Abstract
Dendritic spines are neuron-specific actin-rich subcellular structures and are the location of excitatory synapses. Neurotransmitters released from presynaptic terminals activate the signals modifying the F-actin dynamics and stability and thus control dendritic spine morphology. Many ubiquitously expressed actin-associated proteins, including cortactin, have been shown to regulate dendritic spine morphology and density. Since dendritic spines are neuron-specific structures, neuron-specific proteins are expected to control F-actin cytoskeletons and dendritic spinogenesis. Recently, we demonstrated that cortactin-binding protein 2 (CTTNBP2), a neuron-specific protein, regulates the mobility and distribution of cortactin and controls the density of dendritic spines. This is the first example of a neuron-specific protein that controls the mobility of an F-actin associated protein and influences the dendritic spines. It provides a platform to explore the specific pathway triggering dendritic spinogenesis.
Keywords: Cortactin, CTTNBP2, CTTNBP2-NL, dendritic spine formation, F-actin-associated protein
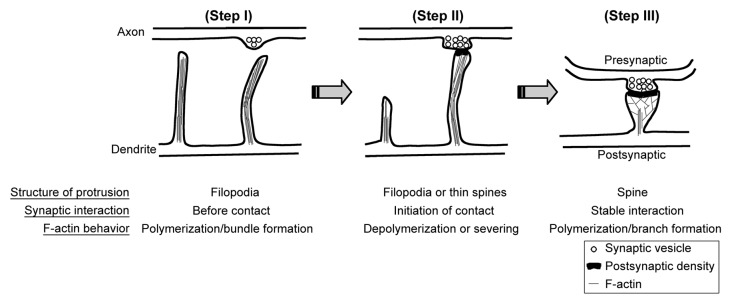
Dendritic spines are the small protrusions (~1–2 μm in length and ~0.5–1 μm in width) that extend from dendrites. The tips of dendritic spines are the major locations of excitatory synapses.1 Dendritic filopodia are proposed to be the primordial structures of dendritic spines. After forming a synaptic contact with an axon, filopodia transform to mature dendritic spines by shortening their length and enlarging their heads (Fig. 1). Since F-actins are the major components of the cytoskeleton that support the morphology of dendritic spines, F-actin dynamics coordinate the changes of dendritic spine morphology. Depolymerization of F-actin eventually results in elimination of dendritic spines (Fig. 1).2 Since dendritic spines are neuron-specific subcellular structures, neuron-specific actin-associated proteins are expected to control formation and morphology of dendritic spines. Recently, we demonstrated that cortactin-binding protein 2, a neuron-specific F-actin associated protein, regulates dendritic spinogenesis and maintenance.3 Cortactin was originally identified as a ubiquitously expressed F-actin associated protein that is highly enriched at the cell cortex and interacts with F-actin as well as the actin-related protein 2/3 (Arp2/3) complex. It has been suggested that it stabilizes F-actin polymers and branches and thus maintains lamellipodiar structures of cells.4,5 In cultured hippocampal neurons, knockdown of cortactin reduces the density of dendritic spines and makes more filopodia-like dendritic spines.6 In our recent study, we found that CTTNBP2 regulates dendritic spinogenesis through interaction with cortactin, because expression of the CTTNBP2 mutant with a weaker affinity for cortactin cannot rescue the effect of CTTNBP2 knockdown on impairment of dendritic spinogenesis and because overexpression of cortactin rescues the effect of CTTNBP2 knockdown. Moreover, knockdown of CTTNBP2 reduces dendritic spine distribution of cortactin. All of the evidence suggested that CTTNBP2 regulates cortactin distribution in neurons and controls dendritic spinogenesis.
Figure 1. F-actin dynamics and dendritic spine formation. (Step I) Dendrite first extends filopodia to explore the environment. The F-actin bundle is the component of the cytoskeleton that supports the structure of filopodia. (Step II) Once filopodia make contact with the presynaptic button and initiates the synaptic interaction, F-actin cytoskeletons undergo remodeling by depolymerization and severing. Filopodia will then withdraw and transform to mushroom-like dendritic spines. Without synaptic contact, filopodia will withdraw and disappear later. Since axonal contact with dendritic filopodia induces calcium influx of dendritic filopodia, it is very likely that calcium triggers the transformation of filopodia to dendritic spines. (Step III) To enlarge the dendritic spine, F-actin cytoskeletons increase the branching level. Therefore, molecules promoting F-actin branching are expected to play a role in regulation of spine morphology or density.
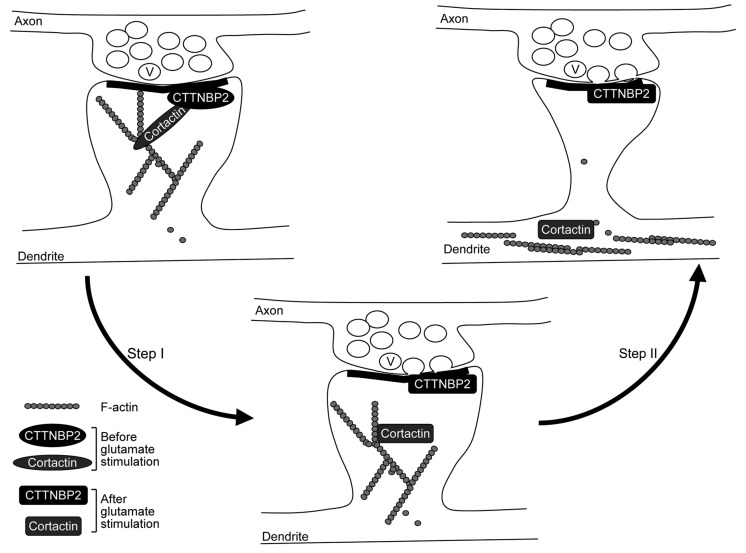
In addition to spine formation, cortactin was also suggested to play a role in remodeling dendritic spines upon synaptic stimulation. The activation of the N-methyl-D-aspartate receptor (NMDAR) by glutamate results in redistribution of cortactin from the dendritic spines to the dendritic shaft.6 In contrast, CTTNBP2 stably resides in the dendritic spines even after glutamate or NMDA stimulation (Fig. 2). Our data suggested that CTTNBP2 serves as an anchoring site for cortactin at the dendritic spines and modulates the mobility of cortactin in dendritic spines.
Figure 2. Coordination of neuronal activity and CTTNBP2-dependent F-actin remodeling. Cortactin binds both F-actin and Arp2/3 and thus stabilizes F-actin polymers and branching; CTTNBP2 stably resides in the dendritic spines and may anchor the cortactin-F-actin cytoskeleton in the dendritic spine. The interaction of cortactin and CTTNBP2 regulates dendritic spine formation. Additionally, when postsynaptic glutamate receptors are activated by glutamate released from the presynaptic button, the signal may then posttranslationally modify CTTNBP2 and/or cortactin and result in dissociation of CTTNBP2 and cortactin (Step I). Consequently, cortactin and F-actin will then move to the dendritic shaft (Step II). The process is expected to regulate remodeling of dendritic spines upon synaptic stimulation. V, synaptic vesicle.
Although our study indicated that CTTNBP2, a neuron-specific actin-associated protein, is critical for dendritic spinogenesis, ectopic expression of CTTNBP2 did not induce spine-like structures in COS cells.3 This suggests that in addition to CTTNBP2, other neuron-specific proteins or signaling are required for coordination with CTTNBP2 to trigger dendritic spine formation. Identification of other neuron-specific proteins could potentially further elucidate the molecular mechanism of dendritic spinogenesis. On the other hand, since calcium influx at dendritic filopodia is triggered by presynaptic contact,7 signals activated by neurotransmitters may modulate F-actin dynamics at dendritic filopodia and thus impact dendritic spinogenesis. Because NMDAR activation induces dissociation between CTTNBP2 and cortactin, it is obvious that neuronal activity modifies the protein-protein interactions and/or function of CTTNBP2, which may therefore regulate dendritic spinogenesis and maintenance (Fig. 2).
At this moment, it is not clear how CTTNBP2 proteins are stably localized in the dendritic spines. One possibility is that CTTNBP2 interacts strongly with core components of the postsynaptic density and thus resides in dendritic spines. It will certainly be interesting to identify more CTTNBP2 interacting proteins in neurons, which may elucidate how CTTNBP2 is enriched in dendritic spines. Additionally, if CTTNBP2 is truly critical for dendritic spinogenesis, it is reasonable to speculate that CTTNBP2 may coordinate multiple signal pathways to control spine formation. Interestingly, a protein similar to CTTNBP2, CTTNBP2 N-terminal like (CTTNBP2NL), has been shown to interact with the protein phosphatase 2A (PP2A) complex containing striatin.8 The striatin protein family contains three members, which are all highly enriched at the dendritic spines.9,10 Since PP2A plays a critical role in neuronal plasticity,11,12 it will be intriguing to learn whether CTTNBP2 associates with striatins and whether CTTNBP2 regulates synaptic distribution of PP2A and thus plays a role in synaptic signaling. The preliminary evidence that supports this idea is the differential expression level of CTTNBP2 and CTTNBP2NL in the brain. According to data from an online database (http://biogps.org/), unlike CTTNBP2 mRNA, which is highly expressed in the brain (biogps.org/#goto=genereport&id=83992), CTTNBP2NL is expressed at a very low level in the brain (biogps.org/#goto=genereport&id=55917). Because CTTNBP2 and CTTNBP2NL share ~50% similarity in amino acid sequence, it will be interesting to discover whether CTTNBP2 replaces the role of CTTNBPNL in its interaction with the PP2A complex in neurons and thus regulates synaptic signaling in neurons.
Although it is clear that CTTNBP2 regulates dendritic spinogenesis, it is still unknown whether CTTNBP2 plays a critical role in the transition from dendritic filopodia to dendritic spines (Fig. 1, Step II to III). It is also unclear how neuronal activity regulates the function and protein-protein interactions of CTTNBP2. More investigations are required to further elucidate the molecular regulation of CTTNBP2.
Acknowledgments
Y.-P.H. is supported by Academia Sinica (AS-100-TP-B09) and National Science Council (NSC 98-2321-B-001-002, NSC 99-2321-B-001-032, NSC 100-2321-B-001-022 and NSC 98-2311-B-001-012-MY3).
Glossary
Abbreviations:
- Arp2/3
actin-related protein 2/3
- CTTNBP2
cortactin-binding protein 2
- CTTNBP2NL
CTTNBP2 N-terminal like
- PP2A
protein phosphatase 2A
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/20364
References
- 1.Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–97. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pontrello CG, Ethell IM. Accelerators, Brakes, and Gears of Actin Dynamics in Dendritic Spines. Open Neurosci J. 2009;3:67–86. doi: 10.2174/1874082000903020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen YK, Hsueh YP. Cortactin-binding protein 2 modulates the mobility of cortactin and regulates dendritic spine formation and maintenance. J Neurosci. 2012;32:1043–55. doi: 10.1523/JNEUROSCI.4405-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren G, Crampton MS, Yap AS. Cortactin: Coordinating adhesion and the actin cytoskeleton at cellular protrusions. Cell Motil Cytoskeleton. 2009;66:865–73. doi: 10.1002/cm.20380. [DOI] [PubMed] [Google Scholar]
- 5.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hering H, Sheng M. Activity-dependent redistribution and essential role of cortactin in dendritic spine morphogenesis. J Neurosci. 2003;23:11759–69. doi: 10.1523/JNEUROSCI.23-37-11759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–60. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Goudreault M, D’Ambrosio LM, Kean MJ, Mullin MJ, Larsen BG, Sanchez A, et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol Cell Proteomics. 2009;8:157–71. doi: 10.1074/mcp.M800266-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaillard S, Bailly Y, Benoist M, Rakitina T, Kessler JP, Fronzaroli-Molinières L, et al. Targeting of proteins of the striatin family to dendritic spines: role of the coiled-coil domain. Traffic. 2006;7:74–84. doi: 10.1111/j.1600-0854.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Haeberlé AM, Castets F, Bombarde G, Baillat G, Bailly Y. Immunogold localization of phocein in dendritic spines. J Comp Neurol. 2006;495:336–50. doi: 10.1002/cne.20895. [DOI] [PubMed] [Google Scholar]
- 11.Tian Q, Wang J. Role of serine/threonine protein phosphatase in Alzheimer’s disease. Neurosignals. 2002;11:262–9. doi: 10.1159/000067425. [DOI] [PubMed] [Google Scholar]
- 12.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:D961–72. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]