compared to past published reports. Age does appear to impact survival with one’s native liver. Early clearance of jaundice is similarly predictive of transplant-free survival. Our observations establish North American benchmarks for future studies and provide a framework to guide clinicians and families regarding prognosis and the potential need for transplantation.
Acknowledgements
We thank all the ChiLDREN investigators, the research coordinators, and most importantly the patients and families who agreed to participate in our studies. We thank Drs. Ron Sokol and Pat Robuck for their support and guidance in the process of manuscript preparation and Ms. Cat Goodhue for proofreading. This work was supported by the National Center for Research Resources, NIH (5M01 RR00069 [Denver], UL1RR025780 [Denver], UL1RR024153[Pittsburgh], UL1RR024134 [Philadelphia], UL1RR024131 [San Francisco], UL1RR025005 [Baltimore], UL1RR025741 [Chicago]), and the National Institute of Diabetes, Digestive and Kidney Diseases (DK 62453 [Denver], DK 62497 [Cincinnati], DK 62481 [Philadelphia], DK 62470 [Houston], DK 62500 [San Francisco], DK 62530 [Baltimore], DK 62445 [Mt Sinai], DK 62466 [Pittsburgh], DK 62456 [Michigan], DK 62452 [St. Louis], DK 62436 [Chicago], DK 84538 [Los Angeles], DK 84585 [Atlanta]).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study describes the clinical and anatomic features of infants enrolled in the prospective Childhood Liver Disease Research and Education Network undergoing Kasai portoenterostomy for biliary atresia, and to examine associations between these parameters and outcomes.
References
- 1.Sokol RJ, Mack C, Narkewicz MR, et al. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37(1):4–21. doi: 10.1097/00005176-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Karrer FM, Price MR, Bensard DD, et al. Long-term results with the Kasai operation for biliary atresia. Arch Surg. 1996;131(5):493–496. doi: 10.1001/archsurg.1996.01430170039006. [DOI] [PubMed] [Google Scholar]
- 3.Davenport M. Biliary atresia. Semin Pediatr Surg. 2005;14(1):42–48. doi: 10.1053/j.sempedsurg.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Khalil BA, Perera MT, Mirza DF. Clinical practice: management of biliary atresia. Eur J Pediatr. 169(4):395–402. doi: 10.1007/s00431-009-1125-7. [DOI] [PubMed] [Google Scholar]
- 5.Shivakumar P, Campbell KM, Sabla GE, et al. Obstruction of extrahepatic bile ducts by lymphocytes is regulated by IFN-gamma in experimental biliary atresia. J Clin Invest. 2004;114(3):322–329. doi: 10.1172/JCI21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyler KL, Sokol RJ, Oberhaus SM, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology. 1998;27(6):1475–1482. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 7.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174(1):8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Wildhaber BE, Majno P, Mayr J, et al. Biliary atresia: Swiss national study, 1994–2004. J Pediatr Gastroenterol Nutr. 2008;46(3):299–307. doi: 10.1097/MPG.0b013e3181633562. [DOI] [PubMed] [Google Scholar]
- 9.Harper P, Plant JW, Unger DB. Congenital biliary atresia and jaundice in lambs and calves. Aust Vet J. 1990;67(1):18–22. doi: 10.1111/j.1751-0813.1990.tb07385.x. [DOI] [PubMed] [Google Scholar]
- 10.Mack CL, Sokol RJ. Unraveling the pathogenesis and etiology of biliary atresia. Pediatr Res. 2005;57(5 Pt 2):87R–94R. doi: 10.1203/01.PDR.0000159569.57354.47. [DOI] [PubMed] [Google Scholar]
- 11.Caponcelli E, Knisely AS, Davenport M. Cystic biliary atresia: an etiologic and prognostic subgroup. J Pediatr Surg. 2008;43(9):1619–1624. doi: 10.1016/j.jpedsurg.2007.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Kasai M, Suzuki SA. A new operation for "non-correctable" biliary atresia-Portoenterostomy. Shijitsu. 1959;13:733–739. [Google Scholar]
- 13.Altman RP, Lilly JR, Greenfeld J, et al. A multivariable risk factor analysis of the portoenterostomy (Kasai) procedure for biliary atresia: twenty-five years of experience from two centers. Ann Surg. 1997;226(3):348–353. doi: 10.1097/00000658-199709000-00014. discussion 353–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport M, Kerkar N, Mieli-Vergani G, et al. Biliary atresia: the King's College Hospital experience (1974–1995) J Pediatr Surg. 1997;32(3):479–485. doi: 10.1016/s0022-3468(97)90611-4. [DOI] [PubMed] [Google Scholar]
- 15.Chardot C, Carton M, Spire-Bendelac N, et al. Prognosis of biliary atresia in the era of liver transplantation: French national study from 1986 to 1996. Hepatology. 1999;30(3):606–611. doi: 10.1002/hep.510300330. [DOI] [PubMed] [Google Scholar]
- 16.Chardot C, Carton M, Spire-Bendelac N, et al. Epidemiology of biliary atresia in France: a national study 1986–96. J Hepatol. 1999;31(6):1006–1013. doi: 10.1016/s0168-8278(99)80312-2. [DOI] [PubMed] [Google Scholar]
- 17.Sokol RJ. New North American research network focuses on biliary atresia and neonatal liver disease. J Pediatr Gastroenterol Nutr. 2003;36(1):1. doi: 10.1097/00005176-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Russo P, Magee JC, Boitnott J, et al. Design and Validation of the Biliary Atresia Research Consortium Histologic Assessment System for Cholestasis in Infancy. Clin Gastroenterol Hepatol. 2011;9(4):357–362. doi: 10.1016/j.cgh.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shneider BL, Brown MB, Haber B, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467–474. doi: 10.1016/j.jpeds.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 20.Myers B. Case of Persistent Jaundice in an Infant; Atresia of the Common Bile-duct and Biliary Cirrhosis. Proc R Soc Med. 1923;16(Sect Study Dis Child):17–18. [PMC free article] [PubMed] [Google Scholar]
- 21.Suruga K, Miyano T, Arai T, et al. A study on hepatic portoenterostomy for the treatment of atresia of the biliary tract. Surg Gynecol Obstet. 1984;159(1):53–58. [PubMed] [Google Scholar]
- 22.Suruga K, Tsunoda S, Deguchi E, et al. The future role of hepatic portoenterostomy as treatment of biliary atresia. J Pediatr Surg. 1992;27(6):707–709. doi: 10.1016/s0022-3468(05)80096-x. [DOI] [PubMed] [Google Scholar]
- 23.Altman RP. Portal decompression by interposition mesocaval shunt in patients with biliary atresia. J Pediatr Surg. 1976;11(5):809–814. doi: 10.1016/0022-3468(76)90107-x. [DOI] [PubMed] [Google Scholar]
- 24.Todani T, Watanabe Y, Mizuguchi T, et al. Portal hypertension after successful Kasai's operation for biliary atresia--special reference to esophageal varices. Z Kinderchir. 1981;34(3):240–248. doi: 10.1055/s-2008-1063353. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim M, Miyano T, Ohi R, et al. Japanese Biliary Atresia Registry, 1989 to 1994. Tohoku J Exp Med. 1997;181(1):85–95. doi: 10.1620/tjem.181.85. [DOI] [PubMed] [Google Scholar]
- 26.Kasai M, Ohi R. Long-term follow-up results of patients with biliary atresia. Indian J Pediatr. 1983;50(403):209–217. doi: 10.1007/BF02821444. [DOI] [PubMed] [Google Scholar]
- 27.Lien TH, Chang MH, Wu JF, et al. Effects of the infant stool color card screening program on 5-year outcome of biliary atresia in Taiwan. Hepatology. 2011;53(1):202–208. doi: 10.1002/hep.24023. [DOI] [PubMed] [Google Scholar]
- 28.Davenport M, De Ville de Goyet J, Stringer MD, et al. Seamless management of biliary atresia in England and Wales (1999–2002) Lancet. 2004;363(9418):1354–1357. doi: 10.1016/S0140-6736(04)16045-5. [DOI] [PubMed] [Google Scholar]
- 29.Davenport M, Caponcelli E, Livesey E, et al. Surgical outcome in biliary atresia: etiology affects the influence of age at surgery. Ann Surg. 2008;247(4):694–698. doi: 10.1097/SLA.0b013e3181638627. [DOI] [PubMed] [Google Scholar]
- 30.Duche M, Fabre M, Kretzschmar B, et al. Prognostic value of portal pressure at the time of Kasai operation in patients with biliary atresia. J Pediatr Gastroenterol Nutr. 2006;43(5):640–645. doi: 10.1097/01.mpg.0000235754.14488.f9. [DOI] [PubMed] [Google Scholar]
- 31.Nio M, Ohi R, Miyano T, et al. Five- and 10-year survival rates after surgery for biliary atresia: a report from the Japanese Biliary Atresia Registry. J Pediatr Surg. 2003;38(7):997–1000. doi: 10.1016/s0022-3468(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 32.Raval MV, Dzakovic A, Bentrem DJ, et al. Trends in age for hepatoportoenterostomy in the United States. Surgery. 2010;148(4):785–791. doi: 10.1016/j.surg.2010.07.028. discussion 791–2. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber RA, Barker CC, Roberts EA, et al. Biliary atresia: the Canadian experience. J Pediatr. 2007;151(6):659–665. 665 e1. doi: 10.1016/j.jpeds.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 34.Serinet MO, Wildhaber BE, Broue P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123(5):1280–1286. doi: 10.1542/peds.2008-1949. [DOI] [PubMed] [Google Scholar]
- 35.Azarow KS, Phillips MJ, Sandler AD, et al. Biliary atresia: should all patients undergo a portoenterostomy? J Pediatr Surg. 1997;32(2):168–172. doi: 10.1016/s0022-3468(97)90173-1. discussion 172–4. [DOI] [PubMed] [Google Scholar]
- 36.DeRusso PA, Ye W, Shepherd R, et al. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology. 2007;46(5):1632–1638. doi: 10.1002/hep.21923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moyer K, Kaimal V, Pacheco C, et al. Staging of biliary atresia at diagnosis by molecular profiling of the liver. Genome Med. 2010;2(5):33. doi: 10.1186/gm154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy P, Chatterjee U, Ganguli M, et al. A histopathological study of liver and biliary remnants with clinical outcome in cases of extrahepatic biliary atresia. Indian J Pathol Microbiol. 2010;53(1):101–105. doi: 10.4103/0377-4929.59194. [DOI] [PubMed] [Google Scholar]
- 39.Shteyer E, Ramm GA, Xu C, et al. Outcome after portoenterostomy in biliary atresia: pivotal role of degree of liver fibrosis and intensity of stellate cell activation. J Pediatr Gastroenterol Nutr. 2006;42(1):93–99. doi: 10.1097/01.mpg.0000189324.80323.a6. [DOI] [PubMed] [Google Scholar]
- 40.Davenport M, Savage M, Mowat AP, et al. Biliary atresia splenic malformation syndrome: an etiologic and prognostic subgroup. Surgery. 1993;113(6):662–668. [PubMed] [Google Scholar]
- 41.Muraji T, Higashimoto Y. The improved outlook for biliary atresia with corticosteroid therapy. J Pediatr Surg. 1997;32(7):1103–1106. doi: 10.1016/s0022-3468(97)90408-5. discussion 1106–7. [DOI] [PubMed] [Google Scholar]
- 42.Kasai M. Advances in treatment of biliary atresia. Jpn J Surg. 1983;13(4):265–276. doi: 10.1007/BF02469507. [DOI] [PubMed] [Google Scholar]
- 43.Ure BM, Kuebler JF, Schukfeh N, et al. Survival With the Native Liver After Laparoscopic Versus Conventional Kasai Portoenterostomy in Infants With Biliary Atresia: A Prospective Trial. Annals of Surgery. 2011;253(4):826–830. doi: 10.1097/SLA.0b013e318211d7d8. [DOI] [PubMed] [Google Scholar]