Abstract
The autonomous generation of phenotypic diversity in embryonic cell populations can be explained by Waddington’s landscape. The landscape proposes that intra- and inter-cellular interactions mediate the generation of cellular diversity. Recently, we implemented, in a population of Escherichia coli, a synthetic diversification, which is governed by inter-cellular signaling mediated by acyl-homoserine lactone (AHL). The cells with the diversity generator diversified into two distinct cell states, “high” and “low,” if all of the cells started from the low state. The ratio of the states after the diversification was affected by the velocity of autonomous signal accumulation, which depends on the cell density and the AHL production rate of individual cells. The dependency of the ratio on the initial cell density is reminiscent of the community effect, which is observed in animal development and is important for ES-cell differentiation. Therefore, it is worthwhile reviewing the roles of natural animal gene networks with similar topologies to the diversity generator design. The diversity generator design will also be the basis for a tool to direct cell fates on the population level in tissue engineering. Here, we discuss the tunability of the ratio of cell states by our synthetic circuit design.
Keywords: phenotypic diversification, Waddington epigenetic landscape, bifurcation, bioengineering, community effect, inter-cellular signaling, synthetic biology, tissue engineering
Autonomous generation of phenotypic diversity in a cell population plays a key role for the development and regeneration processes in multi-cellular organisms. The diversification is conceptually described as the motion of marbles rolling down Waddington’s landscape.1 In the landscape, bifurcation, which is the change of the number of stable states, occurs as development proceeds. In contrast to the simple concept of the landscape, which can interpret the dynamic phenotypic changes in natural phenotypic diversification from bacteria to mammalian cells,2 the underlying design principles of the diversification, which is regulated by complex networks of biomolecules, are not fully understood, although enormous individual molecules and their interactions are elucidated. Emulating complex biological phenomena with simple and well-characterized synthetic circuits is an efficient approach to investigate the underlying design principles of natural gene networks that give rise to these phenomena.3-6
Recently, using a synthetic genetic circuit named diversity generator in an Escherichia coli population, we achieved synthetic phenotypic diversification,7 in which the cells diversify analogously to the motion of marbles rolling down Waddington’s landscape, mediated by inter-cellular signaling. The diversity generator designed the integration of the mutual inhibitory structure from the genetic toggle switch8 and an inter-cellular signaling system from Vibriro fischeri (Fig. 1). The cells with the diversity generator diversified into two distinct cell states, the high and low states, when the cells were initialized to the low state. Here, we defined the high state as the state where the productions of LacI and AHL production enzyme LuxI are dominant, and the low state as that where the production of CIts is dominant.
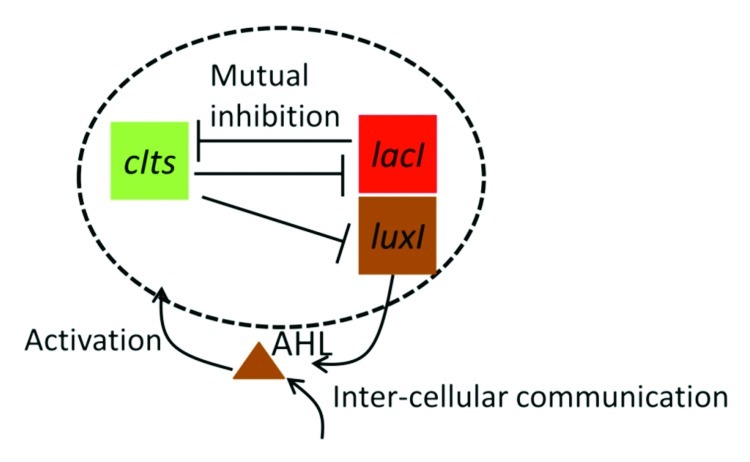
Figure 1. Simplified network diagram of the diversity generator. This network consists of a mutual inhibitory topology of LacI and CIts and inter-cellular signaling system for the activation of the CIts production.
We experimentally showed that the proportion of the low state cells after the diversification depended on the initial cell density and the AHL production rate, which is modulated by the mutation of luxI gene.9 The dependency on the cell density is reminiscent of the community effect,10 which is the experimentally observed dependency of cell fates on the number of cells in developmental systems, and a similar dependency has also been observed in the regulation of ES-cell differentiation.11-13 Therefore, our synthetic diversification achieved by a simple circuit can be a model system to investigate the dynamics of diversifications in development and regeneration.
The design of the diversity generator also has the potential to provide a tool for directing cell fates in tissue engineering because the final proportion of the low state cells with the diversity generator can be tuned by two kinds of modulation: the initial cell density and the AHL production rate. In the context of the application to tissue engineering, the tunability of the proportion of cell states is an important parameter. The proportion is not very sensitive to the initial cell density, so we can fine-tune the proportion. However, the initial cell density is limited in a healthy condition, thereby preventing us from selecting a wide range of proportions. On the other hand, because of the high sensitivity to the AHL synthesis rate, we can tune the proportion roughly by the modulation of the rate. Therefore, by the integration of the two kinds of modulation, we can fine-tune the proportion in a wide range.
There will be many challenges before we can apply the diversity generator design to mammalian cells. However, the fine-tunability of the proportion encourages us to use the design for tissue engineering for the reconstruction of organs where multiple cell states exist, and the ratio of the states is important for the function.
Acknowledgments
This work was supported by the Precursory Research for Embryonic Science and Technology program of Japan Science and Technology to D.K. and the Grant-in-Aid for Scientific Research (KAKENHI) programs from Ministry of Education, Culture, Sports, Science, and Technology (#14085101 to M.H.; #14085203 to M.Y. and D.K.; #23119005 to D.K.; and #23119008 to M.Y.) and Japan Society for the Promotion of Science (#21650018 to M.H. and D.K.; and #23680031 to D.K.). We thank Masahiro Takinoue and other members of the group for helpful discussions.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/20310
References
- 1.Waddington CH. The Strategy of the Genes. London: George Allen & Unwin, 1957:26-41. [Google Scholar]
- 2.Balázsi G, van Oudenaarden A, Collins JJ. Cellular decision making and biological noise: from microbes to mammals. Cell. 2011;144:910–25. doi: 10.1016/j.cell.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner K, Karig DK, Weiss R, Arnold FH. Engineered bidirectional communication mediates a consensus in a microbial biofilm consortium. Proc Natl Acad Sci U S A. 2007;104:17300–4. doi: 10.1073/pnas.0704256104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balagaddé FK, Song H, Ozaki J, Collins CH, Barnet M, Arnold FH, et al. A synthetic Escherichia coli predator-prey ecosystem. Mol Syst Biol. 2008;4:187. doi: 10.1038/msb.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mukherji S, van Oudenaarden A. Synthetic biology: understanding biological design from synthetic circuits. Nat Rev Gen 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ukai-Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144:268–81. doi: 10.1016/j.cell.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Sekine R, Yamamura M, Ayukawa S, Ishimatsu K, Akama S, Takinoue M, et al. Tunable synthetic phenotypic diversification on Waddington’s landscape through autonomous signaling. Proc Natl Acad Sci U S A. 2011;108:17969–73. doi: 10.1073/pnas.1105901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner TS, Cantor CR, Collins JJ. Construction of a genetic toggle switch in Escherichia coli. Nature. 2000;403:339–42. doi: 10.1038/35002131. [DOI] [PubMed] [Google Scholar]
- 9.Kambam PKR, Sayut DJ, Niu Y, Eriksen DT, Sun L. Directed evolution of LuxI for enhanced OHHL production. Biotechnol Bioeng. 2008;101:263–72. doi: 10.1002/bit.21901. [DOI] [PubMed] [Google Scholar]
- 10.Gurdon JB. A community effect in animal development. Nature. 1988;336:772–4. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- 11.Hwang YS, Chung BG, Ortmann D, Hattori N, Moeller HC, Khademhosseini A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 2009;106:16978–83. doi: 10.1073/pnas.0905550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balbach ST, Esteves TC, Brink T, Gentile L, McLaughlin KJ, Adjaye JA, et al. Governing cell lineage formation in cloned mouse embryos. Dev Biol. 2010;343:71–83. doi: 10.1016/j.ydbio.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Choi YY, Chung BG, Lee DH, Khademhosseini A, Kim J-H, Lee S-H. Controlled-size embryoid body formation in concave microwell arrays. Biomaterials. 2010;31:4296–303. doi: 10.1016/j.biomaterials.2010.01.115. [DOI] [PubMed] [Google Scholar]


