Abstract
Chemokine binding to cognate receptors induces actin dynamics that are a major driving force for T cell migration and chemotactic motility. HIV-1 binding to the chemokine coreceptor CXCR4 initiates chemotactic signaling, mimicking chemokine-induced actin dynamics to facilitate infection processes such as entry, early DNA synthesis, and nuclear migration. Recently, we identified that HIV-triggered early actin polymerization is mediated through the Rac1-PAK1/2-LIMK1-cofilin pathway. Inhibition of LIMK1 (LIM domain kinase 1), a kinase phosphorylating cofilin, through shRNA knockdown decreases actin polymerization and T cell chemotaxis toward SDF-1. The LIMK1 knockdown T cells also supported lower viral entry, DNA synthesis and nuclear migration, suggesting a critical role of LIMK1-mediated actin dynamics in the initiation of HIV-1 infection. Surprisingly, LIMK1 knockdown in CEM-SS T cells did not lead to an overall change in the ratio of phospho-cofilin to total cofilin although there was a measurable decrease in the amount of actin filaments in cells. The decrease in filamentous actin in LIMK1 knockdown cells was found to mainly occur in polarized cap region rich in F-actin. These results suggest that LIMK1 may be involved in spontaneous actin polarization in transformed T cells. The inhibition of T cell chemotaxis by LIMK1 knockdown likely result from inhibition of localized LIMK1 activation and cofilin phosphorylation that are required for polarized actin polymerization for directional cell migration. The inhibition of HIV-1 infection by LIMK1 knockdown may also result from the decrease of actin-rich membrane protrusions that may be preferred viral entry sites in T cells.
Keywords: LIMK1, cofilin, chemotaxis, SDF-1, CXCR4, HIV-1, CD4 T cells, Rac1, Pak1, Pak2
HIV-1 entry into CD4 T cells is mediated through viral envelope binding to CD4 and the chemokine coreceptor CXCR4.1,2 This interaction is required for viral fusion with the plasma membrane. HIV-1 binding to these receptors also initiates signal transduction in T cells.3 In particular, HIV-1-mediated signal transduction from the chemokine coreceptor CXCR4 has been shown to trigger actin dynamics critical for viral entry, post entry DNA synthesis and nuclear migration.4-6 It is suggested that in the absence of chemotactic stimulation or T cell activation, the cortical actin in blood resting CD4 T cells is relatively static. This lack of actin activity hinders viral intracellular migration across the actin cortex following fusion. To overcome this limitation, HIV-1 uses CXCR4 signaling to trigger the activation of cofilin, promoting actin treadmilling and viral nuclear migration.4 The importance of actin dynamics in HIV-1 infection has also been highlighted by several recent studies. Induction of the actin activity by treatment of blood CD4 T cells with chemokines such as CCL2 augments gp120-induced F-actin polymerization, which enhances viral DNA synthesis.7 Pretreatment of blood CD4 T cells with CCL19, CXCL9, CXCL10 and CCL20 triggers cofilin activation and changes in actin filaments, which promote viral nuclear localization and DNA integration.8-10 In addition, spinoculation, or infecting CD4 T cells under the condition of centrifugal stress, triggers both cofilin activation and actin dynamics, leading to CXCR4 upregulation and a great enhancement of HIV-1 DNA synthesis and nuclear migration.11
Mechanistically, HIV-1-mediated actin dynamics have been implicated in several early processes in the initiation of HIV infection. Actin binding proteins such as filamin-A and moesin were identified as possible cofactors involved in HIV-1 entry. Filamin-A may anchor CD4 and CXCR4 to F-actin following receptor clustering.12 The Ezrin-Radaxin-Moesin (ERM) family protein moesin is also suggested to promote CD4/CXCR4 receptor clustering following its activation by gp120.13,14 In addition, HIV gp120-mediated cell-cell fusion has been suggested to extensively rely on signal transduction leading to actin dynamics. siRNAs or inhibitors against molecules such as Pyk2, Rac1, GTPase Ras, phospholipase C, protein kinase C, Tiam-1, Abl, IRSp53, Wave2 inhibited gp120-medaited cell-cell fusion.15 Following viral entry, the establishment of an active viral reverse transcription complex may also involve cytoskeletal actin.16 Multiple proteins in the viral preintegration complex are identified to interact with actin. These proteins include the gag nucleocapsid protein (NC),17-20 the large subunit of the viral reverse transcriptase, the viral integrase and Nef.21-24 In addition, HIV-1 intracellular migration and nuclear localization is suggested to be dependent on actin treadmilling mediated by cofilin activity.4
Recently, we also found that HIV-1 binding to resting CD4 T cells triggers a rapid and transient actin polymerization through Rac1-PAK1/2-LIMK1 activation.6 Functionally, HIV-mediated actin polymerization may be required to transiently block CXCR4 internalization for the stabilization of the fusion complex. In addition, LIMK1-mediated actin polymerization is involved in HIV-1 reverse transcription and nuclear migration, as knockdown of LIMK1 inhibited both viral DNA synthesis and nuclear migration. Furthermore, transient treatment of resting CD4 T cells with a pharmacological agent, okadaic acid, activates LIMK1 and promotes HIV-1 latent infection of resting CD4 T cells.6
Given that LIMK is the kinase responsible for the serine 3 phosphorylation of cofilin, which inhibits cofilin activity and its binding to actin filaments,25,26 we expected that knockdown of LIMK1 would lead to an increase in cofilin activity and a decrease in the cortical actin density, as opposed to what we have seen in cofilin knockdown T cells.4 Indeed, LIMK1 knockdown led to a slight decrease of the F-actin intensity in CEM-SS T cells, as judged by flow cytometry6 (Fig. 1B). However, when the status of phospho-cofilin was examined, we did not see a measurable change in the ratio of phospho-cofilin to total cofilin (Fig. 1A). Further examination of the cortical actin staining by confocal microscopy revealed that there was a marked decrease of the F-actin-rich protrusions in the LIMK1 knockdown cells, whereas these spontaneous polarized actin-rich caps were often observed in the control knockdown cells (shNTC) (Fig. 1C). This observation prompted us to hypothesize that in cycling CEM-SS T cells where cofilin activity is constantly required for cytoskeletal remodeling, the maintenance of basal levels of cofilin phosphorylation in the absence of chemotactic stimulation may involve only small amounts of LIMK1. LIMK1 may also be locally enriched in regions where polarized actin caps are localized. Thus, decreasing LIMK1 activity by shRNA knockdown may locally increase cofilin activity, destabilizing these actin-rich caps. Upon chemotactic stimulation, rapid actin polymerization mainly occurs around polarized lamellipodium at the leading edges of migrating cells; rapid LIMK1 activation would be required for transient phosphorylation of cofiin in these locations.27 Thus, a decrease of LIMK1 activity may impair the polarization of actin-rich caps and the ability of T cell for directional migration.6,28 For HIV-1 infection, it has been suggested that actin-rich membrane protrusions such as microvilli may be prefer sites for viral binding and entry in T cells.29 It is attempting to speculate that knockdown of LIMK1 may also result in the decrease of these actin-rich protrusions, leading a less efficient entry and less viral contact with the cytoskeletal actin. This speculation certainly deserves further detailed studies in the future.
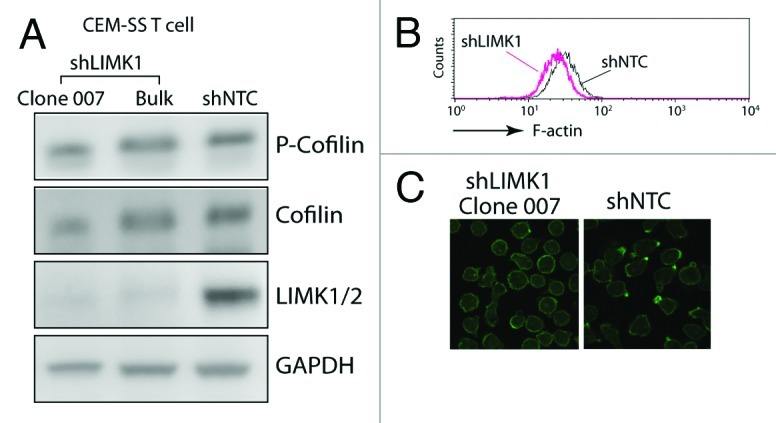
Figure 1. Involvement of LIMK1 in actin polarization in human CD4 T cells. (A) shRNA-mediated LIMK1 knockdown in CEM-SS T cells. Cells carrying stable LIMK1 knockdown or shNTC (a control shRNA against no human genes) were selected in puromycin and analyzed by western blot using antibodies against human phospho-cofilin, cofilin, LIMK1/2 or GAPDH (Bulk, bulk cell populations; clone 007, a derived LIMK1 knockdown cell clone). (B and C) LIMK1 knockdown decreases F-actin in clone 007. The decreases of F-actin in the knockdown cells were measured by FITC-phalloidin staining and flow cytometry (B) or by confocal microscopy imaging (C).
Acknowledgment
This work was supported by grant 1R01AI081568 from NIAID to Y. W.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/20165
References
- 1.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–8. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Yoder A. Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog. 2009;5:e1000520. doi: 10.1371/journal.ppat.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–92. doi: 10.1016/j.cell.2008.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu D, Wang W, Yoder A, Spear M, Wu Y. The HIV envelope but not VSV glycoprotein is capable of mediating HIV latent infection of resting CD4 T cells. PLoS Pathog. 2009;5:e1000633. doi: 10.1371/journal.ppat.1000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vorster PJ, Guo J, Yoder A, Wang W, Zheng Y, Xu X, et al. LIM kinase 1 modulates cortical actin and CXCR4 cycling and is activated by HIV-1 to initiate viral infection. J Biol Chem. 2011;286:12554–64. doi: 10.1074/jbc.M110.182238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell GR, Spector SA. CCL2 increases X4-tropic HIV-1 entry into resting CD4+ T cells. J Biol Chem. 2008;283:30745–53. doi: 10.1074/jbc.M804112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleh S, Solomon A, Wightman F, Xhilaga M, Cameron PU, Lewin SR. CCR7 ligands CCL19 and CCL21 increase permissiveness of resting memory CD4+ T cells to HIV-1 infection: a novel model of HIV-1 latency. Blood. 2007;110:4161–4. doi: 10.1182/blood-2007-06-097907. [DOI] [PubMed] [Google Scholar]
- 9.Cameron PU, Saleh SM, Sallman G, Solomon A, Wightman F, Mark J, et al. Latent HIV infection can be established in resting CD4+ T-cells in vitro following incubation with multiple diverse chemokines which facilitate nuclear import of the pre-integration complex. 4th International Workship in HIV Persistence During Therapy 2009; Dec. 8-11, 2009. [Google Scholar]
- 10.Cameron PU, Saleh S, Sallmann G, Solomon A, Wightman F, Evans VA, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A. 2010;107:16934–9. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Wang W, Yu D, Wu Y. Spinoculation triggers dynamic actin and cofilin activity that facilitates HIV-1 infection of transformed and resting CD4 T cells. J Virol. 2011;85:9824–33. doi: 10.1128/JVI.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiménez-Baranda S, Gómez-Moutón C, Rojas A, Martínez-Prats L, Mira E, Ana Lacalle R, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–46. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 13.Naghavi MH, Valente S, Hatziioannou T, de Los Santos K, Wen Y, Mott C, et al. Moesin regulates stable microtubule formation and limits retroviral infection in cultured cells. EMBO J. 2007;26:41–52. doi: 10.1038/sj.emboj.7601475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrero-Villar M, Cabrero JR, Gordón-Alonso M, Barroso-González J, Alvarez-Losada S, Muñoz-Ferńndez MA, et al. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J Cell Sci. 2009;122:103–13. doi: 10.1242/jcs.035873. [DOI] [PubMed] [Google Scholar]
- 15.Harmon B, Campbell N, Ratner L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010;6:e1000956. doi: 10.1371/journal.ppat.1000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–25. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–4. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 18.Liu B, Dai R, Tian CJ, Dawson L, Gorelick R, Yu XF. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J Virol. 1999;73:2901–8. doi: 10.1128/jvi.73.4.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilk T, Gowen B, Fuller SD. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J Virol. 1999;73:1931–40. doi: 10.1128/jvi.73.3.1931-1940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibarrondo FJ, Choi R, Geng YZ, Canon J, Rey O, Baldwin GC, et al. HIV type 1 Gag and nucleocapsid proteins: cytoskeletal localization and effects on cell motility. AIDS Res Hum Retroviruses. 2001;17:1489–500. doi: 10.1089/08892220152644197. [DOI] [PubMed] [Google Scholar]
- 21.Hottiger M, Gramatikoff K, Georgiev O, Chaponnier C, Schaffner W, Hübscher U. The large subunit of HIV-1 reverse transcriptase interacts with beta-actin. Nucleic Acids Res. 1995;23:736–41. doi: 10.1093/nar/23.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turlure F, Devroe E, Silver PA, Engelman A. Human cell proteins and human immunodeficiency virus DNA integration. Front Biosci. 2004;9:3187–208. doi: 10.2741/1472. [DOI] [PubMed] [Google Scholar]
- 23.Niederman TM, Hastings WR, Ratner L. Myristoylation-enhanced binding of the HIV-1 Nef protein to T cell skeletal matrix. Virology. 1993;197:420–5. doi: 10.1006/viro.1993.1605. [DOI] [PubMed] [Google Scholar]
- 24.Fackler OT, Kienzle N, Kremmer E, Boese A, Schramm B, Klimkait T, et al. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur J Biochem. 1997;247:843–51. doi: 10.1111/j.1432-1033.1997.00843.x. [DOI] [PubMed] [Google Scholar]
- 25.Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–12. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- 26.Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–9. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- 27.Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol. 2005;171:349–59. doi: 10.1083/jcb.200504029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishita M, Aizawa H, Mizuno K. Stromal cell-derived factor 1alpha activates LIM kinase 1 and induces cofilin phosphorylation for T-cell chemotaxis. Mol Cell Biol. 2002;22:774–83. doi: 10.1128/MCB.22.3.774-783.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, et al. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/S0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]


